Drake G.W.F. (editor) Handbook of Atomic, Molecular, and Optical Physics
Подождите немного. Документ загружается.


Relativistic Atomic Structure References 357
22.74 J. Kaniauskas, Z. B. Rudzikas: J. Phys. B 13,3521
(1980)
22.75 G. Gaigalas, S. Fritzsche: Comput. Phys. Commun.
134, 86 (2001)
22.76 S. Fritzsche: Comput. Phys. Commun. 103,51
(1997)
22.77 S. Fritzsche, S. Varga, D. Geschke, B. Fricke: Com-
put. Phys. Commun. 111, 167 (1998)
22.78 G. Gaigalas, S. Fritzsche, B. Fricke: Comput. Phys.
Commun. 135, 219 (2001)
22.79 T. Inghoff, S. Fritzsche, B. Fricke: Comput. Phys.
Commun. 139, 297 (2001)
22.80 S. Fritzsche, T. Inghoff, T. Bastug, M. Tomaselli:
Comput. Phys. Commun. 139, 314 (2001)
22.81 L. V. Chernysheva, V. L. Yakhontov: Comput. Phys.
Commun. 119, 232 (1999)
22.82 C. F. Fischer, T. Brage, P. Jönsson: Computational
Atomic Structure. An MCHF Approach (Institute of
Physics Publishing, Bristol, Philadelphia 1997)
22.83 E. R. Davidson: J. Comput. Phys. 17, 87 (1975)
22.84 E. R. Davidson: Comput. Phys. Commun. 53,49
(1989)
22.85 A. Stathopoulos, C. F. Fischer: Comput. Phys. Com-
mun. 79, 268 (1994)
22.86 J. Olsen, M. R. Godefroid, P. Jönsson, P. Å. Malm-
qvist, C. F. Fischer: Phys. Rev. E 52, 4499 (1995)
22.87 P. Jönsson, C. F. Fischer: Phys. Rev. A 57, 4967 (1998)
22.88 Y Zou, C. F. Fischer: Phys. Rev. Lett. 88, 183001
(2002)
22.89 J. P. Desclaux, private communication, December
2003
22.90 J. Biero
´
n, P. Jönsson, C. F. Fischer: Phys. Rev. A 53,
1 (1995)
22.91 J. Biero
´
n, I. P. Grant, C. F. Fischer: Phys. Rev. A 58,
4401 (1998)
22.92 J. Biero
´
n, C. F. Fischer, I. P. Grant: Phys. Rev. A 59,
4295 (1999)
22.93 P. Jönsson, C. F. Fischer: Comput. Phys. Commun.
100, 81 (1997)
22.94 S. Fritzsche: J. Elec. Spec. Rel. Phenom. 114–116,
1155 (2001)
22.95 S. Fritzsche: Phys. Scr. T 100, 37 (2002)
22.96 S. Fritzsche, J. Anton: Comput. Phys. Commun. 124,
353 (2000)
22.97 S. Fritzsche, C. F. Fischer, C. Z. Dong: Comput. Phys.
Commun. 124, 340 (2000)
22.98 The CPC Program Library home page is at
http://www.cpc.cs.qub.ac.uk/cpc/
Part B 22
359
Many-Body Th
23. Many-Body Theory of Atomic Structure
and Processes
All atoms except hydrogen are many-body
systems, in which the interelectron interaction
plays an important or even decisive role. The aim
of this chapter is to describe a consistent method
for calculating the structure of atoms and the
characteristics of different atomic processes, by
applying perturbation theory to take into account
the interelectron interaction. This method involves
drawing a characteristic diagram based on the
structure or process. This is then used to create an
analytical expression to the lowest order in the
interelectron interaction. Higher-order corrections
are subsequently generated.
This technique was invented about half
a century ago in quantum electrodynamics by
Feynman [23.1], then modified and adjusted for
multiparticle systems by a number of authors.
Its application to atomic structure and atomic
processes required further modifications, which
were initiated at the end of the fifties (see,
e.g., [23.2]) and later. The corresponding technique
was successfully applied to the calculation of
a wide variety of characteristics and processes
in many papers and several review articles
[23.3, 4]. The increasing amount of experimental
data available has led to improved accuracy
for this technique, so that it can be applied to
current problems considering not only atoms
and ions, both positive and negative [23.5–8],
but also molecules [23.9], clusters [23.10]and
fullerenes.
23.1 Diagrammatic Technique...................... 360
23.1.1 Basic Elements.......................... 360
23.1.2 Construction Principles
for Diagrams............................. 360
23.1.3 Correspondence Rules................ 362
23.1.4 Higher-Order Corrections
and Summation of Sequences..... 363
23.2 Calculation of Atomic Properties ........... 365
23.2.1 Electron Correlations
in Ground State Properties ......... 365
23.2.2 Characteristics
of One-Particle States ................ 366
23.2.3 Electron Scattering .................... 367
23.2.4 Two-Electron and Two-Vacancy
States ...................................... 369
23.2.5 Electron–Vacancy States............. 370
23.2.6 Photoionization
in RPAE and Beyond .................. 371
23.2.7 Photon Emission
and Bremsstrahlung .................. 374
23.3 Concluding Remarks ............................ 375
References .................................................. 376
The elements of the diagrammatic technique,
which form a convenient and simple “language”,
are given together with the rules for creating
“sentences” using basic “words”. A kind of
“dictionary” helps to translate diagrammatic
“sentences” into analytical expressions suitable
for calculations.
An essential part of the program is to learn how the sim-
plest approximation can be improved, and what are the
mechanisms and processes connected with, and respon-
sible for, higher-order corrections.
When the diagrammatic technique of many-body
theory is used, it is unnecessary to be restricted to a finite
number of lowest-order terms in the interelectron inter-
action. On the contrary, some infinite sequences may
be taken into account. The sum of all many-body di-
agrams is completely equivalent to the many-particle
Schrödinger equation. Therefore, taking all of them into
account is just as complicated as solving the corre-
sponding equation. Compared with other approaches,
the diagrammatic technique can easily uncover hidden
approximations and transparently demonstrate possible
sources of corrections.
