Crank J. Free and Moving Boundary Problems
Подождите немного. Документ загружается.

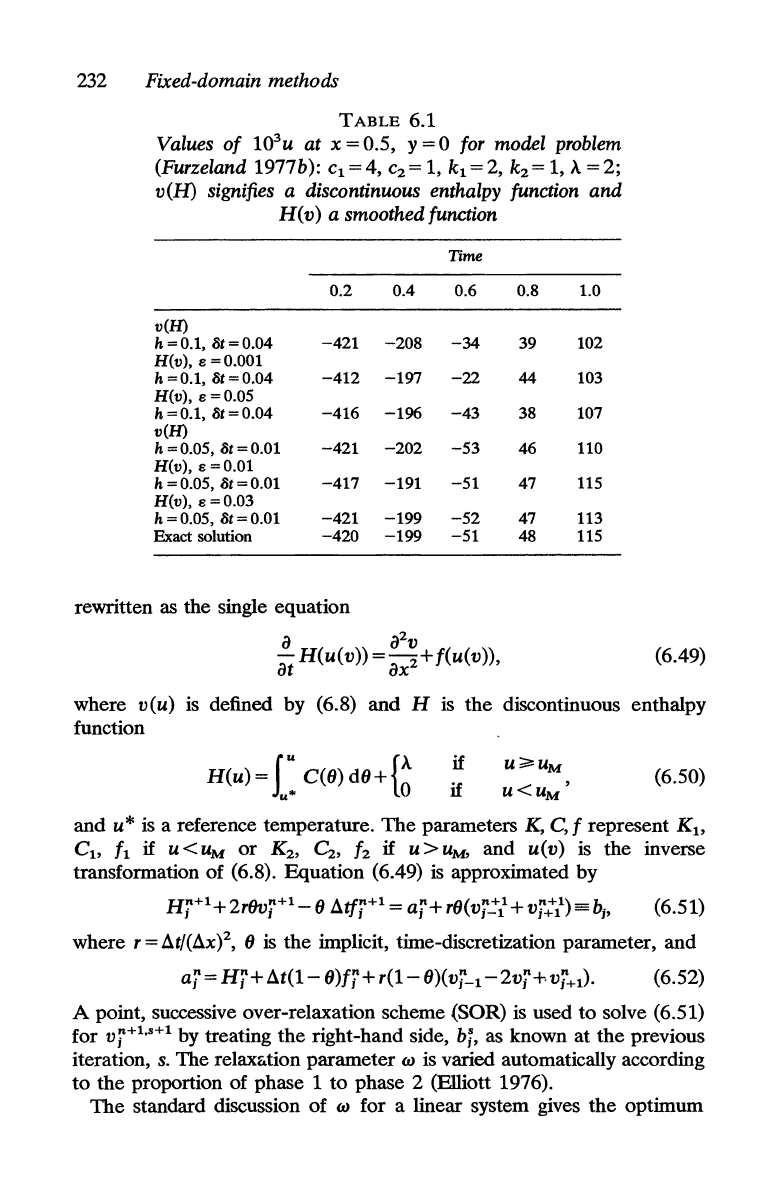
232 Fixed-domain methods
TABLE
6.1
Values
of
10
3
u at x = 0.5, y = 0 for model problem
(Furzeland 1977b):
Cl
=4,
C2=
1,
kl
=2,
k2= 1, A
=2;
v(H)
signifies a discontinuous enthalpy function
and
H(
v)
a smoothed function
Time
0.2 0.4 0.6 0.8 1.0
v(H)
h = 0.1, 8t = 0.04
-421
-208
-34
39
102
H(v), 8
=0.001
h = 0.1, 8t = 0.04
-412
-197
-22
44 103
H(v), 8 =
O.OS
h=0.1,8t=0.04
-416
-196
-43
38 107
v(H)
h =
O.OS,
8t = 0.01
-421
-202
-S3
46
110
H(v), 8 = 0.01
h =
O.OS,
8t = 0.01
-417
-191
-Sl
47
l1S
H(V),8=0.03
h =
O.OS,
8t = 0.01
-421
-199
-S2
47
113
Exact solution
-420 -199
-Sl
48
l1S
rewritten as the single equation
a a
2
v
at
H(u(v))
= ax2
+f(u(v)),
(6.49)
where
v(u)
is defined by (6.8) and H
is
the discontinuous enthalpy
function
H(u)
=
t:
C(8)d8+{~
if
if
(6.50)
and
u*
is
a reference temperature.
