Creagh D., Bradley D. (Eds.) Physical Techniques in the Study of Art, Archaeology and Cultural Heritage. Volume 2
Подождите немного. Документ загружается.


as shall be explained later. Both effects give information on: the oxidation state of the
target atom, its local coordination geometry, the type and number of its neighbouring
atoms, its bond lengths, the angle between bonds, and molecular orientation.
XAFS is an interference effect caused by the interaction of the ejected photoelectron
with its surroundings. The first step in XAFS theory is to determine the value of c(E) at
each energy step in the XAFS pattern.
where m
l0
(E) is the extrapolation of the “pre-edge” or “free atom” region to the edge
energy. This is done by spline fitting the data.
As mentioned, XAFS is an interference effect caused by the interaction of the ejected
photoelectron when a photon (l = 2p/k) is absorbed. Here:
It is convenient to describe the c(E) in terms of the photoelectron’s momentum, k.
where the summation occurs over the shells of atoms that surround the target atom; N
i
is
the number of atoms in the ith shell; r
i
is the distance of the shell from the target atom; f
i
(k)
is the scattering amplitude to which is associated a phase-shift j
i
(k); f
i
(k) depends only on
the backscattering atom, whereas the phase depends on contributions from both the scat-
tering and the absorbing atom; j¢
i¢
(k) = j¢
j¢
(k) + j¢
i¢
(k)-1p, where l = 1 for K and L1
edges and 2 or 0 for L2 and L3 edges; and exp (s
i
2
k
2
) is related to harmonic vibrations
(referred to as the Debye–Waller factor).
The data reduction procedure is extensive for XAFS data. However, the maturity of
XAFS data reduction programs ensures that this causes a minimum of discomfort to
the researcher. All major research facilities will maintain these programs. For example,
for the Australian Synchrotron Research Program’s data reduction program XFIT, see
http://www.ansto.gov.au/natfac/asrp7_xfit.html.
Steps in the data reduction process are:
∑ converting measured intensities to m(E),
∑ subtracting a smooth pre-edge function,
∑ normalizing m(E) to the range 0–1,
∑ fitting a curve to the post-edge m(E) values to approximate m
0
(E),
∑ calculating c(E), and
∑ identifying the threshold energy E
o
and converting to k-space to determine c(k).
χσ
ρ
() ( )| ()|exp sin
222
kNkrfk k
r
ij i
i
i
=∑ −
⎛
⎝
⎜
⎞
⎠
⎟
[[2 ( )]kr k
ii
+
ϕ
k
m
EE=−
⎛
⎝
⎜
⎞
⎠
⎟
2
()
2
o
0.5
π
χ
()
() ()
()
ll0
l0
E
µE µ E
µE
=
−
72 D. Creagh
Having done this, the XAFS data is in a form from which information can be extracted.
Figure 10(e) shows further steps in the data reduction process:
∑ c(k) is weighted by a factor of k
3
, so that the low-amplitude, high-energy part of the
XAFS oscillations are increased in amplitude (referred to as c(R));
∑ electronic filter window is placed around this to limit the range over which; and
∑ the Fourier transform of c(R).
From this point on, a modelling process is undertaken. Various configurations of envi-
ronments surrounding the target atom are considered and the information is used in the
theoretical equation for c(k). The data for the models may be based on crystallographic
information; for example, if the material under investigation were FeO, the iron atom
would be in an octahedral configuration with the oxygen atoms, and R
1
, the radius of the
first shell, would be 2.14 Å. The modelling proceeds until a match between experiment and
theory is achieved.
Despite all the data manipulations that have to be made, and the fact that the analysis
gives relative rather than absolute information, XAFS is a powerful analytical tool.
Perhaps 60% of all experiments at the ANBF are XAFS experiments.
In one of several experiments that have been undertaken, Pantos et al. (2002) have
demonstrated the use of SRXRD and XAFS for the study of the mineral composition of
painting pigments and pottery glazes.