Part B 23
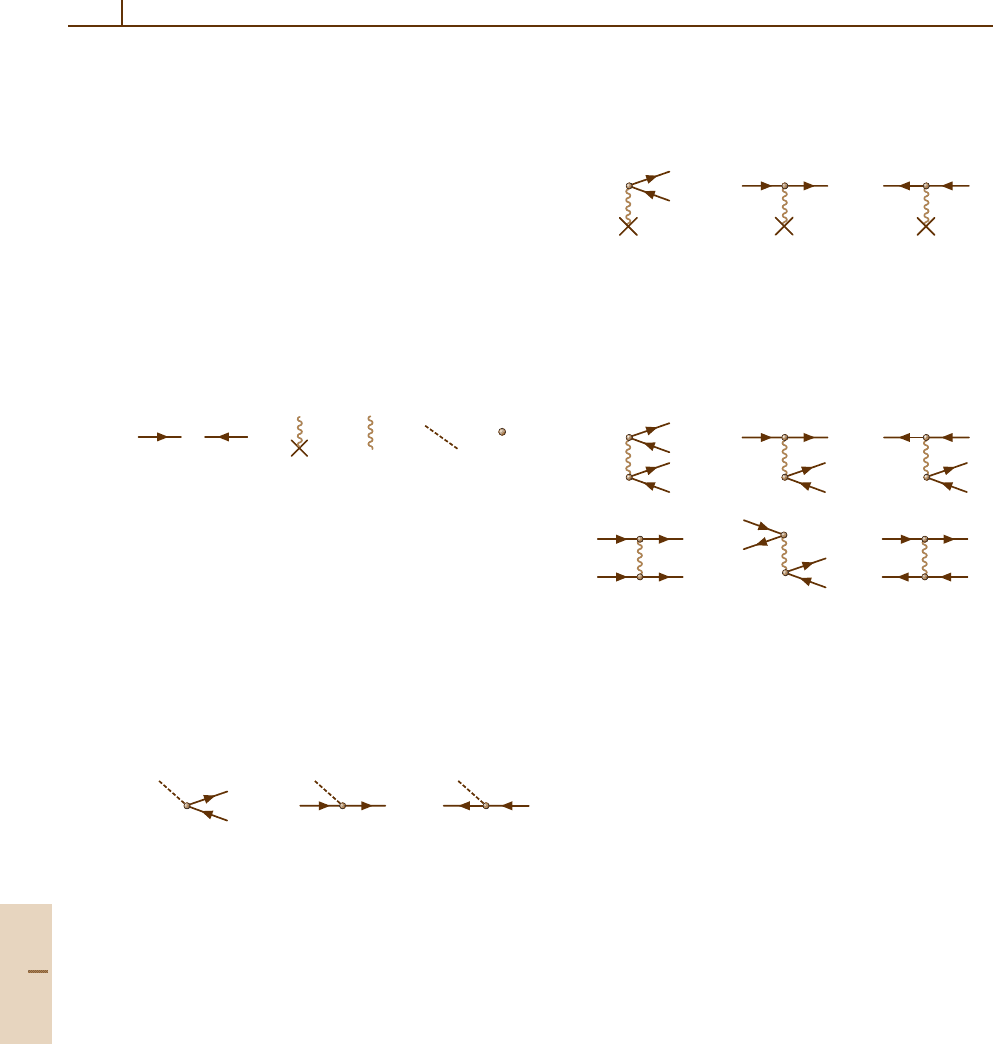
360 Part B Atoms
23.1 Diagrammatic Technique
23.1.1 Basic Elements
Each physical atomic process (or a process with par-
ticipation of a molecule, cluster or fullerenes) involves
an electronic interaction with a projectile or external
field, in general time-dependent, or a mutual inter-
electronic interaction. By convention, the ground state
of the atom (if it is not degenerate) is regarded as the
vacuum state. Then the simplest process in this target is
excitation of an electron to an unoccupied level, leav-
ing behind a vacancy. The basic elements of a diagram
are
a) b) c) d) e) f)
(23.1)
where (a) with an arrow directed to the right represents
an electron excited to a vacant level; (b) with an arrow
directed to the left represents a vacancy; (c) with a cross
represents the static Coulomb interaction; (d) represents
the interelectron Coulomb interaction; (e) represents
interaction with a time-dependent external field, usu-
ally electromagnetic; and (f ) represents the very act of
interaction.
The elements (23.1a–f) in combination can describe
the following real or virtual basic processes
a) b) c)
(23.2)
which represent (a) photon absorption by the vacuum
with electron–vacancy pair creation; (b) electron ex-
citation; and (c) vacancy excitation. Diagrams (23.2)
depict processes as developing in time, shown in-
creasing from left to right. A vacancy can be
thought of as an antiparticle to the electron, mov-
ing backward in time. The time-reverse of processes
(23.2) represent processes of photon emission due
to annihilation of an electron–vacancy pair, va-
cancy transition, and electron inelastic scattering,
respectively.
A static [for example, Coulomb (23.1c)] field can
virtually create an electron–vacancy pair, or affect the
moving electron or vacancy, as shown in the following
diagrams:
a) b) c)
(23.3)
Just as for (23.2), diagrams (23.3) have their time-
reversed counterparts.
Inclusion of interelectron interaction leads to a num-
ber of processes of which some examples are
a) b) c)
d) e) f)
(23.4)
Here, (23.4a) describes creation of two electron–
vacancy pairs, (23.4b) represents the simplest picture
of electron inelastic scattering, (23.4c) depicts vacancy
decay with electron–vacancy pair creation, (23.4d)
stands for electron–electron scattering, (23.4e) repre-
sents a process which can be called electron–vacancy
annihilation and creation, while (23.4f ) shows electron–
vacancy scattering.
23.1.2 Construction Principles for Diagrams
The foundations of diagrammatic techniques are dis-
cussed in a number of books such as [23.11]. This
chapter presents recipes for the construction and eval-
uation of diagrams corresponding to various atomic
processes [23.12].
The basic procedure is to connect the initial and final
states of the atom, drawn at the left and right sides of the
diagram, using any of the elements in (23.1). In doing
so, the following rules apply:
1. At each dot (23.1f), only three lines can meet: wavy
(or dashed) and electron–vacancy.
2. A vacancy cannot be transformed into an electron or
vice versa.
Part B 23.1
Many-Body Theory of Atomic Structure and Processes 23.1 Diagrammatic Technique 361
3. Electrons and vacancies can be created only pairwise
from the vacuum.
4. Only linked diagrams are allowed; i. e. only those
having no parts entirely disconnected from one an-
other.
The simplest or initial approximation to a process
is represented by a diagram which includes the lowest
possible number of elements (23.1–23.4). Higher-order
corrections can be derived by including additional
elements of interaction with the static field of the
nucleus (23.1c) and between electrons and/or vacancies
(23.1d).
As an illustration of the method, consider the fol-
lowing three processes:
1. one-electron photoionization – the initial state is
a photon while the simplest final state is an electron–
vacancy pair. They can be combined together giving
the basic diagram (23.2a).
2. elastic electron scattering – the initial and final states
are single electrons. To describe the simplest scatter-
ing process, the interaction with the Coulomb field
must be taken into account, leading to (23.3b). To
account for interelectron interaction, the simplest
element
(23.5)
must be introduced. It is a modification of (23.4b)
accounting for the interaction of an incoming elec-
tron with all target electrons individually, not altering
their states. This is emphasized by the loop in which
the same vacancy leaving the lower dot reenters it.
Indistinguishability of all electrons as fermions is
taken into account by permutation of the electron
(vacancy) line ends, as illustrated in the following
diagrams:
a) or b)
(23.6)
Diagram (23.6a) is obtained from (23.5)byper-
mutation of the electron lines on one side of the
interelectron interaction. Diagram (23.6b) is equiva-
lent to (23.6a), but is simpler to draw.
3. inelastic electron scattering – the initial state is
a single electron. For the final state we choose one
with two electrons and therefore a single vacancy.
The simplest diagram in this case is given by (23.4b).
