Crank J. Free and Moving Boundary Problems
Подождите немного. Документ загружается.


12
Moving-boundary problems: formulation
and
appropriate initial conditions, where
fICD,
f2(T) are given fwlctions.
An
inverse problem would be to find ft(T),
MT)
such that a prescribed
motion
S(T)
is
produced.
The
automatic, real-time control problem
posed by this inverse situation is
of
practical importance, e.g., in steel
casting, and is elaborated by Hoffman and Sprekels (1982)
'Who
present
numerical solutions. A wider survey of the application of control techni-
ques
to
parabolic systems is given by Hoffmann and Niezgodka (1983).
1.3.6. Multi-phase problems
The
two-phase problem formulated in § 1.2.2 above can
be
easily
extended
to
more than two phases and moving interfaces. More equations
of type (1.7) and more Stefan conditions (1.9) together with appropriate
conditions
on
fixed boundaries and
at
t = 0 determine
U1>
U
2
,
•••
, Un+l
and
S1>
S2,
...
,5,..
The
essential feature of a multi-phase problem
is
that
each domain and
the
solution of
the
corresponding parabolic equation is
connected
to
every neighbouring domain and solution through a set
of
- relations expressing
the
physics
or
chemistry of the problem being consi-
dered, e.g. the Stefan condition. A simple example is provided by a
collection of ice cubes in a glass
of
wa~er.
There is one heat-flow equation
in
the
water phase and one in each ice cube.
In
the
most general case
the
'cubes' have different
heat
parameters, and equations and solutions in
each one are linked with
the
water-domain equation and solution by
Stefan conditions on each cube-water, interface. Cannon (1978) relates
the
analysis of multi-phase problems to a problem in which two sub-
stances in solution diffuse
and
react quickly and completely on a moving
boundary.
The
ablation of
the
alloy walls of a space vehicle leads
to
a three-phase
problem with solid, liquid, and vapour phases and two moving boundaries
(Koh
et
al. 1969).
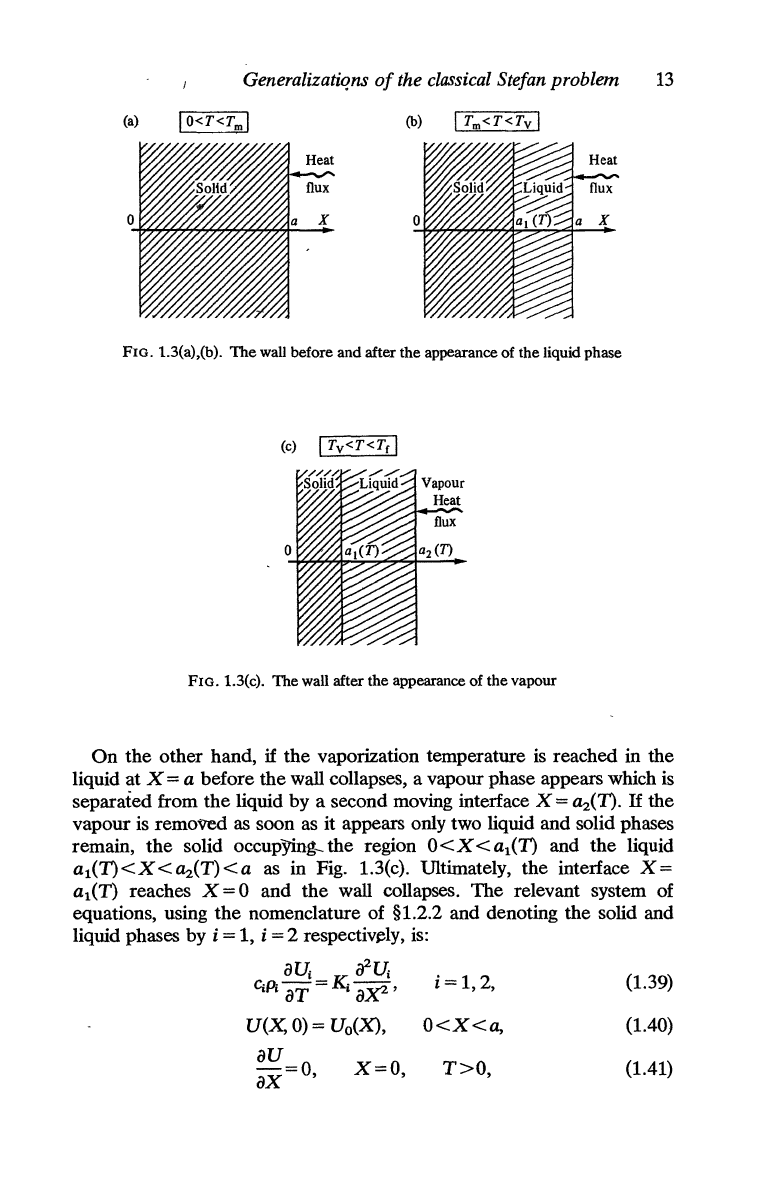
Bonnerot and Jamet (1981) discuss a simple, one-dimensional problem
involving three phases, solid, liquid, and vapour, which can appear
and
disappear. They consider a solid material which initially occupies
the
region 0 < X <
a,
where X is
the
space coordinate in
the
direction
perpendicular
to
the
wall.
