Dunn Colin E. Biogeochemistry in Mineral Exploration
Подождите немного. Документ загружается.


As a second example, Table 6-V shows the effect of different grinding times, hence
fineness of particle size, on the analysis of Acacia stems from samples collected in
central Africa.
In Table 6-V gold shows lack of hom ogeneity at the sub-ppb levels. This is typical
for one-gram samples of milled tissue, although gold is more evenly distributed in
tissues of some species than in others. Gold has an acropetal tendency in leaves – i.e.,
it tends to migrate toward leaf tips. Consequently, the fine fraction of mil led leaves is
TABLE 6-V
Stems of Acacia milled for different durations in a grinder with rotary cutting blades. One-
gram samples digested in HNO
3
then aqua regia with ICP-MS finish
20 s grind 40 s grind 60 s grind
Coarse Medium Sieved fines Coarse residue Fine+coarse
Ag (ppb) 2 4 4 3 4
Au (ppb) o 0.2 0.6 0.3 0.2 0.7
B (ppm) 9 11 19 7 14
Ba (ppm) 28 29 62 23 44
Ca (%) 0.99 1.05 2.2 0.9 1.47
Ce (ppm) 0.03 0.02 0.07 0.03 0.05
Co (ppm) 0.01 0.01 0.02 0.01 0.03
Cr (ppm) 1.37 1.55 1.92 1.52 1.68
Cu (ppm) 3.98 4.78 6.57 3.88 5.1
Fe (%) 0.002 0.002 0.004 0.002 0.003
K (%) 0.99 1.12 1.57 1.02 1.33
La (ppm) 0.06 0.04 0.11 0.06 0.09
Li (ppm) 0.02 0.01 0.04 0.03 0.06
Mg (%) 0.089 0.106 0.223 0.087 0.139
Mn (ppm) 11 12 20 13 13
Mo (ppm) 0.47 0.33 0.66 0.43 0.6
Na (%) 0.007 0.008 0.016 0.01 0.01
Nb (ppm) o0.001 0.001 0.009 0.002 0.006
Ni (ppm) 1 1.3 1.8 0.9 1.6
P (%) 0.094 0.106 0.185 0.084 0.132
Pb (ppm) 0.02 0.07 0.02 0.08 0.07
Rb (ppm) 12.4 14.3 19.3 12.1 15.8
S (%) 0.1 0.1 0.17 0.08 0.15
Sb (ppm) 0.05 0.08 0.12 o0.02 0.12
Se (ppm) 0.5 0.4 0.6 0.3 0.6
Sr (ppm) 209 219 443 197 271
Y (ppm) 0.01 0.006 0.017 0.007 0.015
Zn (ppm) 10.5 11.6 22.3 12.4 16
162 Sample Preparation and Decomposition
likely to contain the relatively soft and fragile leaf tips, whereas the coarser residue is
made up of the harder vein tissues which typically contain less gold. The result is that
higher gold values tend to be obtained from fine fractions. This relationship holds,
too, for other tissue types and other elements. Pickering et al. (2000, 2003) used a
Synchrotron to study the distribution and chemical speciation of Se in leaves of the
Se hyperaccumulator plant Astragalus bisulcatus (two-grooved poison or milk vetch).
This fundamental investigation showed, among other observations, that selenate is
located almost exclusively in the soft tissue of mature leaves, rather than the veins of
the leaves (Fig. 6-6).
SAMPLE DECOMPOSITION
Once samples have been prepared for analysis, the next consideration becomes
whether to analyse dry tissue directly or first reduce tissues to ash. If low and ac-
ceptable detection levels can be obtained from the direct analysis of dry tissue, and
the selected method (e.g., ICP-MS, INAA) can generate high-quality data (good
precision and accuracy) for all elem ents of interest, then reducing samples to ash is an
unnecessary step. However, plant tissues contain only minute traces of many ele-
ments, and so from the direct analysis of dry tissue by such methods as AAS and
ICP-ES many values, particularly some of those of relev ance to mineral exploration,
are recorded as ‘below detection’ and ashing may be warranted. The various an-
alytical methods and their merits are discussed in the next chapter, in which dis-
cussions centre on the sophisticated analytical instruments that are becoming
increasingly available for determining ‘ultra-trace’ levels of almost all elements.