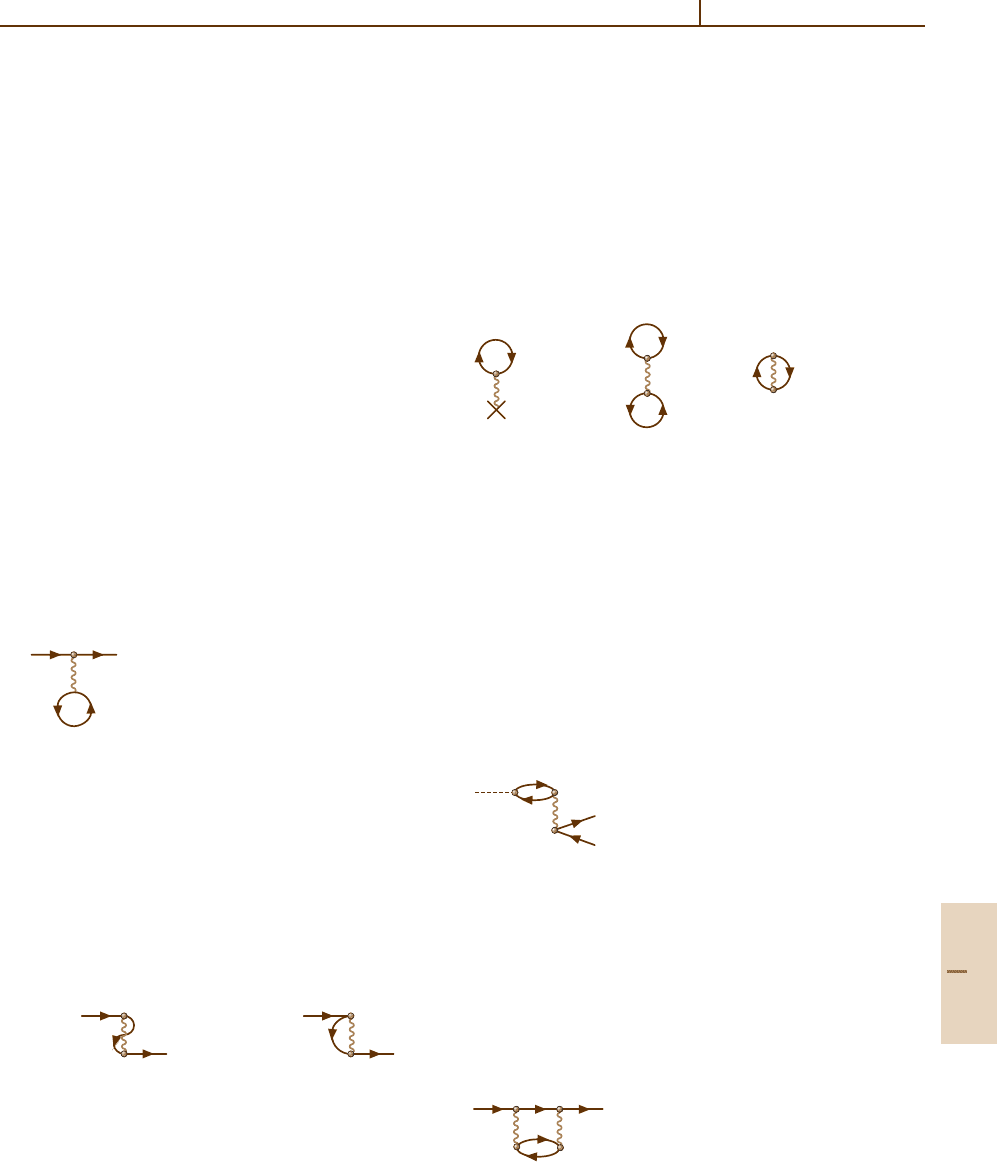
To illustrate the description of the ground state char-
acteristics, consider the contributions to the ground state
energy of an atom. If this state is not degenerate, its po-
tential energy is given by vacuum diagrams which have
no free lines in the initial or final states. The simplest
vacuum diagrams are
a) b) c)
(23.7)
Higher-order corrections to all these diagrams can be
obtained by adding elements such as a static external
field (23.3) or interelectron interaction (23.4) without
changing the initial and final states of the processes.
The lowest-order processes are represented by (23.2a),
(23.5), (23.6), (23.4b), and (23.7). There are many cor-
rections even in the next order of interaction, either with
an external field or with electrons or vacancies. To il-
lustrate, only one correction to each process will be
presented:
1. Simple photoionization (23.2a) may be combined
with (23.4e) to obtain
(23.8)
This describes the effect of the creation of another
electron–vacancy pair, after annihilation of the first
one formed by absorption of the initial photon.
2. Simple elastic electron scattering (23.5) can be
combined with an extra interaction term (23.4f) be-
tween the incoming electron and the vacancy of the
loop (23.5), to obtain
(23.9)
Part B 23.1
362 Part B Atoms
3. Simple inelastic electron scattering (23.4b) can also
be combined with (23.4f), accounting for the in-
teraction of an electron and vacancy created in the
lowest-order process, to obtain
(23.10)
4. The ground state energy term (23.7b) can be com-
bined with (23.7b) and the element (23.3c) of the
interaction between the vacancy in the (23.7b) loops
and the static field to obtain
(23.11)
Higher-order corrections can be constructed step by
step by introducing further elements of interaction. In
some cases, classes of diagrams may be taken into ac-
count up to infinite order by solving closed systems of
integral or differential equations.
23.1.3 Correspondence Rules
These rules describe how to obtain an analytical ex-
pression corresponding to a given diagram. One starts
by choosing a zero-order approximation which can be
that of independent electrons moving in the Coulomb
field of an atomic nucleus. Atoms with completely occu-
pied shells, or subshells having a non-degenerate ground
state, can be chosen as the vacuum. Electron (vacancy)
states are characterized in this case by the quantum
numbers n, , m
,andσ =±1/2.
The first correspondence rule is to substitute a matrix
element for each interaction:
Diagram (23.2) →p|W|q ,
Diagram (23.3) →p|U|q ,
Diagram (23.4) →pt|V|qs , (23.12)
where W is the interaction potential of an electron with
the external time-dependent field, U is the interaction
potential of an electron (vacancy) with an external static
field, for example that of the nucleus, and V is the
Coulomb interelectron interaction. Each of the letters
p, q, t, s represents a full set of n, , m
, σ quantum
numbers. Vacancy states are below (and include) the
highest occupied energy level, called the Fermi level,
so that p ≤ F. Electron states are above the Fermi level
so that q > F. Thus diagram (23.2a) is represented by
p|W|q with p ≤ F and q > F.
Apart from initial and final states, each diagram can
have sections, i. e., intervals between successive inter-
actions. For instance (23.9)and(23.10) each have one
section. Each section is represented by an inverse energy
denominator ε
−1
d
. It includes the sum over all vacancy
energies
vac
ε
i
minus the sum of the electron energies
el
ε
n
to which the entrance energy E of the diagram
(e.g.
ω for a time-dependent field) must be added:
ε
−1
d
=
vac
ε
i
−
el
ε
n
+ E
−1
. (23.13)
The second correspondence rule is to identify sections
and write down their energy denominators. After at-
tributing to each electron (vacancy) line a letter, denoting
its state, the analytical expression for a diagram is given
by
Analytical Expression
= (the product of all interaction matrix elements)
× (all energy denominators)
−1
× (−1)
L
summed over all intermediate
electron and vacancy states,
(23.14)
where L is equal to the sum of the total number of
vacancy lines and closed vacancy or electron–vacancy
loops.
Although electrons are fermions, the summation
in (23.14) has no additional restrictions caused by the
Pauli principle. It runs over all electron (> F) and va-
cancy (≤ F) states, including those where two or more
electrons (or vacancies) are in the same state. The cor-
respondence rules (23.12), (23.13), and (23.14) can be
illustrated by giving as examples the analytical expres-
sions of two diagrams (23.8)and(23.9).