The
wall
is
at
a known temperature initially
and then it is heated from
the
right (Fig. 1.3(a) by a given heat flow on
X
=
a.
The
surface X = 0 is thermally insulated.
When
the
temperature
on
the
right side of the wall, X =
a,
reaches the
melting temperature a liquid phase appears. Assuming no density change
on melting,
the
solid occupies
the
space
O<X
< a 1(1') and
the
liquid is in
al(T)
<X<a,
where
X=al(T)
is
the
melting interface (Fig.1.3b). This
interface moves
to
the
left and temperature increases at any fixed point.
If
the
melting interface X = al(T) reaches the left side
of
the wall X = 0
at T = T
f
before
the
temperature on X = a reaches
the
vaporization
temperature, the solid phase disappears and
the
wall collapses.
Generalizatiqns
of
the classical Stefan problem
13
FIG. 1.3(a),(b).
The
wall before and after the appearance of the liquid phase
(c) I
Tv<T<Tr
I
o
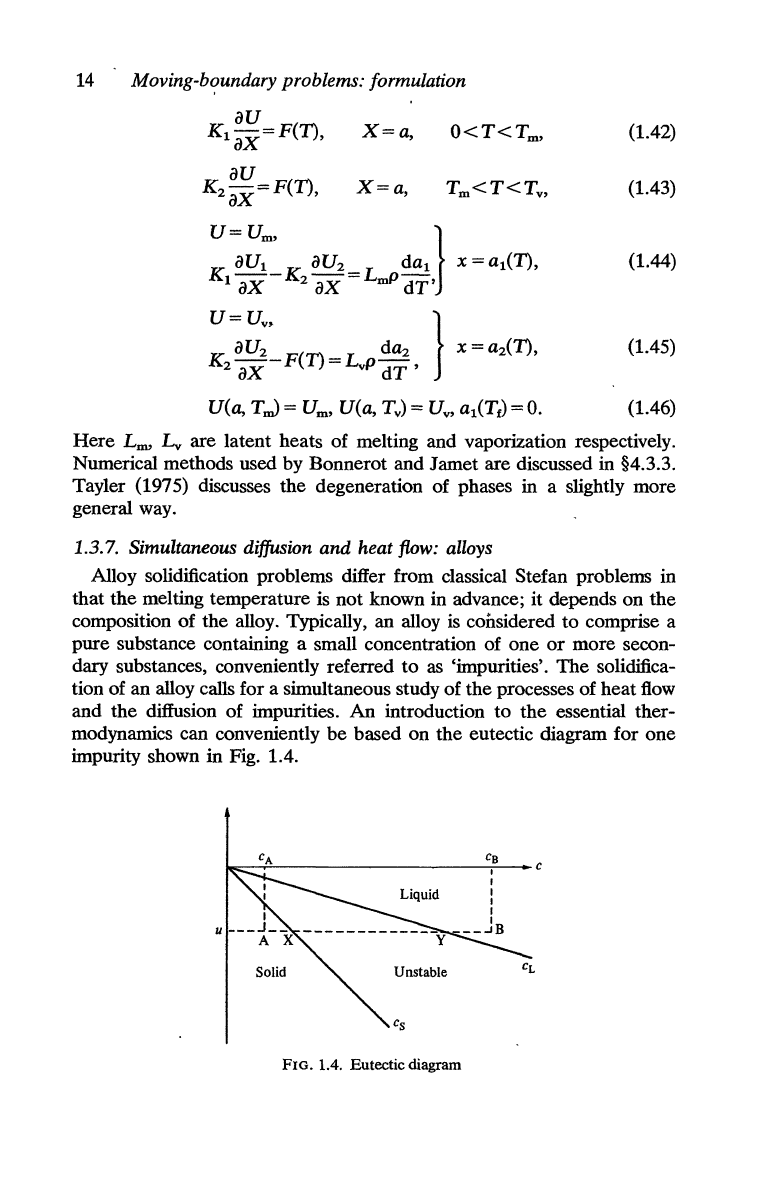
FIG. 1.3(c).