5.4.2. X-ray absorption near edge structure (XANES)
In the case of XAFS, the photoelectron is ejected from the atom and is, therefore, trans-
formed from a bound state to the continuum. The electron interacts with neighbouring
atoms, the high-energy electrons undergoing single scatter, and the low-energy electrons
undergoing multiple scatter in their passage away for the target atom (Fig. 10(g)). For
XANES, the situation is different because the transitions may be to virtual energy states,
or to other bound states. Therefore, XANES gives information about the coordination
chemistry of the target atom.
Most experiments have been performed using focussed beams. The strong dependence
of pre-edge and near-edge structure on ionization state is shown in Fig. 10(h) for the oxides
of iron (Foran, 2005). These measurements are a precursor to the study of iron speciation
on iron-gall inks on parchment (Lee et al., 2006).
De Ryck et al. (2003) have used XANES to study oxidation state maps involving the
copper ions in corrosion products formed in a bronze object.
5.5. Infrared (IR) techniques
The use of the infrared (IR) component of the synchrotron spectral output for research is
relatively recent (less than 10 years). This field of research, pioneered initially by Dr.Gwyn
Williams at the NSLS (Williams, 1990), has led to the establishing of beamlines at a
number of synchrotron radiation sources. Synchrotron radiation infrared radiation (SRIR)
has applications to the fields of surface science, geology, cell biology, materials science,
conservation science, and so on. Initially, this research was performed using synchrotron
Synchrotron Radiation and its Use in Cultural Heritage Studies 73
radiation from sources of less than 1 GeV energy, but more recently storage energies of up
to 8 GeV have been used (ESRF, Spring8). IR beamlines are currently being designed for
SOLEIL (2.75 GeV) and the Australian Synchrotron (3 GeV).
SRIR sources have a number of advantages over conventional IR (black body) sources.
Table 1 sets out a comparison of the capabilities of radiation sources of various types. A
conventional source radiates over a large area, and into all space. SRIR is up to 1000 times
more intense over the whole wavelength range (10–100 mm) than a conventional source
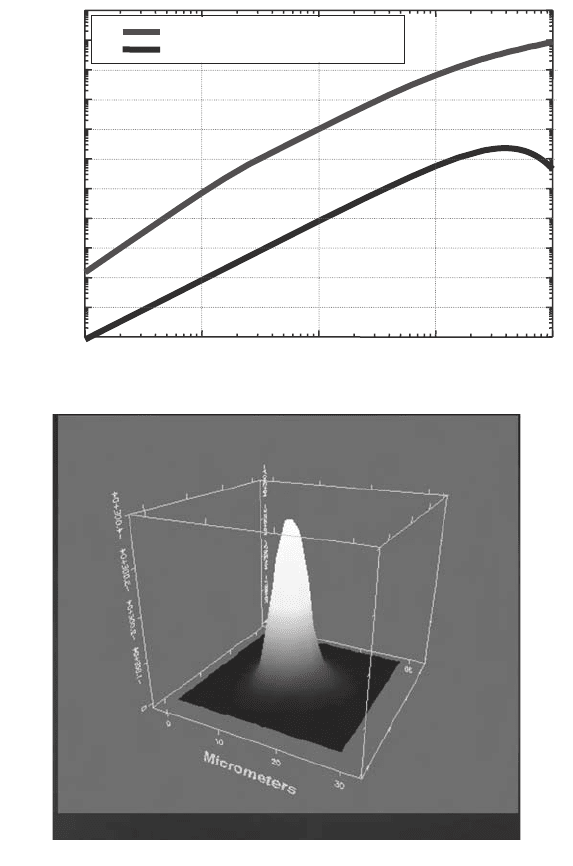
(Fig. 11(a)(i)). And it delivers a collimated beam from a source of small size (Fig. 11(a)(ii)).
This shows the unapertured beam profile at the sample stage at the IR Beamline 11 at CLRC
Daresbury (Tobin, 2006). The mapped area is 30 ¥ 30 mm
2
. The brightness of the synchro-
tron radiation source makes it especially useful in microscope/microprobe experiments.
5.5.1. Design requirements and constraints
The functional requirements of an IR beamline at the sample is for the provision of radia-
tion in the wavelength range, typically 0.4–100 mm, with a photon flux of around 5 ¥ 10
13
photons/s/0.1% bandwidth. In newer facilities, it is a requirement that both the high-
resolution spectrometer and the microscope–spectrometer be able to be used simultane-
ously by research groups.