Attributing letters denoting electron and vacancy
states, diagram (23.8) becomes
ω
r
t
f
i
(23.15)
Part B 23.1
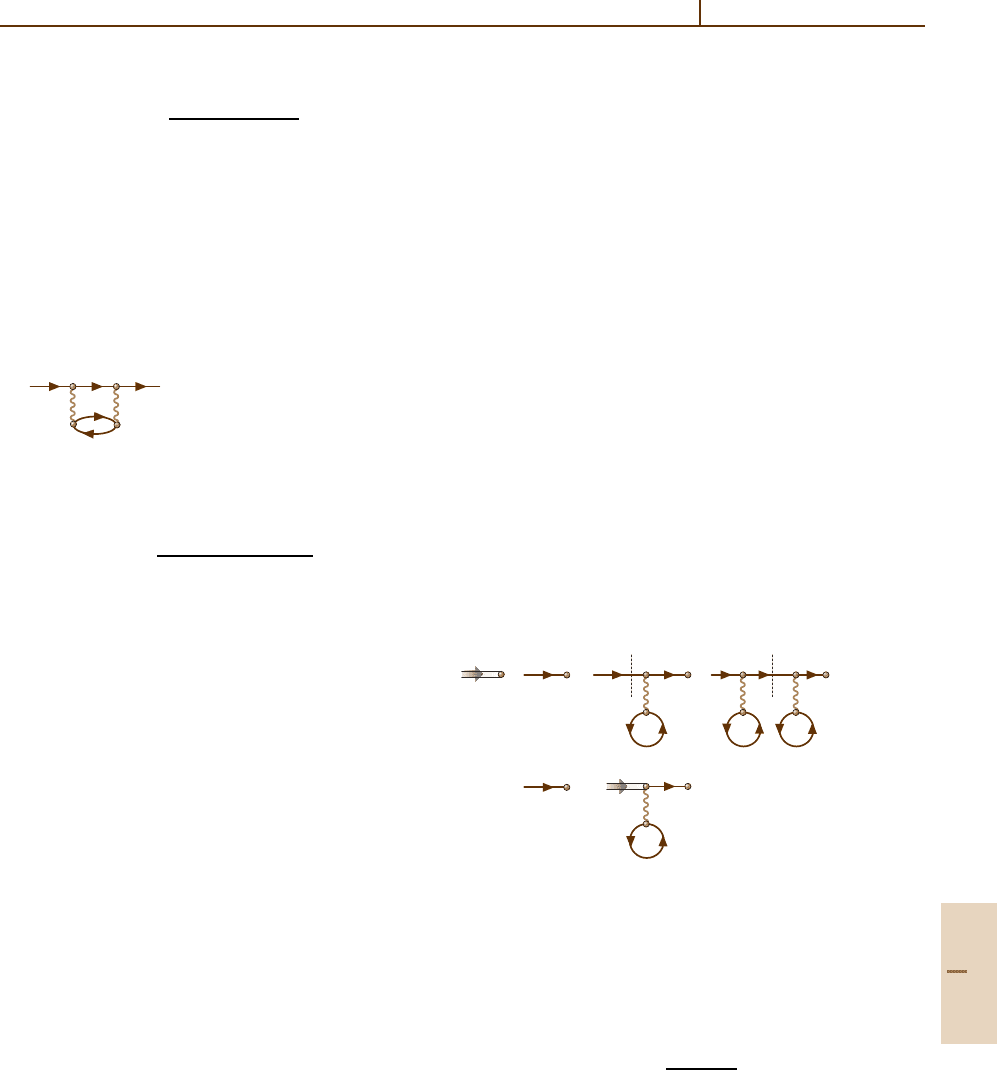
Many-Body Theory of Atomic Structure and Processes 23.1 Diagrammatic Technique 363
Accordingto(23.12–23.14), the analytical formula
A
if
(ω) =
r>F,t≤F
t|W|rri|V|tf
ε
t
− ε
r
+ ω
(−1)
2+1
(23.16)
is obtained. The symbol
includes summation over
discrete levels and integration. In (23.16), the interme-
diate state is r > F, t ≤ F and the diagram has two
vacancies (t and i) and one loop rt. Integration must
be performed over those states r which belong to the
continuum.
Assigning letters denoting states, (23.9) appears as
pq p'
r
t
(23.17)
where p, q, p
, r > F, while t ≤ F. According to
(23.14),
∆E =
r,q>F;t≤F
pt|V|qrqr|V| p
t
ε
t
− ε
q
− ε
r
+ ε
p
(−1)
1+1
,
(23.18)
where the intermediate states are q, r > F and t ≤ F.It
has one vacancy and one electron–vacancy loop rt.
An intermediate state in a diagram can be real or
virtual. It is real if the energy conservation law can be
fulfilled, i. e. if for some values of the section energy the
following relation holds:
E =
el
ε
n
−
vac
ε
i
. (23.19)
If (23.19) can be fulfilled, a prescription for avoiding
the singularity in (23.13) is to substitute the expression
ε
−1
d
Q,where
Q = lim
η→0
E −
el
ε
n
+
vac
ε
i
+ iη
−1
=P
E −
el
ε
n
+
vac
ε
i
−1
− iπδ
E −
el
ε
n
+
vac
ε
i
,
(23.20)
for ε
−1
d
.Here P denotes that the principal value is to
be taken on integration over intermediate state energies.
The result of (23.20) can thus be complex.
An intermediate state is virtual if the energy con-
servation law (23.19) is violated for all values of the
section energy. In general, the bigger the virtuality,
i. e. the difference E −
el
ε
n
+
vac
ε
i
, the smaller the
contribution to the amplitude of the process.
23.1.4 Higher-Order Corrections
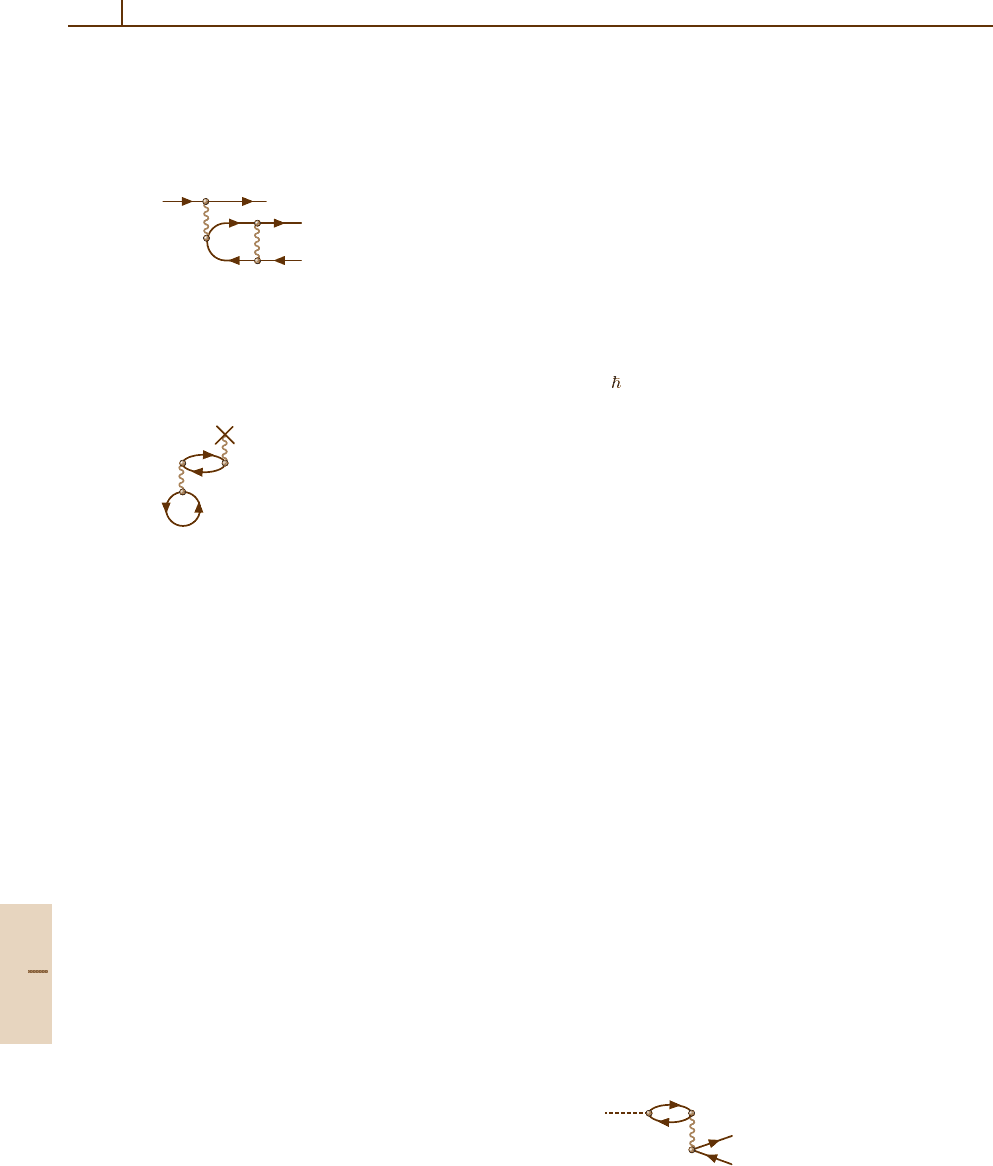
and Summation of Sequences
An important feature of the diagrammatic technique is
the convenience in constructing higher-order corrections
and in the summation of infinite sequences of diagrams.