The
wall after the appearance of
the
vapour
On
the
other hand,
if
the
vaporization temperature
is
reached in the
liquid
at
X = a before
the
wall collapses, a vapour phase appears which
is
separated from
the
liquid by a second moving interface X =
aiT).
If
the
vapour
is
remowd
as
soon as it appears only two liquid and solid phases
remain,
the
solid occupymg_
the
region
O<X
<al(T)
and
the
liquid
al(T)<X<a2(T)<a
as
in Fig. 1.3(c). Ultimately,
the
interface
X==
al
(T) reaches X = 0 and
the
wall collapses.
The
relevant system of
equations, using
the
nomenclature of § 1.2.2 and denoting
the
solid and
liquid phases by
i = 1, i
==
2
respectiv~ly,
is:
au;
~u;
C;P;
aT
=
~
a~
,
i=
1,2,
(1.39)
U(X,
0)
= Uo(X),
O<X<a,
(1.40)
au=o
ax
'
x==o,
T>O,
(1.41)
14
Moving-bf!undary problems: formulation
au
Kl
aX
=
F(T),
X=a,
(1.42)
au
K2
aX
=
F(T),
X=a,
(1.43)
U=U
m
, }
K
aU
1
_
K
aU
2
=L
dal
1
aX
2
ax
mP
dT'
(1.44)
U=U
v
,
aU
2
da2
K2
ax-F(T)=Lvp
dT'
}
x~a,(T).
(1.45)
(1.46)
Here
L
m
,
Lv
are latent heats of melting and vaporization respectively.
Numerical methods used by Bonnerot and Jamet are discussed in §4.3.3.
Tayler (1975) discusses the degeneration of phases
in
a slightly more
general way.
1.3.7. Simultaneous diffusion and heat
flow:
alloys
Alloy solidification problems differ from classical Stefan problems in
that
the
melting temperature is not known in advance; it depends on
the
composition
of
the alloy. Typically, an alloy
is
considered
to
comprise a
pure substance containing a small concentration of one
or
more secon-
dary substances, conveniently referred
to
as
'impurities'.
The
solidifica-
tion of an alloy calls for a simultaneous study of the processes of heat
flow
and
the
diffusion of impurities.
An
introduction
to
the
essential ther-
modynamics can conveniently be based on
the
eutectic diagram for one
impurity shown
in
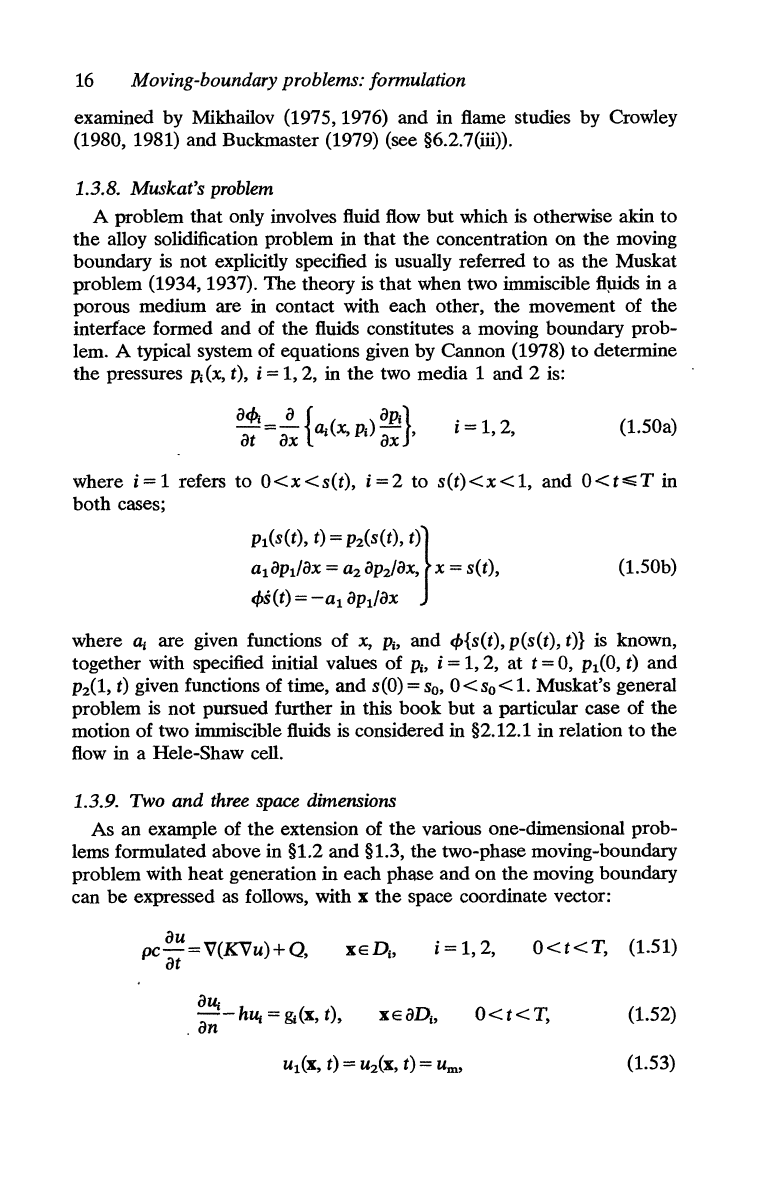
Fig. 1.4.