The
parameters K,
C,
f represent
Kl>
Cl>
it
if
U<UM
or
K
2
,
C
2
,
f2
if
U>UM,
and
u(v)
is
the inverse
transformation
of
(6.8). Equation (6.49)
is
approximated by
(6.51)
where r
= Ilt/(IlX)2, 8
is
the implicit, time-discretization parameter, and
aj=
Hj+
Ilt(1-
8)fj+
r(1-
8)(Vj_1-2vj+vj+1)'
(6.52)
A point, successive over-relaxation scheme (SOR)
is
used
to
solve (6.51)
for
vj+l,s+1
by treating
the
right-hand side, bj, as known at
the
previous
iteration,
s.
The reiax&tion parameter
Cd
is varied automatically according
to
the proportion of phase 1
to
phase 2 (Elliott 1976).
The
standard discussion of
Cd
for a linear system gives
the
optimum
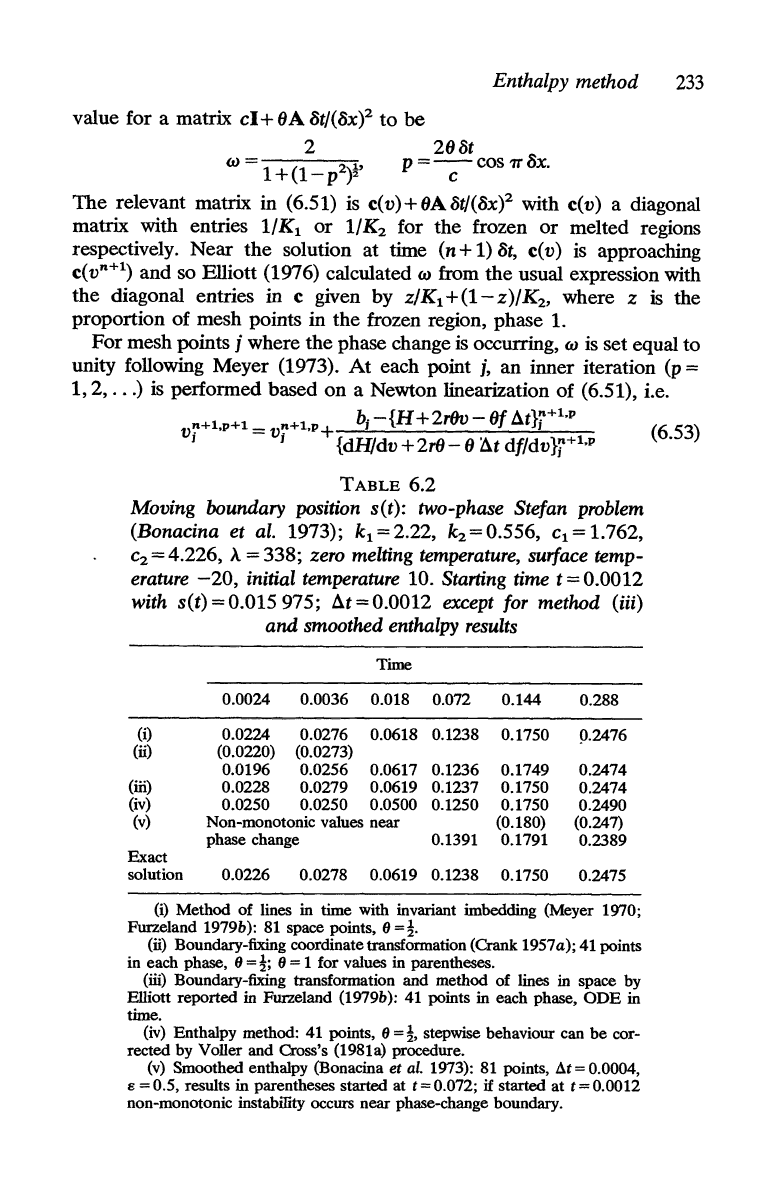
Enthalpy method
233
value for a matrix
cI
+
OA
cSt/(cSx?
to
be
2
205t
w = 1
+(1-p2)~'
P
=-c-cos
7TcSX.