Consequently, the multi-element analysis of dry tissues is steadily gaining a foothold
as the method of choice.
Historically, no single analytical instrument was sufficiently sensitive to provide
data for a comprehensive range of elemen ts of relev ance to mineral exploration. The
non-destructive methods of INAA and XRF require no sample decomposition and
provide excellent data for many elements. However, the main limitations for INAA
are (a) the requirement of access to a nuclear reactor; (b) the inability to provide data
for certain elements; and (c) the need for special irradiations to obtain data for
certain elements. Included in the list of elements that are problematic or not pos sible
to determine by INAA are Be, Bi, Cd, Cl , Cu, Ga, Ge, I, In, Li, Mg, Mn, P, Pb, Pd,
Pt, Re, S, Te, Ti, Tl and V. Other elements have detection levels that are too high for
most vegetation samples – e.g., Ag, Ni and Sr. Yet others may require substantial
corrections for fission products, so if a sample has a high U content, then it may be
impossible to report values for Ba, Mo, Te and some of the rare-earth elements
(REE) (Hoffman, 1992). Furthermore, high level s of Na or Br in halophyte species
necessitate additional corrections and therefore higher detection levels. Similarly,
XRF has its limitations. These non-destructive methods are discussed in the next
163
Biogeochemistry in Mineral Exploration
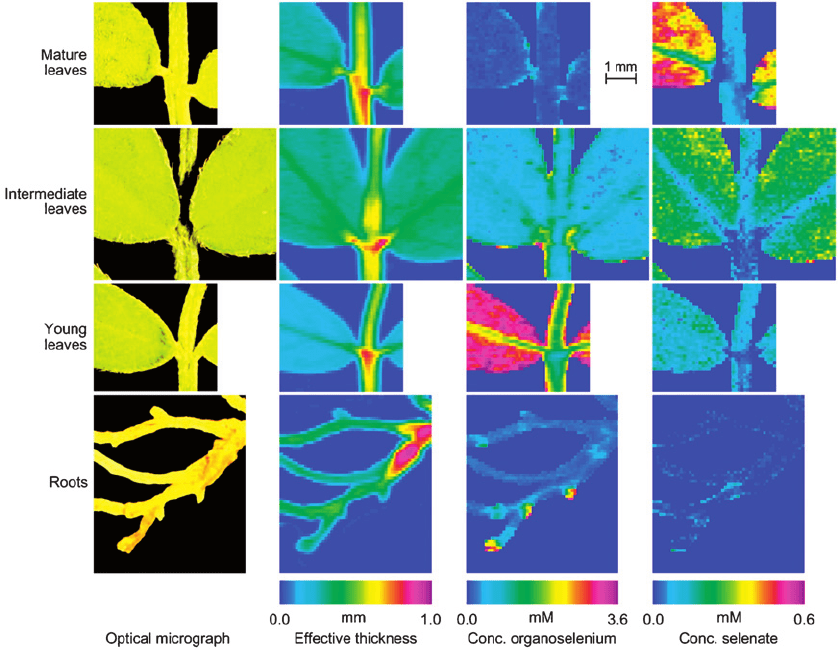
Fig. 6-6. Selenium concentration images of different parts of Astragalus bisulcatus. From left to right: optical micrograph; effective tissue
thickness; concentration of organoselenium; concentration of selenate. Top to bottom: mature, intermediate and young leaves and roots. The
mature leaves, young leaves and roots were taken from the same plant, the mature leaves from the lowest shoot and the young leaves from the
highest shoot of the same plant branch. Reproduced with permission from National Academy of Sciences, USA, Ref. www.pnas.org,
Pickering et al. (2000).
164 Sample Preparation and Decomposition
chapter, and the remainder of this section deals with procedures required to decom-
pose plant tissues prior to their introduction into analytical instruments.
A number of the elements listed above can be determined by other techniques
(e.g., ICP-ES or AAS), but without careful pre-concentration procedures multi-el-
ement scans generate data that are mostly below the level of detection. Many an-
alytical methods have been developed that can address these problems, but they
involve costs that are too high for the implementation of a biogeochemical explo-
ration programme for minerals.