~~~------
____________
~
__
c
FIG. 1.4. Eutectic diagram

Generalizations
of
the classical Stefan problem
15
H at any point in
the
alloy the concentration of impurity
is
CA
and the
temperature
is
U represented by
the
point A in Fig. 1.4 this point
is
in the
solid region.
H the concentration is increased
to
a value CB at the same
temperature, the point
B
is
reached in the liquid region. Equilibrium
between
the
impurity and
the
pure alloy
is
not possible in
the
range
Cs < C <
Cv
This
is
a region of instability
but
liquid and solid phases can
coexist in equilibrium
at
the
same temperature u with respective concent-
rations
cs(u) and
cdu),
e.g.
at
points
X.
Y in Fig. 1.4. Thus, the melting
temperature will depend
on
the
concentration of the impurity and
at
a
phase-change boundary there
will
_be
a discontinuous change in the
concentration related
to
the
melting temperature through the eutectic
diagram, i.e.
CL(U)-CS(u) for a
temp~ture
u.
A simple two-phase alloy soli9ification process can now
be
expressed in
non-dimensional terms as follows, where
Uh
Ch
Kl
refer,
to
the solid
phase and
U2,
etc.
to
the
liquid phase.
aUl a
2
Ul
aCl_
D a
2
Cl
;;t=Kl
ax2'
at
- 1
ax
2
'
aUz
a
2
u
2
aC2_ D a
2
C2
;;t=K2
ax2'
at
- 2
ax
2
'
aU2_
aUl_
ds
'Y2
ax
'Yl
ax
-
dt'
O<x<s(t),
s(t)<x
< 1,
aC2
aCl
ds
x =
s(t),
{32
a;-
{3l
a;=
(Cl - cz)
dt'
Ul
= U
2
=
g,
Cl
= cs(g),
C2
= CL(g),
(1.47)
(1.48)
(1.49)
together with appropriate conditions on
x
=0,
1 and t = 0. Tayler (1975)
discusses some special limiting cases of the general formulation. Fix
(1978) outlines a simplified model of alloy solidification due to Langer
and Turski (1977)
to
illustrate ideas
of
non-iinear instabilities associated
with Mullins and Sekerka (1963,1964) on the melting front and related
to
the
growth of dendrites in multi-dimensional problems.
The
simplifica-
tions in
the
Langer model are: (a) heat conductivity
is
everywhere
infinitely large; (b)
the
diffusion coefficient for the impurity
is
constant;
and
(c)
the
concentration
jump
CL(U,j-CS(U,j
is constant, independent
of
the
melting temperature. Fix then develops an alternative weak varia-
tional formulation of
the
complete alloy solidification problem. Ockendon
(1978) also derived a weak formulation in
one
space dimension. An
enthalpy formulation of alloy solidification and some solutions are pre-
sented in §6.2.7(ii).
Other examples of simultaneous diffusion and heat
flow
have been
16
Moving-boundary problems: formulation
examined by Mikhailov (1975,1976) and in flame studies by Crowley
(1980, 1981) and Buckmaster (1979) (see §6.2.7(iii)).
1.3.8.
Muskat's
problem
A problem that only involves fluid
flow
but
which is otherwise akin
to
the
alloy solidification problem in that
the
concentration on
the
moving
boundary
is
not explicitly specified
is
usually referred
to
as
the
Muskat
problem (1934,1937).