The
relevant matrix in (6.51) is c(v)+OA5t/(cSx? with c(v) a diagonal
matrix with entries
l/Kl
or
1/K2 for the frozen or melted regions
respectively. Near the solution at time
(n + 1)
cSt,
c(v)
is
approaching
c(v
n
+1)
and so Elliott (1976) calculated w from the usual expression with
the diagonal entries in c given by
Z/Kl
+(1-
z)/K
2
,
where z is the
proportion of mesh points in the frozen region, phase 1.
For
mesh points j where the phase change
is
occurring, w
is
set equal to
unity following Meyer (1973).
At
each point j, an inner iteration (p
=:
1,2,
...
) is performed based on a Newton linearization of (6.51), i.e.
V!'+l,p+l
=
vn+l,p+
b
i
-{H
+2r6v -
Of
at}j+l,p (6.53)
I j {dH/dv + 2r6 - 0 ;1t df/dv}j+1,p
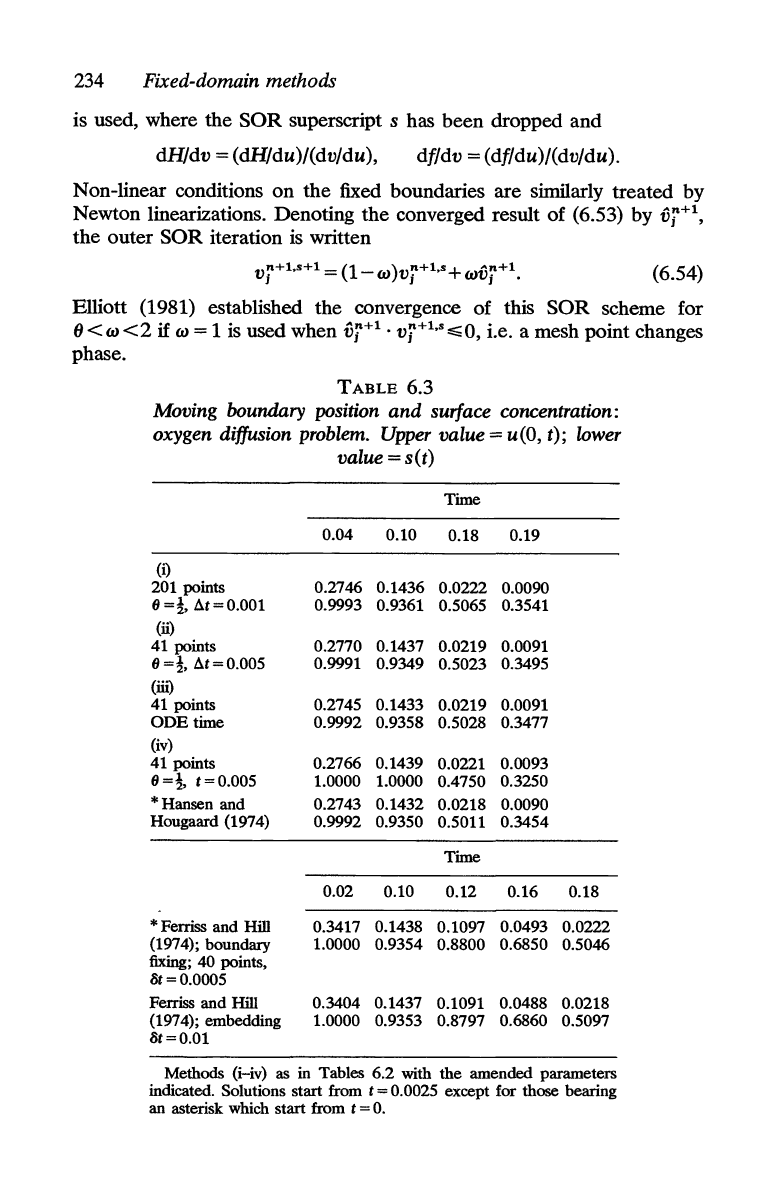
TABLE
6.2
Moving boundary position s(t): two-phase Stefan problem
(Bonacina et al.