According to (23.13), each new interaction line leads
to an additional interaction matrix element, extra en-
ergy denominator and summation over new intermediate
states.
An important example of infinite summation
is that of determining the one-electron states.
The interaction with the nucleus (23.3b) and
with atomic electrons (23.5)and(23.6) is not
small and must be taken into account non-
perturbative; i. e., these elements must be iterated
infinitely. To simplify the drawing, only the elem-
ent (23.5) is repeated, leading to the diagrammatic
equation:
= + + + ...
pppqpqq'
p
=
+
p
q
i
i
ii'
12
(23.21)
Indeed, everything in the infinite sum which is in front
of the dashed line repeats the infinite sum itself, thus
leading to a closed equation of the form
˜
p|=p|+
q>F
˜
pi|V|qi
1
−ε
q
+ ε
p
q| . (23.22)
The two interactions leading to (23.21) can be permuted,
so that the interaction 1 can be after 2. This leads to
Part B 23.1
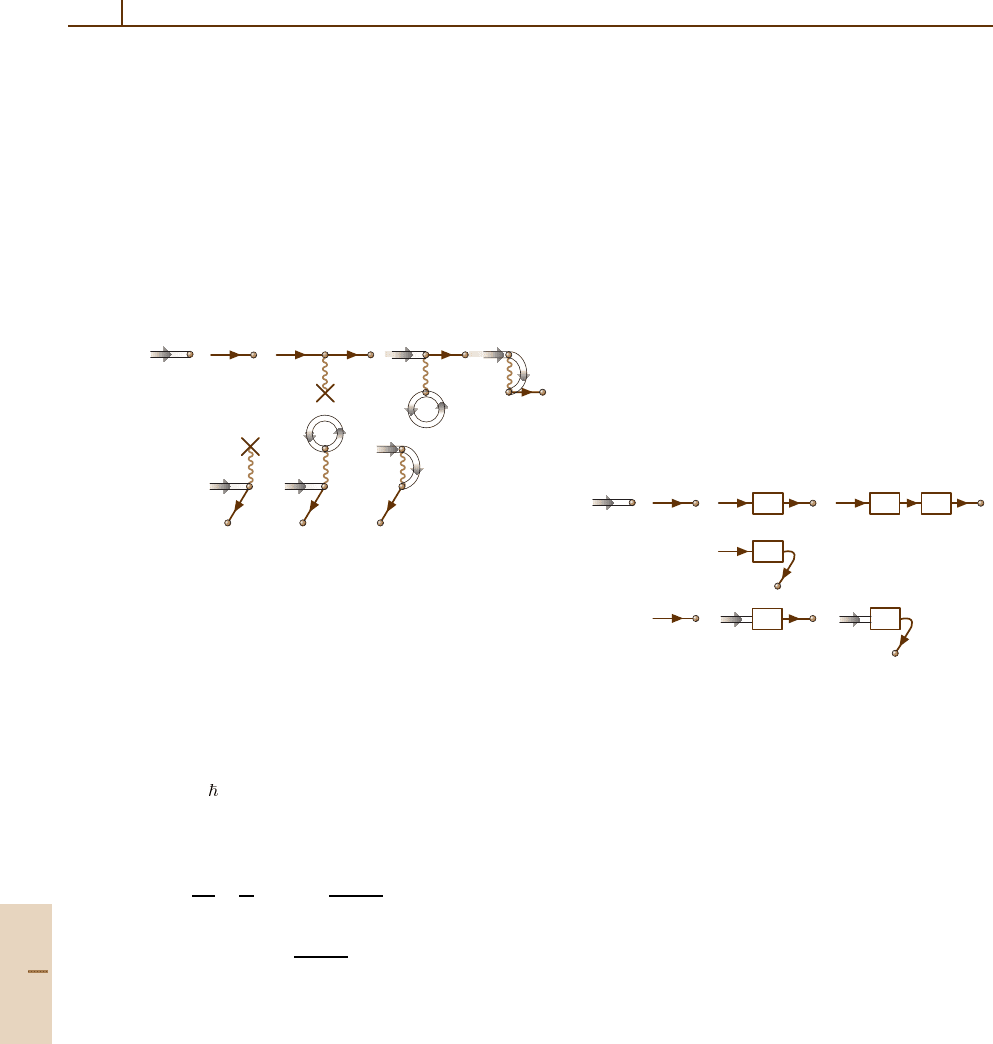
364 Part B Atoms
extension of the sum to include states with q ≤ F.As
a result, the summation in (23.22) must be performed
over all states q.
Interaction with the nucleus and the other elec-
trons affects also the occupied (or vacancy) states i
in (23.21) and therefore the latter must be modified by
inserting the elements (23.3b), (23.5), and (23.6)into
them. Here again, the vacancy line in (23.5)and(23.6)
must be modified by including the corrections (23.3b),
(23.5), (23.6) and so on. Finally, the diagrammatic
equation
=+ +
pppqpq
p
+
+
i
q
+
p
i
q
p
q
i
+
p
i
q
(23.23)
is obtained. The doubled line for i emphasizes that the
vacancy wave function is determined by an equation
similar to (23.21). The corresponding analytical equa-
tion looks like (23.22), but includes also the Coulomb
interaction with the nucleus and the exchange interac-
tion with other atomic electrons. The summation over q
in this equation is extended over all q, not only q > F.
Multiplying the corresponding equation by (
ˆ
H
0
− ε
p
)
from the right (atomic units are used in this chapter:
e = m
e
= = 1),where
ˆ
H
0
=−∇
2
/2, and using the
completeness of the functions
q
|qq|=δ(r − r
),
results in the equation
−
∇
2
2
−
Z
r
+
i≤F
dr
|r
−r|
|φ
i
(r
)|
2
− ε
p
φ
p
(r)
=
i≤F
dr
|r
−r|
φ
∗
i
(r
)φ
p
(r
)φ
i
(r) (23.24)
for the electron wave function φ
p
(r).Hereφ
i
(r) are
wave functions determined by equations similar to
(23.24). These are the Hartree–Fock (HF) equations.
HF includes a part for interelectron interaction ma-
trix elements, namely that given by (23.5)and(23.6).
The rest is called the residual interaction, and its in-
clusion leads beyond the HF frame, accounting for
correlations.
When a perturbative approach is used, it is essential
to define the zero-order approximation. In this chapter,
and very often in the literature, the Hartree–Fock ap-
proximation is used in this role. To simplify the drawing
of diagrams, from now on single (rather than double)
lines will represent electrons (vacancies), whose wave
functions are determined in the HF approximation by
(23.24). Obviously, in this case elements (23.3a), (23.5)
and (23.6) should not be added to any other diagrams.
Theprocedureusedinderiving(23.21)and(23.23)
is in fact more general. Let us separate all diagrams de-
scribing elastic scattering which do not include a single
one-electron or one-vacancy state as intermediate. De-
picting their total contribution by a square, the precise
one-particle state is determined by an infinite sequence
of iterative diagrams which can be summed, similarly to
(23.21), by
=+ +
pppq
q
pqq'
Σ
ˆ
Σ
ˆ
Σ
ˆ
+…
+
p
Σ
ˆ
+
…
=
p
+
p
Σ
ˆ
q
+
q
p
Σ
ˆ
(23.25)
Here the single line stands for an HF state. Using the
correspondence rule (23.14), an analytical equation sim-
ilar to the Schrödinger equation can be derived with the
operator
ˆ
playing the role of an external potential.
The essential difference is, however, that this “poten-
tial” depends in principle upon the energy and state of
the particle. The same kind of iterative procedure lead-
ingto(23.21)or(23.23) will be used several times in
this chapter.