The
theory
is
that when two immiscible
fl~ds
in a
porous medium are in contact with each other, the movement of
the
interface formed and of
the
fluids constitutes a moving boundary prob-
lem. A typical system of equations given by Cannon (1978)
to
determine
the
pressures Pt(x, t), i = 1, 2, in
the
two media 1 and 2 is:
i=
1, 2,
(1.50a)
where
i=1
refers
to
O<x<s(t),
i=2
to
s(t)<x<l,
and O<t~T in
both cases;
PI(S(t), t) = P2(S(t),
t)}
al
apI/aX
=
a2
ap2/aX,
x = s(t),
#(t)
=
-al
apI/aX
(1.50b)
where
a;
are given functions
of
x,
Pb
and
</>{s(t),
p(s(t), t)} is known,
together with specified initial values of
Pb
i = 1, 2, at t = 0,
PI(O,
t) and
pil,
t) given functions of time,
and
s(O)
=
So,
0 <
So
< 1. Muskat's general
problem
is
not pursued further in this
book
but a particular case of
the
motion of two immiscible fluids
is
considered in §2.12.1 in relation
to
the
flow in a Hele-Shaw cell.
1.3.9.
Two and three space dimensions
As an example of
the
extension of
the
various one-dimensional prob-
lems formulated above in §1.2 and §1.3, the two-phase moving-boundary
problem with heat generation in each phase and on
the
moving boundary
can
be
expressed as follows, with x
the
space coordinate vector:
au
pc-=V(KVu)+Q,
at
au;
-;;--hu;
= gt(x, t),
. un
i=
1, 2,
O<t<T,
(1.51)
O<t<T,
(1.52)
(1.53)

Generalizations
of
the
classical
Stefan
problem
17
FIG. 1.5. Two-phase region with a moving boundary f(x, t) =
O.
and
[
au]2
aU2
aUI
K-
=K
2
--K
1
-=-pLv,.+q
an
I
an
an
on
the
moving boundary f(x, t} = 0, 0 < t <
T,
u(x,
O}
=
uo(x},}
t =
o.
f(x,
O}
=
fo(x),
(1.54)
(1.55)
With reference
to
Fig. 1.5, D =
DI
U D
2
,
aD
=
aD
I
U
aD
2
denote the
interior and boundary of the relevant domain.
The
operator V encompas-
ses different coordinate systems, e.g. rectangular, cartesian, cylindrical
and spherical polar, and generalized curvilinear.
In
heat conduction
nomenclature
Uj denotes
the
temperature in each phase, i = 1, 2, U
m
the
phase-change temperature, L
the
latent heat, n
the
outward normal
to
the
moving boundary f(x, t) = 0, and
v"
the velocity of this boundary
along
the
normal.
The
thermal properties
c,
p,
K and
the
heat generation
terms
Q,
q may
be
functions of
u,
x, and t and may
be
different in
the
two
phases,
Patel (1968) expressed
the
condition (1.54)
on
the
moving boundary in
the
more usable form
{
(
as)2 (as)2}
I
auI
2
as
1+
- + -
K-
=-pL-
z:;s(x
y
t)
ax
ay
az
I
at'
, ,
(1.56)
for
the
case q =
O.
This relationship connects explicitly
au/az
with deriva-
tives'"'
of
the
moving-boundary surface f(x,
t}
= 0 written as z = s(x,
y,
t}.
Similar relations hold for
the
other two components
au/ax
and
au/ay.
Patel's derivation of (1.56) for q = 0 is as follows, denoting temperatures
by
Uj, i = 1, 2,
and
considering the three-dimensional case in which the
18 Moving-boundary problems: formulation
moving boundary is given by f(x,
y,
z, t) = 0. Thus we have
u;(x,
y,
z, t) =
Urn,
i = 1, 2,
(1.56a)
K
aUI_
K
au
2
=
!Lv
I
an
2
an
p
'"
(1. 56b)
on
f(x,
y,
z, t) = 0, (1.56c)
where n is
the
outward normal and
Vn
the
normal velocity.
Then
n =
Vl/IVfl
=
VU;/IVu;I,
au;
-=Vu;
'n=(Vu;
• Vf)/IVfl =
IVu;1
an
'
v,.
=v
'n=(v'
Vf)/IVfI =
(v'
Vu;)/IVu;I.
Remembering from (1.56c)
that
df=
af dx+ af
dy+
af
dz+
af dt=O,
ax
ay
az
at
and hence
that
. af . af . af af °
x-+y-+z-+-=
,
ax
ay
az
at
we
see
from (1.56f)
that
IVfl
(
'
.•.
k·)(·af+.af+ka
f
) af
v,.=IX+JY+
z
1-)-
-
=--,
,
ax
ay
az
at
and
s~ilarly
for
IV
U;
I
v'"
so
that
Vn
= -(aflat)/IVfI =
-(aU;/at)/lvu;l.