1973);
kl
= 2.22, k2 = 0.556,
Cl
= 1.762,
C2 = 4.226, ,\ = 338; zero melting temperature, surface temp-
erature
-20,
initial temperature 10. Starting time t = 0.0012
with s(t)
=0.015
975;
at=O.0012
except for method (iii)
and
smoothed enthalpy results
Time
0.0024 0.0036 0.018 0.072 0.144
0.288
(i)
0.0224 0.0276
0.0618 0.1238 0.1750
9·2476
(ii)
(0.0220) (0.0273)
0.0196
0.0256 0.0617
0.1236 0.1749 0.2474
(iii)
0.0228
0.0279
0.0619 0.1237
0.1750
0.2474
(iv)
0.0250 0.0250
0.0500 0.1250 0.1750 0.2490
(v)
Non-monotonic values near
(0.180) (0.247)
phase change 0.1391 0.1791
0.2389
Exact
solution
0.0226 0.0278 0.0619 0.1238 0.1750 0.2475
(i)
Method of lines in tinle with invariant inlbedding (Meyer 1970;
Furzeland
1979b):
81
space points, 6
=!.
(ii) Boundary-fixing coordinate transformation (Crank 1957a); 41 points
in each phase, 6
=!;
6 = 1 for values in parentheses.
(iii) Boundary-fixing transformation and method of lines
in
space by
Elliott reported in
Funeland
(1979b);
41
points in each phase,
ODE
in
tinle.
(iv)
Enthalpy method:
41
points, 6
=~,
stepwise behaviour can
be
cor-
rected by Voller and Cross's (1981a) procedure.
(v)
Smoothed enthalpy (Bonacina et
al.
1973):
81
points,
At
= 0.0004,
B = 0.5, results in parentheses started at t = 0.072;
if
started at t = 0.0012
non-monotonic instability occurs near phase-change boundary.
234 Fixed-domain methods
is used, where the
SOR
superscript s has been dropped and
dH/dv
= (dH/du)/(dv/du),
df/dv
= (df/du)/(dv/du).
Non-linear conditions
on
the
fixed boundaries are similarly treated by
Newton linearizations. Denoting the converged result of (6.53) by
ilj+l,
the
outer
SOR
iteration
is
written
(6.54)
Elliott (1981) established the convergence
of
this
SOR
scheme for
6 <
w
<2
if
w = 1
is
used when
vj+l
. vj+1,s
~O,
i.e. a mesh point changes
phase.