Other zero-order approximations can be chosen.
But then diagrams with corrections of the type (23.3a)
must be included, with the external static field potential
equal to the difference between the HF and the chosen
one.
To calculate the numerical value of a given dia-
gram or a sequence of diagrams one needs to know,
according to the description given above, the matrix
elements of external fields and interelectron interac-
tions obtained with the help of one-electron HF wave
functions. The required calculational procedures are
described in [23.13].
Part B 23.1
Many-Body Theory of Atomic Structure and Processes 23.2 Calculation of Atomic Properties 365
23.2 Calculation of Atomic Properties
23.2.1 Electron Correlations
in Ground State Properties
A major advantage of the diagrammatic technique in
many-body theory is that it is usually unnecessary to
know the total wave function of the atom. On the con-
trary, only actively participating electrons or vacancies
appear in a diagram. The HF zero-order approxima-
tion for one-electron and one-vacancy wave functions
is used in what follows. All atomic characteristics and
cross sections for atomic processes calculated with HF
form the one-electron approximation. Everything be-
yond the HF frame, i. e., caused by residual interaction,
are called correlation corrections or correlations. They
can be calculated using the many-body perturbation the-
ory (MBPT) [23.3], random phase approximation (RPA)
[23.14] and random phase approximation with exchange
(RPAE) or its generalized version GRPAE [23.12, 13].
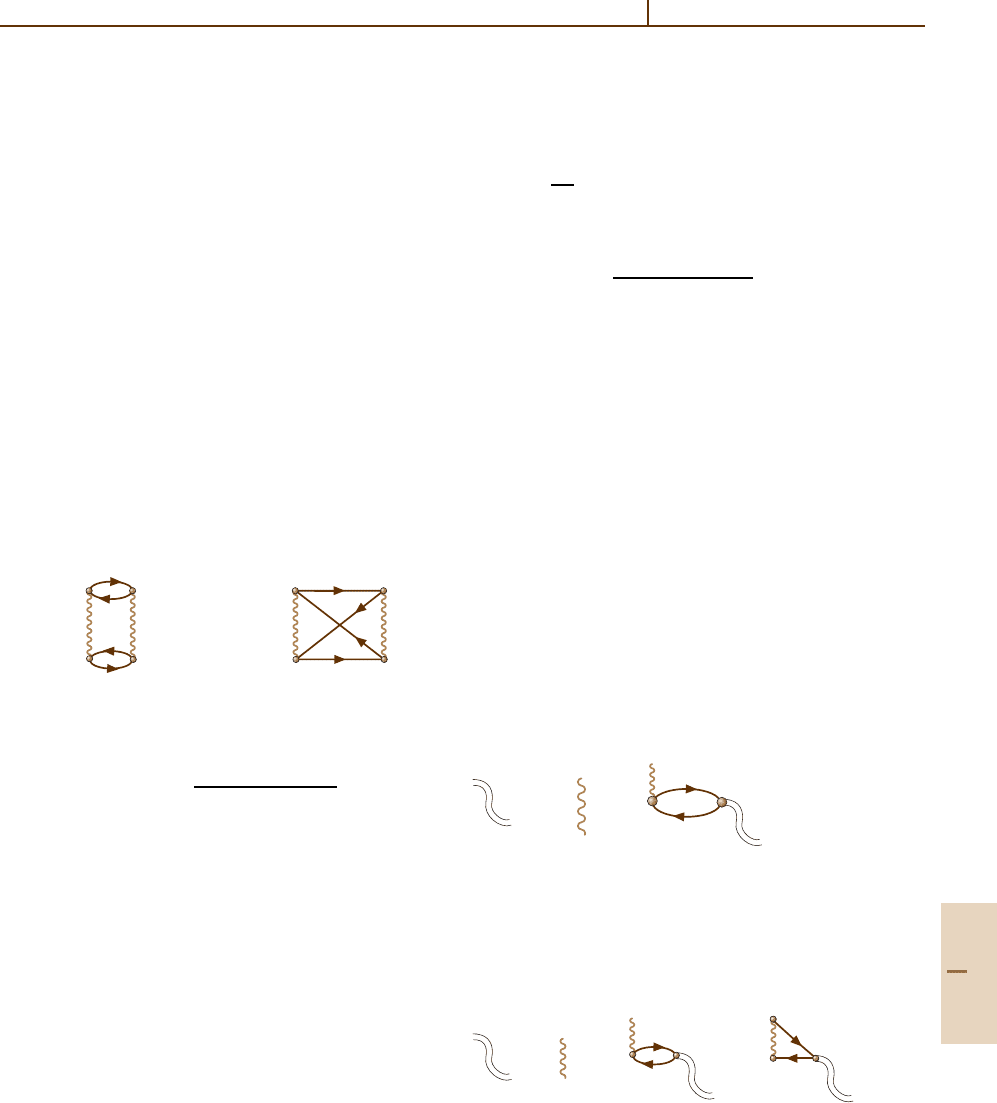
The simplest diagrammatic expression for the corre-
lation energy is given by the two diagrams
a) b)
k
i
j
n
k
j
n
i
(23.26)
The analytical expression ∆E
(2)
corr
for (23.26a) is
∆E
(2)
corr
=
k,n>F;i, j≤F
ij|V|knkn |V|ij
ε
i
+ ε
j
− ε
k
− ε
n
. (23.27)
The analytical expression for (23.26b) differs from
(23.27) by the sign and an exchange matrix element
kn|V| ji instead of a direct kn|V|ij one. The contri-
bution (23.26) overestimates the correlation energy by
≈10%.
Diagrams (23.26) can also be used to describe the
interaction potential of two atoms, designated A and B.
Let the ki states belong to A and njto B. At large dis-
tances R between the atoms, the contribution of (23.26b)
is exponentially small. Because the vacancies i and j
are located inside atoms A and B respectively, the
interelectron potential V =|r
A
−r
B
+ R|
−1
at large dis-
tances R R
A,B
,(R
A,B
are atomic radii), can be
expanded as a series in powers of R
−1
.Thefirst
term giving a non-vanishing contribution to (23.27)is
V R
−3
[(r
A
· r
B
) − 3(r
A
· n)(r
B
· n)], n being the unit
vector in the direction of R. Substituted into (23.26),
this potential leads to the expression
U(R) =−
C
6
R
6
(23.28)
for the interatomic potential [23.15], where
C
6
≈
k,n>F;i, j≤F
|i|r|k|
2
| j|r|n|
2
(ε
i
+ ε
j
− ε
k
− ε
n
)
.
(23.29)
Calculations [23.16] show that the inclusion of
higher-order corrections is important for obtaining ac-
curate values for ∆E
corr
and C
6
. However, to improve
accuracy by taking into account the corrections to dia-
grams (23.26) requires considerable effort. Indeed, there
are several types of corrections to (23.26)suchas(i)
screening of the Coulomb interelectron interaction by
the electron–vacancy excitations; (ii) interaction be-
tween vacancies ij ; (iii) interaction between electrons
and vacancies ki(nj)(kj(ni)); and (iv) interaction be-
tween electrons kn. Corrections to the HF field itself
which acts upon electrons k, n and vacancies i, j are
discussed in Sect. 23.2.3.
Screening of the Coulomb interelectron interaction
is very important, and in many cases must be taken into
account non-perturbative. The simplest way to do this
is to use RPA, which defines the effective interelectron
interaction
˜
Γ as a solution of an integral equation, shown
diagrammatically by
=+
k
i
Γ
~
Γ
~
(23.30)
If V in (23.27) is replaced by
˜
Γ , an expression for ∆E
corr
in RPA can be derived.