A solution to the problem of plant tissues having very low levels of many elements
of exploration interest is to pre-concentrate them. This can be done by first reducing
samples to ash by controlled ignition to drive off organic compounds, so that only
the inorganic constituents remain for analysis. This ‘ashing’, as it will be referred to
here, substantially reduces the volume of the plant material so that a sample that has,
for example 1 ppm Ni in twig tissue, will have 50 ppm Ni in ash, because the ash yield
of the twig is only 2% (i.e., a 50-fold concentration). From an exploration point of
view, it is more comforting to see a value of 50 ppb Au (in ash) than 1 ppb Au (in dry
material). In practical exploration terms, it is more appeali ng to an exploration
manager to see an ‘anomaly’ of 50 ppb Au than the same ‘anomaly’ at 1 ppb Au in
dry-weight equivalent.
Prior to discussing the merits of analysis of ‘dry’ versus ‘ash’, it is worth noting
that modern analytical equipment (ICP-MS) can now provide remarkably good
multi-element data from the analysis of just 1 g of dry tissue, and so the need to ash
samples is dimi nishing. However, for the reasons discussed above, results of past
surveys were quite commonly reported as concentrations in ash and, since the ex-
pensive state-of-the-art instrumentation will not be universally available for some
years to come, the ashing process warrants discussion.
As is noted repeatedly in this book, in biogeochemical exploration, the absolute
concentrations of elements are of lesser importance than their distribution patterns.
Provided there is a direct relationship between the element content of dry tissue and
the element co ntent of ashed tissue, it makes no difference to the spatial distribution
of anomalous zones of concentrations, and the data therefore provide the same focus
for defining exploration targets.
DRY ASHING
Element volatilization
The most important consideration in dry ashing is the potential loss of some
elements due to volatilization. For Hg, Br, Cl, F and I only a small percentage, if any,
remains in ash after controlled ignition at 475 1C. Although there is partial loss of
many elements during ashing the critical factor for exploration is that, for a given
plant species, the amount of an element lost remains constant. The question is, if, for
165
Biogeochemistry in Mineral Exploration
example, twigs from species ‘A’ are found to lose 20% of their As at one sample
station, do they also lose 20% of their As at another? Provided this loss remains
constant, then the relative concentrations remain the same and it becomes possible to
generate realistic plots of As distribution patterns. These plots of relative concen-
trations are critical to interpreting biogeochemical exploration data, because they
may define stratigraphic, lithological and structural trends, in addition to zones of
mineralization.
Volatilization depends on the chemical form of the element as well as the com-
position of the matrix (i.e., the type of plant tissue). Lead remains in ash if it is present
as its sulphate, nitrate or oxide, but significant loss occurs if it is present as the
chloride (Hall, 1995). Furthermore, during heating to decompose vegetation tissues an
element may react with an organic or inorganic constituent to produce a volatile
chemical species. Various ashing aids can be added to control chemical reactions. The
complexity of potential chemical reactions during the heating phase is enormous and
chemists can point to many combinations of physico-chemical parameters that could
take place to render the ashing process an untenable option. Some of these are
summarized in Hall et al. (1991) and Hall (1995). Fortunately, as will be shown in the
following pages, the actual losses of elements are surprisingly consistent and quan-
tifiable (for most species), confirming that ashing is a viable option. Consistency in the
approach to ashing is, like so many situations in science, the key to obtaining mean-
ingful results that the exploration biogeochemist can plot to reveal structure in the
data that can be attributed to underlying geological conditions.
In order to minimize and standardize elemental losses, the ashing parameters need
to be closely controlled. Ashing should take place by slowly ramping up the tem-
perature of a muffle furnace (or kiln), dedicated to vegetation, to abou t 475 1C. It
rarely makes any difference if a temperature of 500 1C is selected, but it is as well to
be consistent, and 475 1C is about the lowest temperature at which vegetation will be
reduced to ash without a long period of charring. If the temperature is maintained at
450 1C, most types of plant tissue go through a long phase as charcoal before all of
the organic components are released. Increasing the temperature by just 25 1Cto
475 1C greatly accelerates the ashing process.
Borosilicate glass beakers are suitable for ashing vegetation samples; although
after repeated usage the glass be comes etched from reaction with the hot ash and a
few ppm B is transferred from the borosilicate to the ashed samples. However, since
B is commonly present in plant ash in hundreds of ppm, the slight addition of boron
from the beakers does not usually significantly affect natural distribution patterns of
boron.