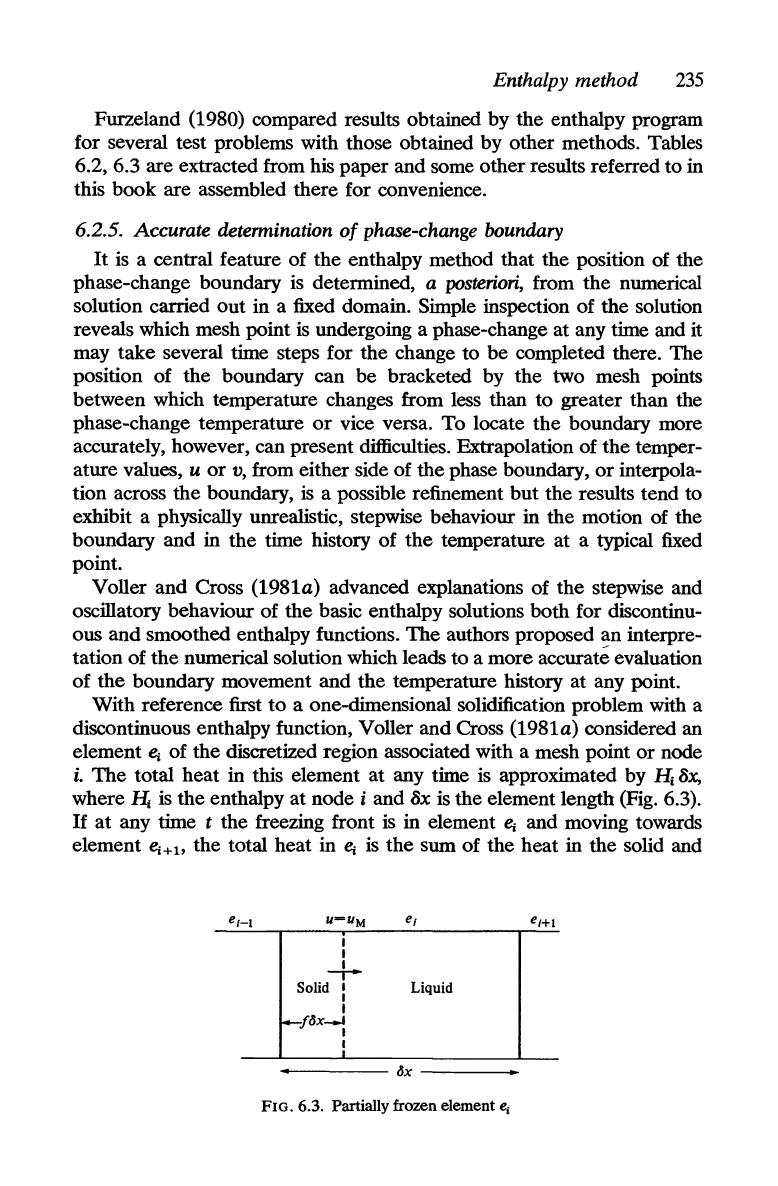
TABLE
6.3
Moving boundary position
and
surface concentration:
oxygen diffusion problem. Upper value =
u(O,
t); lower
value = s(t)
(i)
201 points
8=~,
At=O.OOl
(ii)
41 points
8=~,
At=0.005
(iii)
41 points
ODE
time
(iv)
41 points
8=~,
t=0.005
*Hansen and
Hougaard (1974)
* Ferriss and Hill
(1974); boundary
fixing; 40 points,
&=0.0005
Ferriss and Hill
(1974); embedding
8t=0.01
Time
0.04 0.10 0.18 0.19
0.2746 0.1436
0.0222 0.0090
0.9993 0.9361
0.5065
0.3541
0.2770
0.1437
0.0219
0.0091
0.9991 0.9349
0.5023
0.3495
0.2745
0.1433
0.0219
0.0091
0.9992 0.9358
0.5028
0.3477
0.2766
0.1439
0.0221
0.0093
1.0000
1.0000
0.4750
0.3250
0.2743
0.1432
0.0218
0.0090
0.9992 0.9350
0.5011
0.3454
Time
0.02 0.10 0.12 0.16 0.18
0.3417
0.1438
0.1097
0.0493 0.0222
1.0000 0.9354
0.8800
0.6850 0.5046
0.3404
0.1437
0.1091
0.0488
0.0218
1.0000 0.9353 0.8797 0.6860 0.5097
Methods (i-iv) as
in
Tables 6.2 with the amended parameters
indicated. Solutions start from
t = 0.0025 except for those bearing
an asterisk which start from
t =
O.
Enthalpy method
235
Furzeland (1980) compared results obtained by the enthalpy program
for several test problems with those obtained by other methods. Tables
6.2, 6.3 are extracted from his paper and some other results referred
to
in
this book are assembled there for convenience.