Using (1.56e,g),
eqn
(1.56b) becomes
KIVUI
.
Vf-K
2
VU
2
' Vf=-pL(aflat),
and
by
differentiating (1.56a), (1.56c) we find
..
aU;
aflax
au; au;
aflayau;
ax
= aflaz
az
'
ay
=
afliJz
az
'
and hence by substituting (1.56i) into (1.56h)
[
1+faflax)2+(aflay)2][K aUI_K aU2]=_!L
aflat
\aflaz
aflaz
1
az
2
az
P aflaz'
(1.56d)
(1.56e)
(1.56f)
(1.56g)
(1.56h)
(1.56i)
(1.56j)
which is
the
same as (1.56) above for f(x,
y,
z,
t)
= z -
sex,
y,
t)
= 0.
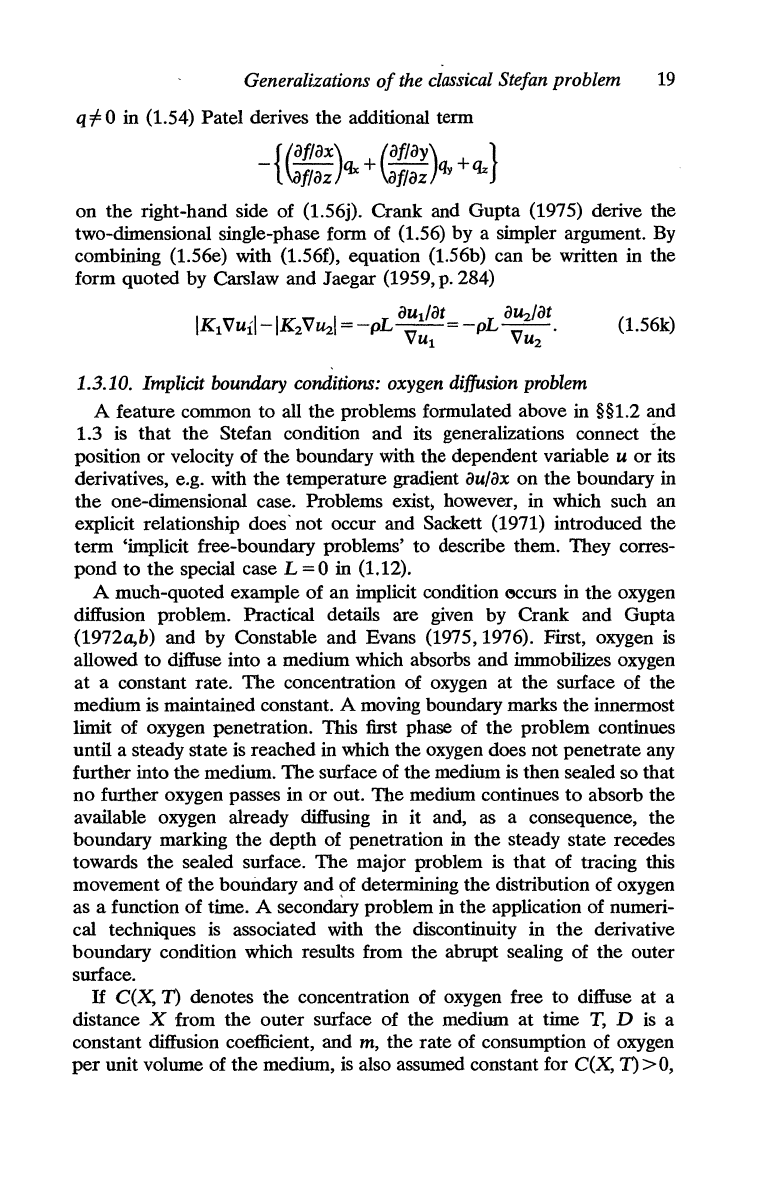
For
Generalizations
of
the classical Stefan problem
19
q
1=
0 in (1.54) Patel derives
the
additional tenn
{
la/lax)
(a/lay)
}
-
VJ/laz
llx
+
a/laz
qy
+ 4:
on
the
right-hand side of (1.56j). Crank and Gupta (1975) derive the
two-dimensional single-phase
fonn
of (1.56)
by
a simpler argument.