IR beamlines differ significantly from other beamlines found at synchrotron radiation
sources because the mechanism for the extraction of the radiation involves the positioning
of the extracting mirror within the vacuum chamber of the synchrotron. This places severe
constraints on the design of the system. The design requirements for all IR beamline
systems are the:
∑ maximization of the cone of radiation extracted (which, in practice, is related to the
design of the vacuum chamber);
∑ preservation of the integrity of the ultra-high vacuum within the ring; the minimization
of vibration in the mirror system;
∑ need to control the heat load on the mirror due to the hard component of the synchro-
tron radiation beam; and
∑ need for accurate mirror positioning.
All this has to be achieved within the constraints posed by the bending magnet and asso-
ciated beam-focussing magnets (Fig. 11(b)). The vacuum chamber had to be modified to
enable the extraction mirror to be fitted. In almost all SRIR beamlines a design solution
enabling vertical extraction of the beam is possible. However, in the case of the Australian
Synchrotron, because the configuration of the vacuum chambers was optimized for X-ray
production, the only modification to the chambers that would enable a suitably large
extraction aperture involves the use of a horizontally mounted mirror. It is important to
accept a large solid angle since the flux at long wavelengths is sensitive to the angle of
acceptance.
Modifications were made to the dipole vacuum chamber to enable an extraction area of
17 mrad (vertical) and 58 mrad (horizontal) to be obtained. This is 40% larger than what
might have been obtained using vertical extraction. This results in a gain in brightness at
100 mm of around 100%.
74 D. Creagh
Synchrotron Radiation and its Use in Cultural Heritage Studies 75
1 10 100 1000 10000
1E-11
1E-10
1E-9
1E-8
1E-7
1E-6
1E-5
1E-4
1E-3
0.01
0.1
1
SOLEIL 20x78 mrad I= 500 mA
BlackBody 2000
°
K
Watts/cm
-1
/mm
2
sr
Wavenumbers / cm
-1
a(ii)
a(i)
Fig. 11. (a) (i) Comparison of the brightness of radiation from a bending-magnet synchro-
tron radiation source operating at 3 GeV, 200 mA, with that of a black body source operat-
ing at 2000 K. (a) (ii) The unapertured beam profile at the sample stage at the IR Beamline
11 at CLRC Daresbury (Tobin, 2006). The mapped area is 30 ¥ 30 µm
2
.
76 D. Creagh
(b)
c(i)
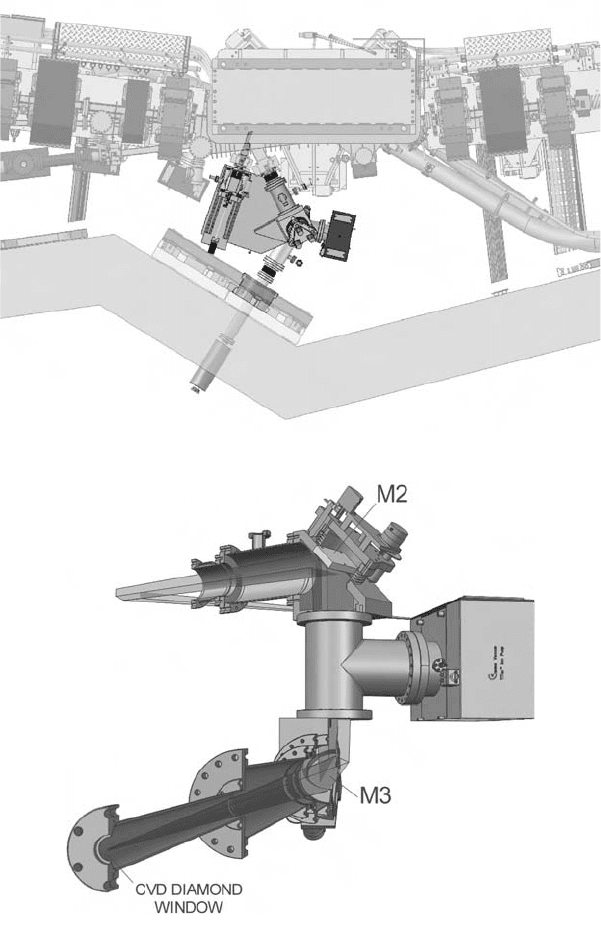
Fig. 11. (b) Bending and focussing magnets surrounding the vacuum chamber. (c) (i) The
beam path through the front end mirror system at the Australian Synchrotron.