Exchange is very important in atoms and molecules,
so diagram (23.30) can be modified to include this
effect, thus leading to the effective interaction Γ in
RPAE [23.12, 13, 16]:
=+
k
i
Γ
~
Γ
~
+
Γ
~
k
i
(23.31)
Replacing V in (23.27)byΓ gives a rather accurate
expression for ∆E
corr
in RPAE. Taking into account
Part B 23.2
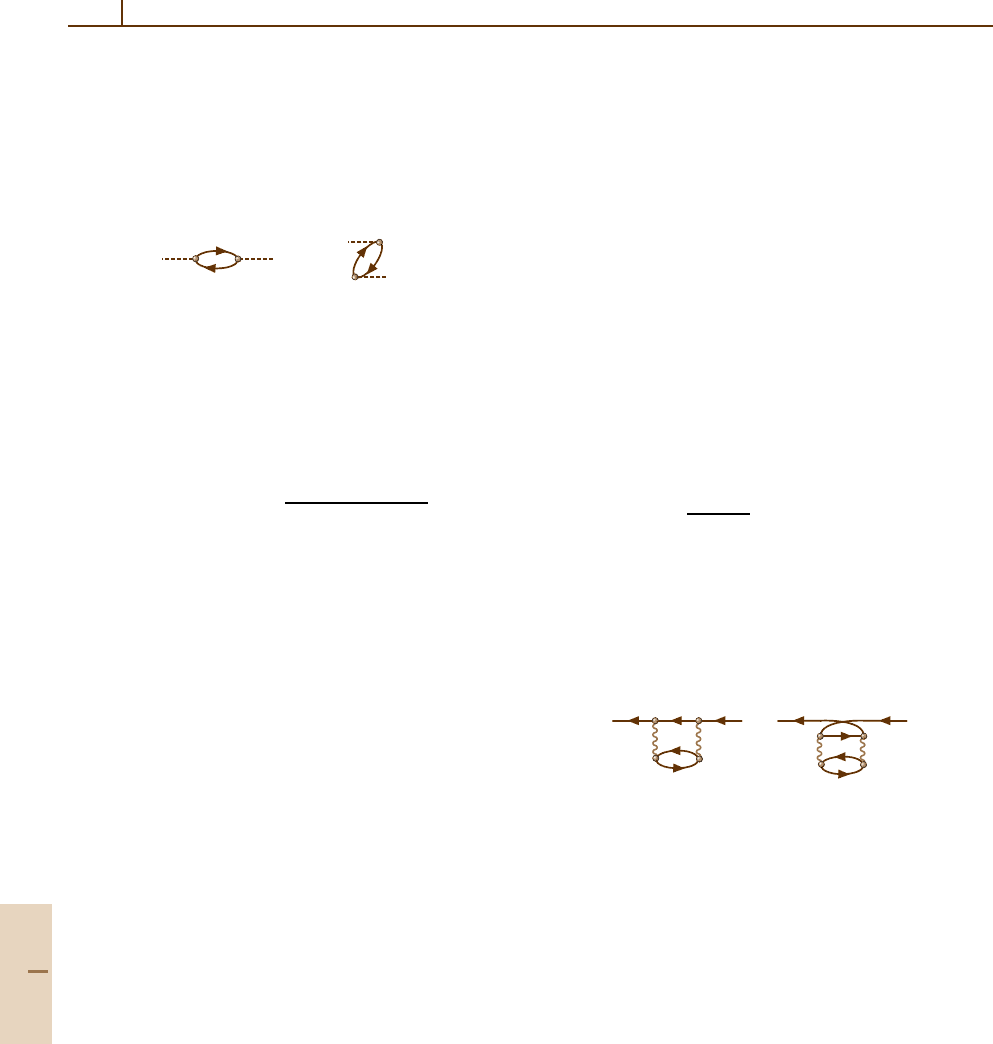
366 Part B Atoms
screening also affects the long-range interatomic in-
teraction considerably by altering the constant C
6
in (23.28).
The ground state energy of an atom or molecule is
modified by an external field. For a not too intense elec-
tromagnetic field, the simplest correction to the ground
state energy is given by the diagrams
k
i
+
k
i
(23.32)
Considering a dipole external field, its interaction with
the atomic electrons is given by W =
i≤F
E· r
i
, E be-
ing the strength of the field. The ground state energy
shift is given by ∆E =−α(ω)E
2
/2, where α(ω) is the
dynamical dipole polarizability and ω is the frequency
of the field. According to (23.32), α(ω) is determined
by
α(ω) =
k>F;i≤F
2|i|z|k|
2
(ε
k
− ε
i
)
(ε
k
− ε
i
)
2
− ω
2
, (23.33)
where z is a component of the vector r.
RPAE corrections to α(ω) are discussed in
Sect. 23.2.5 in connection with the photoionization
process.
Non-dipole polarizabilities of other multipolarities
can be obtained in the lowest order of interelectron
interaction using (23.32) with a properly chosen interac-
tion operator between the electromagnetic field and an
electron, instead of W =
i≤F
E· r
i
.
23.2.2 Characteristics of One-Particle States
A single vacancy or electron can propagate from one in-
stant of interaction to another, as described to zero order
by elements (23.1b) [or (23.1a)] with dots (23.1f) atthe
ends. This line represents an HF one-particle state with
a given angular momentum, spin, and total momentum.
Accounting for virtual or real atomic excitations leads,
for a vacancy, to a diagram similar to (23.25) but with
oppositely directed arrows. Because the interaction with
these excitations is usually much smaller than the en-
ergy distance between shells, in the sum over q only the
term q = i, i being the considered vacancy state, need be
taken into account. Interaction with the vacuum leaves
the angular momentum, spin,and total momentum un-
altered. It can however change the energy, and lead to
a finite lifetime for a vacancy state.
Analytically, the vacancy propagation in the HF ap-
proximation is described by the one-particle HF Green’s
function G
HF
:
G
HF
i
(E) = 1/(ε
i
− E). (23.34)
Solving (23.25) for a vacancy i with only Σ
ii
terms
included gives
G
i
(E) = 1/[ε
i
+ Σ
ii
(E) − E] . (23.35)
The pole in G(E) which determines the vacancy energy
is shifted from E = ε
i
to E
i
= ε
i
+ Σ
ii
(E
i
). The quan-
tity Σ
ii
(E) is called the self-energy, and is in general
a complex function of energy, its imaginary part deter-
mining the lifetime of the vacancy i. Near E
i
,(23.35)
can be written in the form
G
i
(E) ≈ F
i
/
[
ε
i
+ Σ
ii
(E
i
) − E
]
= F
i
/(E
i
− E),
(23.36)
where
F
i
=
1 −
∂Σ
ii
(E)
∂E
E=E
i
−1
(23.37)
is called the spectroscopic factor. It characterizes the
probability for more complicated configurations to be
admixed into a single vacancy state i [23.12].
An important problem is to calculate the self-energy
part Σ(E). The first nonzero contributions are
a) b)
+
i' j' i i' i
j
n
n
j
k
+
exchange
terms.
(23.38)
Specific calculations [23.16] demonstrate that if the in-
termediate electron states n [in (23.38a)] and kn [in
(23.38b)] are found in the field of vacancies jj
and ii
j,
the diagrams (23.38) are able to reproduce the values
of the correlation energy shift with about 5% accuracy.
For outer subshell vacancies, the contributions (23.38a)
and (23.38b) are almost equally important, to a large ex-
tent cancelling each other. For example, (23.38a) shifts
the outer 3p vacancy in Ar to lower binding ener-
gies by 0.1 Ry, while the contribution of (23.38b) is
−0.074 Ry. The total value 0.026 Ry is small and close
to the experimental one, which is 0.01 Ry. For inner va-
cancies, (23.38a) is dominant because the intermediate
states in (23.38b) have large virtualities and are therefore
small. The main contribution to the sum over j
comes
from the term j
= i
= i, which gives for the energy
Part B 23.2
Many-Body Theory of Atomic Structure and Processes 23.2 Calculation of Atomic Properties 367
shift of level i
∆ε
i
= Σ
(2)
ii
(ε
i
) =
n>F; j≤F
|n|r
−1
| j|
2
ε
n
− ε
j
. (23.39)
The value (23.39) is positive. Most important higher-
order corrections will be included if V in (23.38a) is
replaced by Γ from (23.31).