By
combining (1.56e) with (1.56f), equation (1.S6b) can
be
written in the
form quoted by Carslaw and Jaegar (1959, p. 284)
(1.56k)
1.3.10. Implicit boundary conditions: oxygen diffusion problem
A feature common to all the problems fonnulated above in §§1.2 and
1.3 is that
the
Stefan condition and its generalizations connect the
position
or
velocity
of
the
boundary with the dependent variable u
or
its
derivatives, e.g. with
the
temperature gradient
aulax
on
the
boundary in
the
one-dimensional case. Problems exist, however, in which such an
explicit relationship does' not occur and Sackett (1971) introduced the
tenn
'implicit free-boundary problems'
to
describe them. They corres-
pond
to
the
special case L
=0
in (1.12).
A much-quoted example of an implicit condition
,-,ccurs
in
the
oxygen
diffusion problem. Practical details are given by Crank and Gupta
(1972a,b) and by Constable and Evans (1975,1976). First, oxygen
is
allowed
to
diffuse into a medium which absorbs and immobilizes oxygen
at
a constant rate.
The
concentration of oxygen
at
the surface of the
medium
is
maintained constant. A moving boundary marks the innennost
limit of oxygen penetration. This first phase of
the
problem continues
until a steady state
is
reached in which the oxygen does not penetrate any
further into the medium.
The
surface of the medium
is
then sealed so that
no
further oxygen passes in
or
out.
The
medium continues
to
absorb the
available oxygen already diffusing in it and, as a consequence,
the
boundary marking the depth of penetration in
the
steady state recedes
towards
the
sealed surface.
The
major problem
is
that of tracing this
movement of the boundary and of detennining
the
distribution of oxygen
as a function of time. A secondary problem in
the
application of numeri-
cal techniques
is
associated with
the
discontinuity in
the
derivative
boundary condition which results from the abrupt sealing of the outer
surface.
If
C(X,
1)
denotes the concentration of oxygen free to diffuse
at
a
distance X from
the
outer surface of
the
medium
at
time
T,
D
is
a
constant diffusion coefficient, and
m,
the rate
of
consumption of oxygen
per
unit volume of
the
medium,
is
also assumed constant for C(X,
1)
> 0,

20
Moving-boundary problems: formulation
the steady state
is
defined by a solution of
d
2
C
D
dXZ-m=O
which satisfies
the
conditions
c=ac/ax=o,
where
Xo
is
the
innermost extent of oxygen penetration, and on
the
outer
surface
c =
Co
= constant, X = 0.
The
required solution
is
readily seen
to
be
given by
After
the
surface X = ° has been sealed,
the