6.2.5. Accurate determination
of
phase-change boundary
It
is a central feature of the enthalpy method that the position of the
phase-change boundary is determined,
a
posteriori,
from
the
numerical
solution carried out in a fixed domain. Simple inspection of the solution
reveals which mesh point
is
undergoing a phase-change at any time and it
may
take
several time steps for the change to be completed there. The
position of the boundary can be bracketed by the two mesh points
between which temperature changes from less than to greater than the
phase-change temperature
or
vice versa. To locate
the
boundary more
accurately, however, can present difficulties. Extrapolation of the temper-
ature values,
u
or
v,
from either side of
the
phase boundary,
or
interpola-
tion across the boundary,
is
a possible refinement but the results tend to
exhibit a physically unrealistic, stepwise behaviour in
the
motion of the
boundary and in the time history of
the
temperature at a typical fixed
point.
Voller and Cross (1981a) advanced explanations of
the
stepwise and
oscillatory behaviour of
the
basic enthalpy solutions both for discontinu-
ous
and
smoothed enthalpy functions.
The
authors proposed an interpre-
tation of
the
numerical solution which leads to a more accurate evaluation
of
the
boundary movement and
the
temperature history at any point.
With reference first
to
a one-dimensional solidification problem with a
discontinuous enthalpy function, Voller and Cross (1981a) considered an
element
e;
of
the
discretized region associated with a mesh point or node
i.
The
total heat in this element at any time
is
approximated by
II.
8x,
where
II.
is
the enthalpy at node i and 8x
is
the element length (Fig. 6.3).
If
at
any time t the freezing front
is
in element
e;
and moving towards
element
e;+1,
the total heat in
e;
is
the
sum of the heat in the solid and
I
I
+
ej
Solid : Liquid
I
~fax-l
I
-----
ax
----
FIG. 6.3. Partially frozen element e
j

236
Fixed-domain methods
liquid parts, and so
If;
can
be
approximated by
If;
=
CuJ+(Ct4
+L)(l-f),
(6.55)
where
f is
the
solid fraction
of
the
element
and
the
heat
capacity C is
taken
to
be
the
same
in
both
phases.
When
the
freezing front reaches
the
node
i then
f=!,
U;
=
UM,
the
melting temperature, and (6.55) becomes
(6.56)
Thus, when any nodal enthalpy satisfies (6.56)
the
phase-change
bound-
ary can
be
located approximately
on
the
node
i.
This interpretation
of
the
state
of
an element undergoing a phase-change is physically
more
satisfying
than
the
idea
that
the
whole element is uniformly in some
intermediate
state
betwen
the
solid and liquid phases.
Voller and Cross
(1981a) concluded from (6.56)
that
if
at
any nodal
point
i in
the
basic finite-difference solution H/>CuM+!L and
Hj+l<
CuM+!L, for a freezing problem,
the
phase-change boundary
had
passed
through node
i in
the
time interval between j 8t and
(j
+ 1) 8t.
It"it
is
further assumed
that
the
enthalpy changes linearly in any time interval,
8t,
then
the
time
t;
when
the
phase-change boundary is
on
node
point
i,
which occurs when
If;
=
CuM
+ !L, is given
by
t;
=
(j
+
a)
8t
where
a < 1
and
is estimated
by
a=@L+Cu
M
-Hl)/(H/+1-HD. (6.57)
Clearly,
at
time
t;
the
temperature
at
node i is
UM'
The
temperatures
at
other
node points k
(k1=
i)
are
approximated
by
the
linear
int~rpolation
(6.58)
Voller and Cross
(1981a)
obtained
good agreement between
their
finite-
difference solution
interpreted
using
the
above argument
and
the
analyti-
cal solution for a classical, two-phase Stefan problem.
They
extended
their
algorithm
to
include some two-dimensional problems
and
used
it
to
solve a problem similar
to
the
freezing in a square channel studied
by
Crowley (1978),
but
it was a two-phase
problem
as distinct from Crow-
ley's
one
phase. A refinement
of
the
method
(Voller and Cross 1983)
led
to
a
more
continuous tracking
of
the
phase change.