Quartz vessels are reported to be unsatisfactory for dry-ashing of biological ma-
terials, because Zn (and perhaps other metals) has been found to penetrate the
surface and cannot be readily extracted with acid (Spitzy and Dosudil, 1962). An
alternative is to use large aluminium trays, since they are suitable for ashing mod-
erately large samples (50–100 g) of dry tissue. They can be rinsed between batches of
samples and they can be re-used for many years. Dry tissues of plants commonly
166
Sample Preparation and Decomposition
used in biogeochemical exploration contain tens to hundreds ppm Al. On reduction
to ash, these co ncentrations are magnified 30- to 100-fold, depending on the type of
tissue, so that the content of ash is frequently in the range of 0.1–1% Al. Thus, as in
the case of B, the potential addition to samples of a few or tens ppm Al from contact
with the Al trays is not significant for most purposes. Of course, other vessels should
be used if low-level Al determinations are required.
Temperature control can be particu larly important for a number of reasons, not
least of which is that aluminium trays melt at around 550 1C. Also, at around this
temperature, the carbonate component of the ash may start to dissociate releasing
CO
2
(and possibly some higher temperature phases of metals contained within the
ash), especially if the ash contains a magnesite component. Kovalevsky (1987) notes
that the chemical composition of plant ash approximates that of dolomi tized car-
bonate rocks and so plant ash can be treated as if it is a carbonate.
Tests on dry black spruce samples show that between 100 and 475 1C there is a
weight loss of approximately 98% for twigs and outer bark scales and 97% for
needles; between 475 and 700 1C there is a further loss from the 475 1C ash weight of
15–20%. As the temperature increase continues, additional weight losses occur as
some elemental oxide bonds break down (e.g., PdO), and by the time 900 1Cis
reached all of the carbonate has dissociated releasing CO
2
, and for some tissues a
fused pellet has formed. Elements that show significantly higher concentrations in
ash heated to 900 1C, mostly due to breaking of oxide bonds, include Al, Au, Ba, Eu,
Ga, Ge, Li, Na, Pb, Pd, Yb and W. If ceramic crucibles are used for high-temper-
ature ashing, some tissues are sufficiently reactive at 900 1C for the remaining ash to
further complic ate the situation by fusing with the crucible itself, introducing con-
taminants from the crucible.
The door to the muffle furnace or kiln should remain closed throughout the
ashing process, because if it is opened a rush of fresh oxygen can cause partially
decomposed samples to ignite. This sudden and localized increa se in heat can cause
differential losses of elements among the samples (because of differing temperatures
of element dissociation) with the result that analytical results cannot be effectively
compared. Furthermore, flash fires in the furnace can cause partial or even complete
melting of some aluminium trays. In the former Soviet Union large scale biogeo-
chemical exploration programmes sometimes used open fires to rapidly reduce sam-
ples to ash in the field, claiming that they were able to treat 200–1000 samples per
shift and that the temperature of ashing ranged from 400 to 700 1C with ashing time
ranging from 20 min to 4 h (Kovalevsky, 1987). Kovalevsky noted that
the percentage losses of volatile elements for background and mineralized samples under
standard ashing conditions are similar and have no effect on the main results of ex-
ploration: i.e., the shape, degree of contrast, and intensity of biogeochemical anomalies
and haloes.
Given what is now known about element volatilization at increasing temperatures,
these conditions, although expeditious in the field, are rather too semi-quantitative for
167
Biogeochemistry in Mineral Exploration
some elements and probably too much valuable multi-element information is lost by
not closely controlling the ashing conditions.
Nonaka et al. (1981) determined losses of elements during dry ashing of standard
reference material (SRM ) orchard leaves and bamboo leaves, at stepwise tempe rature
increases from 200 to 800 1C. Their principal findings were [with added comments in
square parentheses] as follows.
Loss of Hg began at 110 1C and increased steadily thereafter [studies using pine
twigs yielded similar results, see Table 6-VI].
[Partial] losses of As and Sb occurred at 200 1C.
There was a sha rp loss of Br, Cl, Cr and Se at 200 1C and again above 500 1C [this
provides further strength to the argument that 475 1C should be a preferred tem-
perature for ashing].
There were no losses of alkaline earths, REE, or Al, Co, Fe, Mn, V and Zn.