The configuration of the beamline inside the radiation shield wall is shown in
Figs. 11(c)(i) and (ii). Figure 11(d)(i) shows the beam path from the sources (one compo-
nent arises from the so-called “edge radiation”, and the other from the bending-magnet
radiation) to the mirror M1, and from there to the ellipsoidal focussing mirror M2 and the
plane deflecting mirror M3. In the final design, a mirror pair will be used to perform the
function of M3. The IR radiation exits the UHV section of the beamline (1 ¥ 10
-9
mbar)
through a CVD window. Figure 11(d)(ii) shows the ancillary beamline components such
as the ion pumps and the gate valves used to isolate the extraction system from the vacuum
in the storage ring.
Synchrotron Radiation and its Use in Cultural Heritage Studies 77
(d)
c(ii)
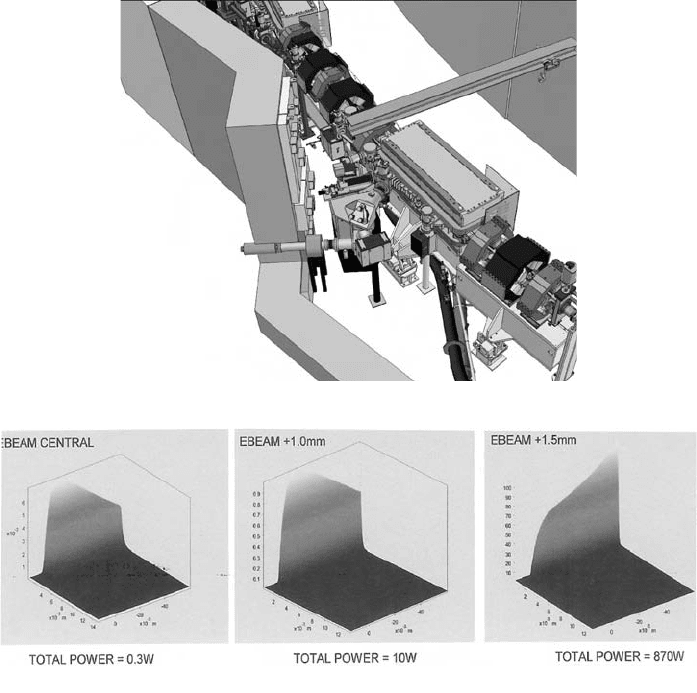
Fig. 11. (c) (ii) Another view of the beam path through the front end mirror system at the
Australian Synchrotron, showing the location of ion pumps. (d) The effect on the heatload-
ing on mirror M1 due to deviations from the true orbit. From left to right: true orbit, 1 mm
deviation; 1.5 mm deviation.
78 D. Creagh
e(ii)
e(i)
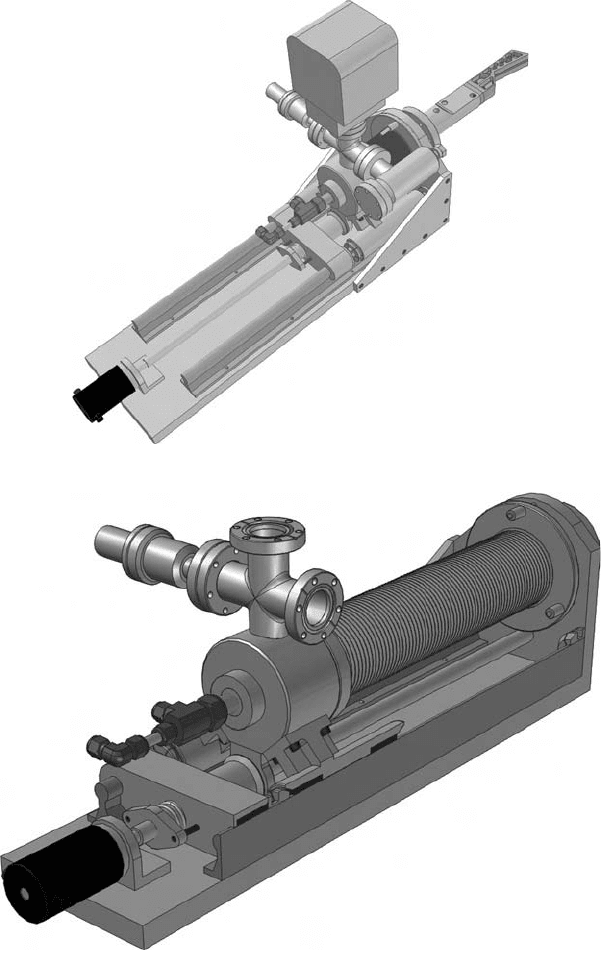
Fig. 11. (e) (i) View of the mirror M1 in its inserted position.