The physical meaning of diagram (23.38a) is trans-
parent: it accounts for configuration mixing of one
vacancy i and “two vacancies jj
– one electron n” states
in the lowest order in the interelectron interaction. Dia-
gram (23.38b) is not as transparent, and for i = i
its
intermediate state appears to violate the Pauli principle.
However, as noted in connection with (23.14), the Pauli
principle should not be considered as a restriction in
constructing intermediate states.
Diagram (23.38a) and its exchange can have an
imaginary part, which gives the probability of Auger
decay γ
(A)
i
, calculated to the lowest order in the inter-
electron interaction. For (23.38a) one has
γ
(A)
i
= Im
[
Σ
ii
(E
i
)
]
=2π
j, j
≤F;n>F
|in|V| jj
|
2
δ(ε
j
+ ε
j
− ε
n
− ε
i
).
(23.40)
The width γ
(A)
i
is usually much smaller than
Re[Σ
ii
(E
i
)], but there are several exceptional cases with
abnormally large Auger widths, among which the most
impressive is the 4p-vacancy in Xe with its γ
4p
≈ 10 eV.
Higher-order corrections include those which are
taken into account when V in (23.40) is replaced by Γ
from (23.31). The others include jj
vacancy–vacancy
interaction, the interaction between vacancies jj
and the
electron n and so on. As noted above, all of these can
be obtained step by step by inserting the elements (23.4)
into (23.38). To select the most important corrections,
a physical idea and/or experience are necessary. For in-
stance, if the energy transferred in the decay process
∆ε = ε
j
− ε
j
is close to some threshold energies of
atomic intermediate or outer shells, corrections which
include virtual excitation of this shell must be taken into
account.
The contribution to the spectroscopic factor
from (23.38a) is given according to (23.37)by
F
(2)
i
≡
1 +
j, j
≤F;n>F
|in|V| j
j|
2
(ε
j
+ ε
j
− ε
n
− ε
i
)
2
−1
.
(23.41)
Generally, for any Fermi particle, F
i
≤ 1 [23.17] because
there cannot be more than one particle in a given state.
Note that the integrand in (23.41) is the lowest-order
admixture of the jj
n state to a pure one-vacancy state i.
AsmallF value means strong mixing. For atoms, F
i
is
usually close to 1, but there are exceptions where F is
small. For example, F
5s
in Xe is about 0.33 [23.12].
The operator Σ
ij(n)
(ε) has non-diagonal matrix
elements, which leads to admixture of other one-
vacancy j or one-electron n states to the vacancy i.
A measure of this admixture is given by the ratio
Σ
ij(n)
(ε
i
)/(ε
j(n)
− ε
i
).
In higher orders, decay processes more complex than
those described by the imaginary part of (23.38a) be-
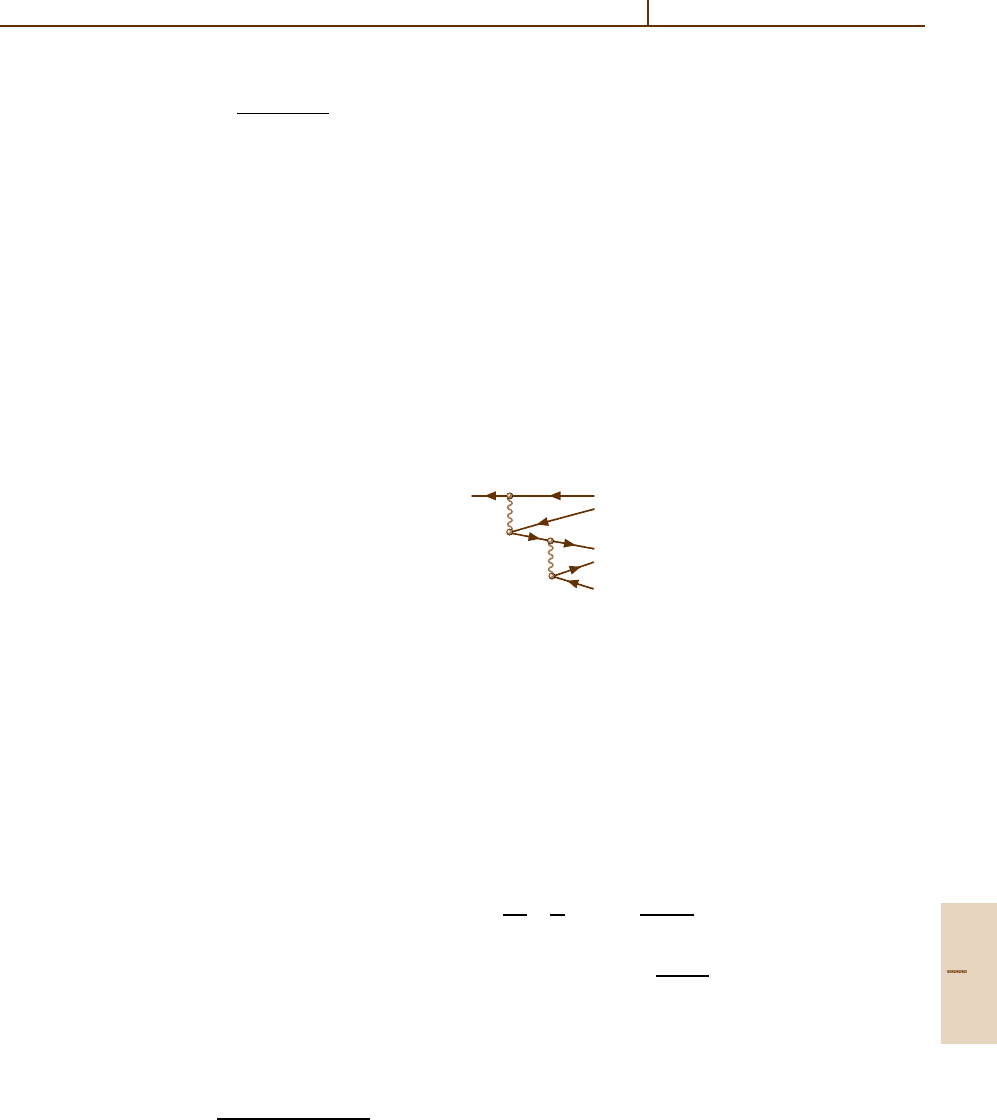
come possible. For example, this could be a two-electron
Auger decay in which the transition energy is distributed
between two outgoing electrons. An example of the
lowest-order diagram for this process is
ij
j
1
j
2
ε
1
ε
2
(23.42)
This is one of those diagrams which describe the mix-
ing of a pure one-vacancy state with a quite complex
configuration jj
1
j
2
ε
1
ε
2
.
23.2.3 Electron Scattering
Propagationofanelectroninadiscretelevelor
in a scattering state can be described in the same
way as for a vacancy. The electron wave function
is determined by (23.25). Using the correspondence
rule (23.14), (23.25) can be expressed analytically in
the form
−
∇
2
2
−
Z
r
+
i≤F
dr
|r
−r|
φ
i
(r
)
2
− ε
ψ
ε
(r)
=
i≤F
dr
|r
−r|
φ
∗
i
(r
)ψ
ε
(r
)φ
i
(r)
+
ˆ
Σ(r, r
, ε)ψ
ε
(r
)dr
.
(23.43)
The terms with the Coulomb interelectron interaction
|r
− r|
−1
determine the Hartree–Fock self-consistent
potential. The last term in (23.43) represents the nonlocal
energy dependent polarization interaction of the contin-
uous spectrum electron with the target atom. Although
Part B 23.2