Losses of alkali elements were dependent upon the crucible material (e.g., severe in
a silica dish) and sample species.
Once samples have been reduced to ash, care should be taken not to expose them
to highly volatile elements such as Br, Cl, F, Hg, I and S, because ash is a good
absorbent of gases (Kovalevsky, 1987). This phenomenon explains why some multi-
element ICP-MS determinat ions of ash occasionally yield a few ppb Hg.
On occasion a perceived ashing loss can actually be an inadequate digestion of the
ash, or the formation of poorly soluble oxide bonds that require high temperatures to
dissociate. Also, apparent ‘ele ment losses’ can be the result of element incorporation
into the insoluble residue, which is dominated by silica in some plant species (Hoenig
and de Borger, 1983), and the HCl and HNO
3
normally used to digest the ash do not
dissolve silica nor the elements associated with it.
TABLE 6-VI
Loss of Hg from pine twigs (Pinus banksiana)
on heating for 24 h at progressively higher tem-
peratures (material comprising control V6).
Analysis by ICP-MS after aqua regia digestion
Temperature
(1C)
Hg (ppb)
Air-dried 40
70 40
80 40
110 30
150 30
200 o3
168 Sample Preparation and Decomposition
Although the ramping rate does not appear to be critical, an increase in tem-
perature of about 100 1 C per hour has proved to be appropriate and, depending on
the amount and nature of the material to be ashed, the furnace is held at 475 1C for
about 24 h. For small samples of leaf tissue 12 h is sufficient. For cones or large
chunks of wood the ashing may take up to 48 h.
These examples serve to demonstrate the complexity of problems that can exist
during reduction of plant tissues to ash at various temperatures. However, to re-
iterate a point made earlier ‘in order to minimize and standardize elemental losses,
the ashing parameters need to be closely controlled’.
Dry ashing – the realities
The last section outlined many of the potential problems that can arise from
ashing samples. They appear sufficient in number and complexity that the explo-
rationist might dismiss the idea of ashing. In hindsight, it is fortuitous that ashing
was once the only feasible approach for determining concentrations of many ele-
ments, until the commercialization in the 1980s of multi-element INAA of pelletized
dry tissues and, more importantly, the introduction by commercial laboratories over
the past 10 years or so of multi-element analysis of small samples of dry tissue by
ICP-MS. The historic necessity ‘to ash’ provided a large amount of information, and
showed that, pr ovided ash ing takes place under controlled conditions, the biogeo-
chemical signatures are remarkably robust. Modern comparisons of ‘ash’ versus ‘dry’
analyses of the same samples permit evaluation of concentrations (and attendant
losses) of a wide array of elements.
Figures 6-7, 6-8 and 6-9 provide some examples of various Acacia tissues from
Western Australia that were analysed both as 50 g samples reduced to ash (from which
a 0.25 g portion was taken for analysis) and a 1 g sample of milled dry tissue. Both were
digested in aqua regia with an ICP-MS finish. The data from analysis of the ash have
been normalized to a dry weight basis by taking the concentration in ash, dividing by
100 and multiplying by the ash yield. These data are shown on the accompanying CD
as Table 6-ID permitting the user to examine the relationship of ash versus dry for all
51 elements that were determined. The digital table also permits sorting into relative
concentrations of different plant tissues. In the examples illustrated here, samples are
sorted from lowest to highest concentrations, with the average for the dataset (n ¼ 53)
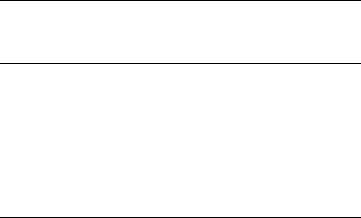
plotted as single symbols on the right of each chart. They illustrate three features.
Figure 6-7 shows examples of elements that exhibit little or no loss from con trolled
ignition to 475 1C. The profiles are almost identical. Gold gives somewhat erratic
results, partly because it is not always all dissolved from dry tissue. This is dis-
cussed in the section on data quality.