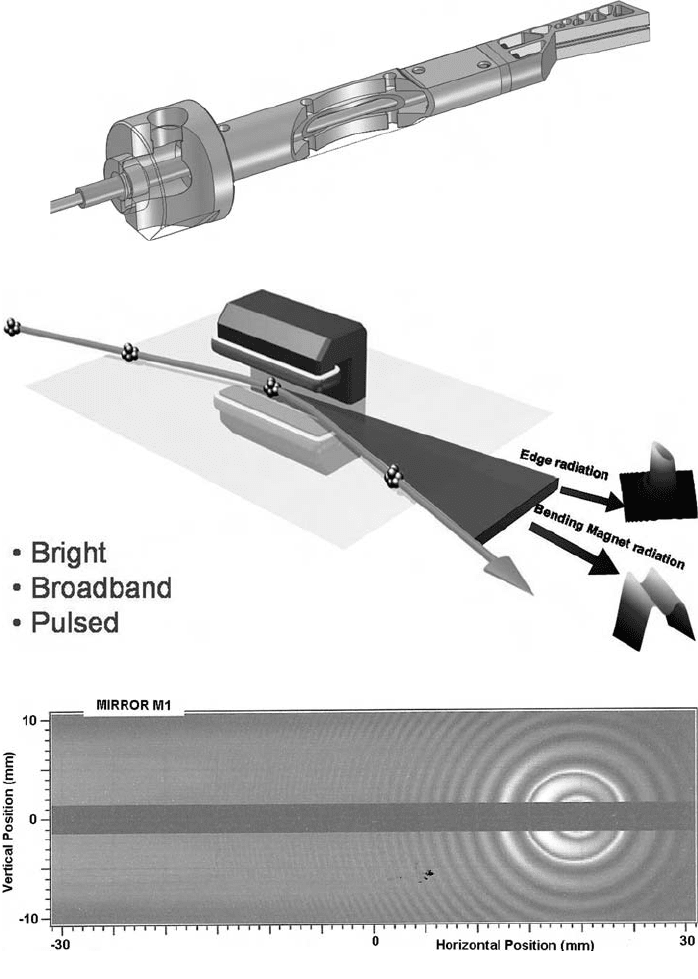
Synchrotron Radiation and its Use in Cultural Heritage Studies 79
e(iii)
(f)
(g)
Fig. 11. (e) (ii) Cutaway view of the proposed concentric water cooling system for
the mirror M1. The machined Glidcop mirror is bolted onto the stainless steel mirror beam.
(f) Illustrates the formation of bending-magnet and edge radiation. (g) Edge (circular
fringes) and bending magnet (horizontal bands) at 3 mm, calculated using the SRW method.