position of the receding
boundary is denoted by,Xo(T) and the problem
to
be solved becomes
aC
a
2
c
aT=D
aXZ-:
m
, O.,;;X.,;;Xo(T),
(1.57a)
aC
=0
X=O,
T;-;.O,
ax
'
(1.58a)
aC
C= ax=o,
X=Xo(T),
T;-;.O,
(1.59a)
m '
C=_(X_X)2
2D
0 ,
T=O,
(1.60a)
where T = °
is
the
moment when the surface
is
sealed. By making
the
changes of variables,
X
x=-
Xo'
D C
c=--=-
~
2C
o
'
and denoting by s(t)
the
value of x corresponding to Xo(T),
the
above
system
is
reduced
to
the
following non-dimensional form:
ac
= a
2
c_
1
at ax
2
'
O,,;;x";;s(t),
(1.57)
ac=o
ax '
x=o,
t;-;.o,
(1.58)
c
=ac/ax=o,
x
=5
s(t),
t;-;.o,
(1.59)
c=t(1-X)2,
O.,;;x.,;;1,
t=o,
(1.60)
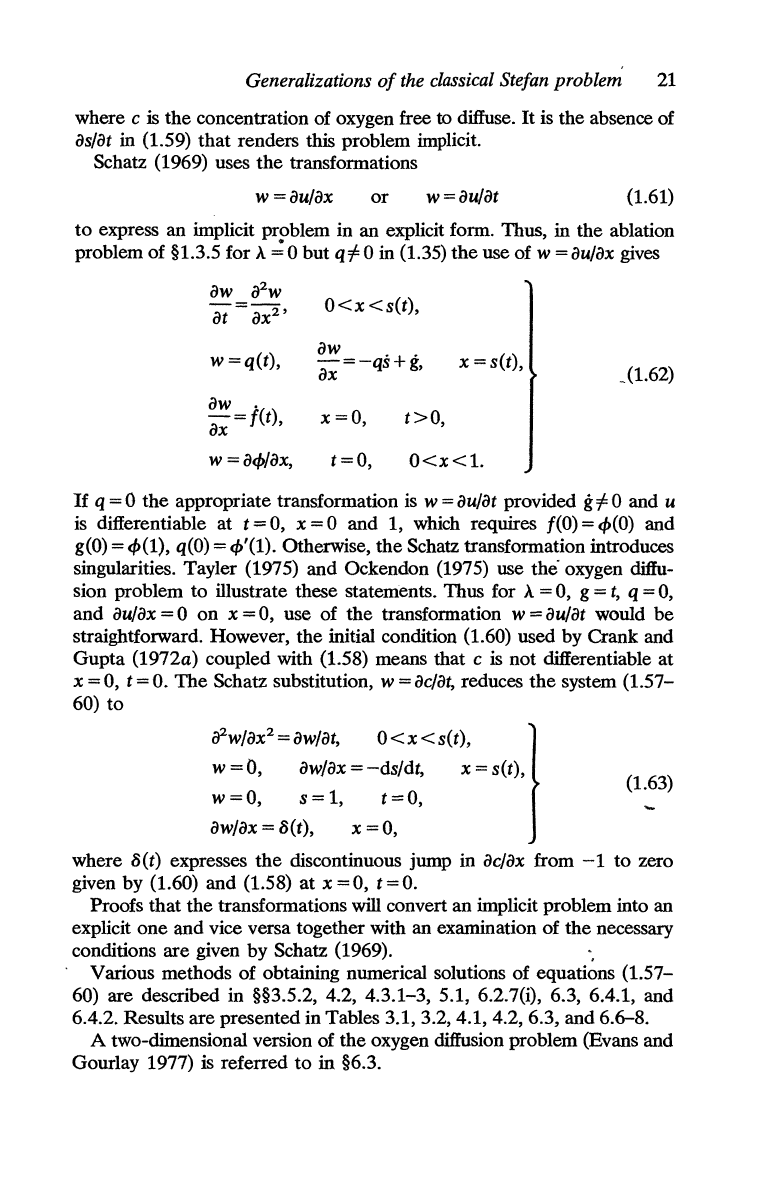
Generalizations
of
the classical Stefan problem
21
where c is
the
concentration of oxygen free to diffuse.
It
is
the
absence of
as/at
in (1.59)
that
renders this problem implicit.
Schatz (1969) uses
the
transformations
w = au/ax
or
w=au/at
(1.61)
to
express
an
implicit problem in
an
explicit form. Thus, in
the
ablation
problem
of
§1.3.5 for
A';O
but
q'l=O
in (1.35)
the
use of w =
au/ax
gives
w=q(t),
O<x
<s(t),
aw
..
-=-qs+g
ax
'
aw
.
-=
[(t), x
=0,
t>O,
ax
x = s(t),
w=a4>/ax,
t=O,
O<x<1.
-<1.62)
If
q = 0
the
appropriate transformation is w = au/at provided
g'l=
0 and u
is differentiable at
t:;:
0, x = 0
and
1, which requires
[(0)
=
4>(0)
and
g(O)
=
4>(1),
q(O)
=
4>'(1).
Otherwise,
the
Schatz transformation introduces
singularities. Tayler (1975)
and
Ockendon (1975) use
the
oxygen diffu-
sion problem
to
illustrate these statements. Thus for A = 0, g =
t,
q = 0,
and
au/ax = 0
on
x = 0,
use
of
the
transfonnation w = au/at would
be
straightforward. However,
the
initial condition (1.60) used by
Crank
and
Gupta
(1972a) coupled with (1.58) means that c is
not
differentiable
at
x = 0, t =
O.
The
Schatz substitution, w =
ac/at,
reduces
the
system
(1.57-
60)
to
ifw/ax
2
=
aw/at,
O<x<s(t),
1
w =
0,
aw/ax = -ds/dt, x = s(t),
(1.63)
w=O,
s=l,
t=O,
aw/ax
= 8(t), x = 0,
where
8(t) expresses
the
discontinuous jump in
ac/ax
from
-1
to
zero
given
by
(1.60)
and
(1.58)
at
x =
0,
t =
O.
Proofs
that
the
transformations will convert
an
implicit problem into an
explicit
one
and
vice versa together with
an
examination
of
the
necessary
conditions
are
given
by
Schatz (1969). ,
Various methods
of
obtaining numerical solutions
of
equations (1.57-
60)
are
described in §§3.5.2, 4.2, 4.3.1-3, 5.1, 6.2.7(i), 6.3, 6.4.1, and
6.4.2. Results
are
presented in Tables 3.1,
3.2,4.1,4.2,6.3,
and
6.6-8.
A two-dimensional version of
the
oxygen diffusion problem (Evans and
Gourlay 1977) is
referred
to
in
§6.3.