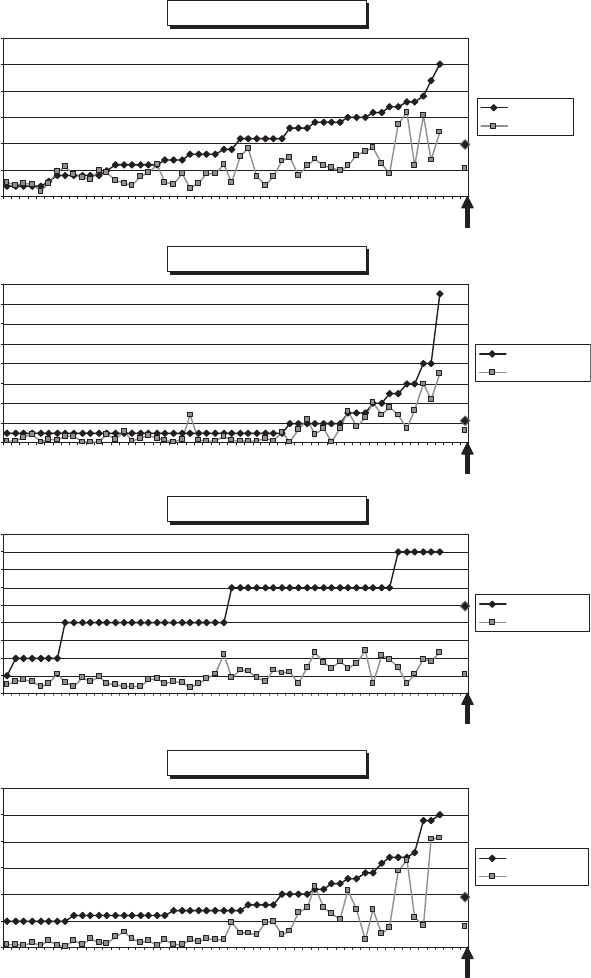
Figure 6-8 shows examples of elements that partially volatilize during ashing.
Figure 6-9 shows elements for which data derived from the analysis of dry tissue
are too close to the detection limit for data structure to be determined. By reducing
169
Biogeochemistry in Mineral Exploration

Dry (diamond) v Ash (square)
Dry (diamond) v Ash (square)
Dry (diamond) v Ash (square)
Dry (diamond) v Ash (square)
0
5
10
15
20
25
30
35
40
45
50
135791113151719212325272931333537394143454749515355
Sequence #
Conc.
0
5
4
3
2
1
6
Conc.
0
5
10
15
20
25
30
Conc.
0
0.1
0.05
0.15
0.2
0.25
0.3
0.35
Conc.
Cu ppm
Cu_Norm ppm
As ppm
As_Norm ppm
Au ppb
Au_Norm ppb
Cd ppm
Cd_Norm ppm
Averages
135791113151719212325272931333537394143454749515355
Sequence #
Averages
135791113151719212325272931333537394143454749515355
Sequence #
Averages
135791113151719212325272931333537394143454749515355
Sequence #
Avera
g
es
Fig. 6-7. Examples of elements showing little or no loss on ignition. Comparison of element
concentrations determined by ICP-MS (aqua regia digestion) in dry Acacia tissues compared
to ashed portions of the same samples. Data normalized to dry weight basis (details on CD –
Table 6-ID).
170 Sample Preparation and Decomposition
S %
S_Norm %
0
0.02
0.04
0.06
0.08
0.1
0.12
0.14
0.16
Sb ppm
Sb_Norm ppm
0
0.05
0.1
0.15
0.2
0.25
0.3
0.35
0.4
0.45
Se ppm
Se_Norm ppm
0
0.05
0.1
0.15
0.2
0.25
0.3
Sn ppm
Sn_Norm ppm
Dry (diamond) v Ash (square)
Dry (diamond) v Ash (square)
Dry (diamond) v Ash (square)
Dry (diamond) v Ash (square)
0
0.05
0.1
0.15
0.2
0.26
0.3
135791113151719212325272931333537394143454749515355
Sequence #
Conc.
Conc.Conc.Conc.
Averages
1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49 51 53 55
Sequence #
Averages
1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49 51 53 55
Sequence #
Averages
1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49 51 53 55
Sequence #
Avera
g
es
Fig. 6-8. Examples of elements showing moderate to significant loss on ignition. Comparison
of element concentrations determined by ICP-MS (aqua reiga digestion) in dry Acacia tissues
compared to ashed portions of the same samples. Data normalized to dry weight basis (details
on CD – Table 6-ID).
171Biogeochemistry in Mineral Exploration