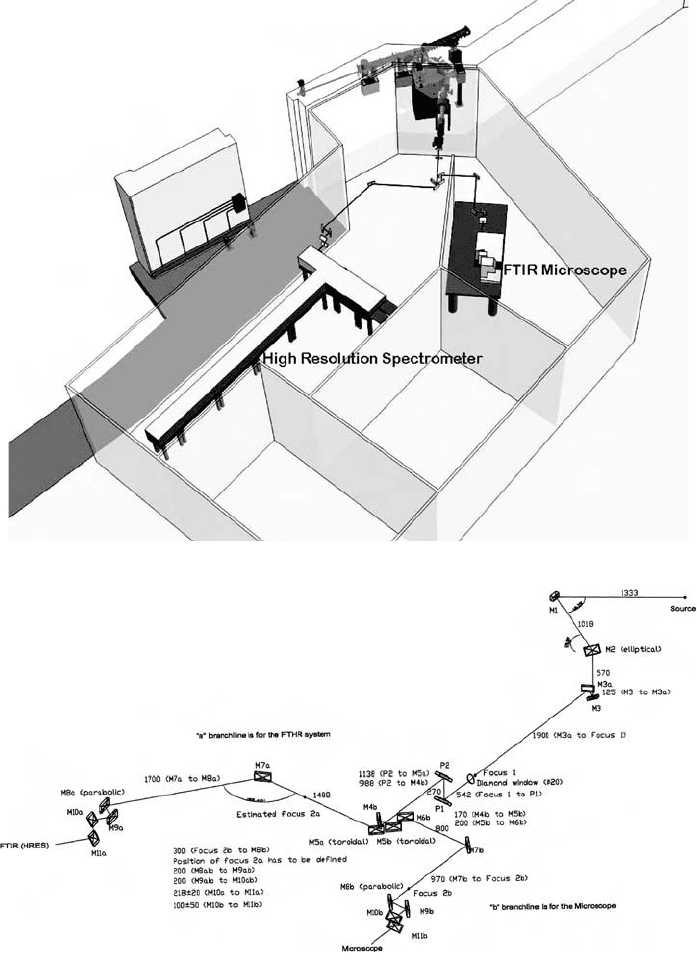
80 D. Creagh
h(ii)
h(i)
Fig. 11. (h) Plan view of the IR beamline at the Australian Synchrotron.
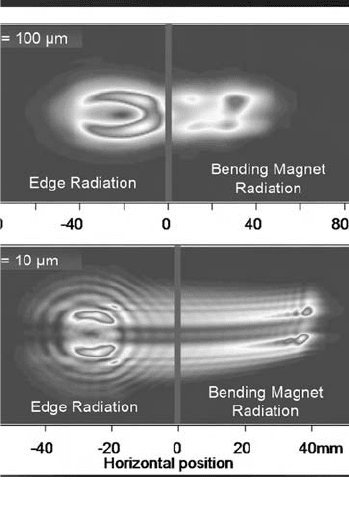
Mechanical vibrations in the mirror systems, particularly M1, lead to a degradation of
performance, and care has been taken to minimize the effect of these vibrations. An analy-
sis of the effects of vibrations from external sources on the mirror M1 has been discussed
elsewhere (Creagh et al., 2005). The mirror M1 for horizontal reflection of the infrared is
a rectangular bar of 30 ¥ 54 ¥ 460 mm, with the reflective face approximately 85 mm long
at an angle of 55∞ to the vacuum chamber straight. A 3-mm slot in the mirror allows the
high-energy radiation to pass through, as shown below. The first two resonant modes,
calculated using finite element analysis (ANSYS Workbench 8.0), are at 181 (pure verti-
cal motion) and 212 Hz (pure horizontal motion). Measurements have been made of the
floor vibrations as they exist at present. They are ±0.05 and ±0.01 mm for the first and
second harmonics, respectively. The motion of the mirror in the fundamental mode is in
the vertical plane, and will not influence the performance of the mirror. The second
harmonic vibration is in the plane of the mirror, and has the capacity to influence the beam
optics because of the effect this has on the phase of the reflected wavefronts. (The wave-
length selection in the instruments is made using Michelson interferometers. Motion in the
horizontal plane can generate phase noise in the overall system.) Other sources of vibration
are associated with the water cooling of the storage ring in general, and the dipole chamber
in particular. Care has been taken to decouple the mirror beam from these sources of radia-
tion. Special care has been taken to provide damping of the support for the mirror beam.
Synchrotron Radiation and its Use in Cultural Heritage Studies 81
(i)
Fig. 11. (i) Partitioning of the wavefields by the beamsplitting box at wavelengths of 10 and
100 mm. The optimum place for this partitioning is 18 000 mm from the diamond window.