Voller and Cross
(1981a) also described
an
implicit scheme for numeri-
cal solution which incorporated
the
idea
of
choosing each
time
step so
that
the
phase-change
boundary
moved from
one
node
point
to
the
next
for each chosen time step. This was
the
strategy adopted
by
Douglas
and
Gallie (1955),
but
they
did
not
apply
it
to
an
enthalpy
equation
(see
§4.3.1).
The
advantage
of
this approach is
that
the
position
of
the
phase-change boundary is known at each time step. This means
that
the
finite-difference formulae,
and
hence
the
nodal enthalpy and
temperature

Enthalpy method
237
distributions, have their normal accuracy and
if
the
heat
parameters are
different
in
the
two phases they can
be
easily
and
accurately accommo-
dated in
the
scheme.
The
essential feature
of
the
algorithm proposed is
to
ensure
that
at
each time step
one
and
only
one
nodal enthalpy has
the
value CuM+!L.
If
at
time t
the
phase-change boundary is
at
node
k,
an
initial guess for
the
next time step,
l>t
k
,
is
taken
as
l>tg
=
l>t
k
-
1
•
An
implicit finite-difference
scheme for equation (6.37), following Longworth (1975) (see equations
(6.36, 37) above) is used
to
evaluate
the
nodal enthalpies for a time
intervall>tg
and
for successive estimates,
l>tl:',
calculated from
the
iterative
formula
(6.59)
where w is a relaxation factor.
When
H~~8{r
has converged
to
CuM+!L,
the
corresponding enthalpy
and
temperature values
at
all nodal points are
adopted as
the
solution for
the
time
t+l>tl:'.
Voller
and
Cross (1981a)
quoted
acceptable results for a simple two-phase freezing problem and
for a spot-welding problem similar
to
that
studied by
Atthey
(1974).
Their
implicit algorithm is much faster than
their
explicit
one
because
there
are
no
stability restrictions
on
the
time step.
Voller
and
Cross (1981b) confirmed that
their
explicit numerical
scheme incorporating (6.56-8) can
be
satisfactorily applied
to
the
freezing
or
melting
of
a circular cylinder.
From
a collection
of
numerical results
they approximated
the
dimensionless solidification time
t~
of
a circu-
lar
cylinder
to
be
given by
the
empirical expression
t~
=
(0.14+0.085u~)+(0.252-0.0025u~)L
*, (6.60)
with
an
accuracy of 1
per
cent
of
the
numerical predictions when
O:SO;
u~:so;
2,
2:so;L
*:so;50.
The
cylinders
of
radius R are solidifying
under
the
following conditions:
U=UO>UM,
O:SO;r:SO;R,
t<O;
U=UW<UM,
r=R,
t;a>O;
UM
is
the
melting temperature.
The
non-dimensional variables in
(6.60) are defined
to
be
t*=Kt/(R2pc),
u~=(Uo-U~/(UM-UW)'
L*=
L/{C(UM- uw)}.
The
enthalpy function has a discontinuous jump L
at
U =
UM'
A
more
tentative approximation for
the
melting time
of
general
symmetrically shaped
but
not
necessarily circular cylinders is based
on
an
upper and a lower bound.
The
former is
the
melting time
of
a circular
cylinder with
the
same cross-section as
the
original cylinder;
the
latter
is
based
on
a circular cylinder which fits totally inside
the
original cylinder.
A reasonable approximation for
the
solidification time
of
any general,
symmetrically shaped cylinder is afforded
by
using
an
approximating
circular cylinder with a radius equal
to
the
mean
of
the
radii associated
with
the
upper
and
lower bounds.
The
sum
of
the
percentage errors in
the
238
Fixed-domain methods
upper
and lower bounds
is
stated
to
be
given by B =
100(T~-
T~)fTa,
where
To>
Te
are the radii of the upper and lower bound approximating cylinders
and
Ta=(Tu+Te)/2.
For
a square channel
B=24
per
cent: for a pentagon
B = 14.5
per
cent.
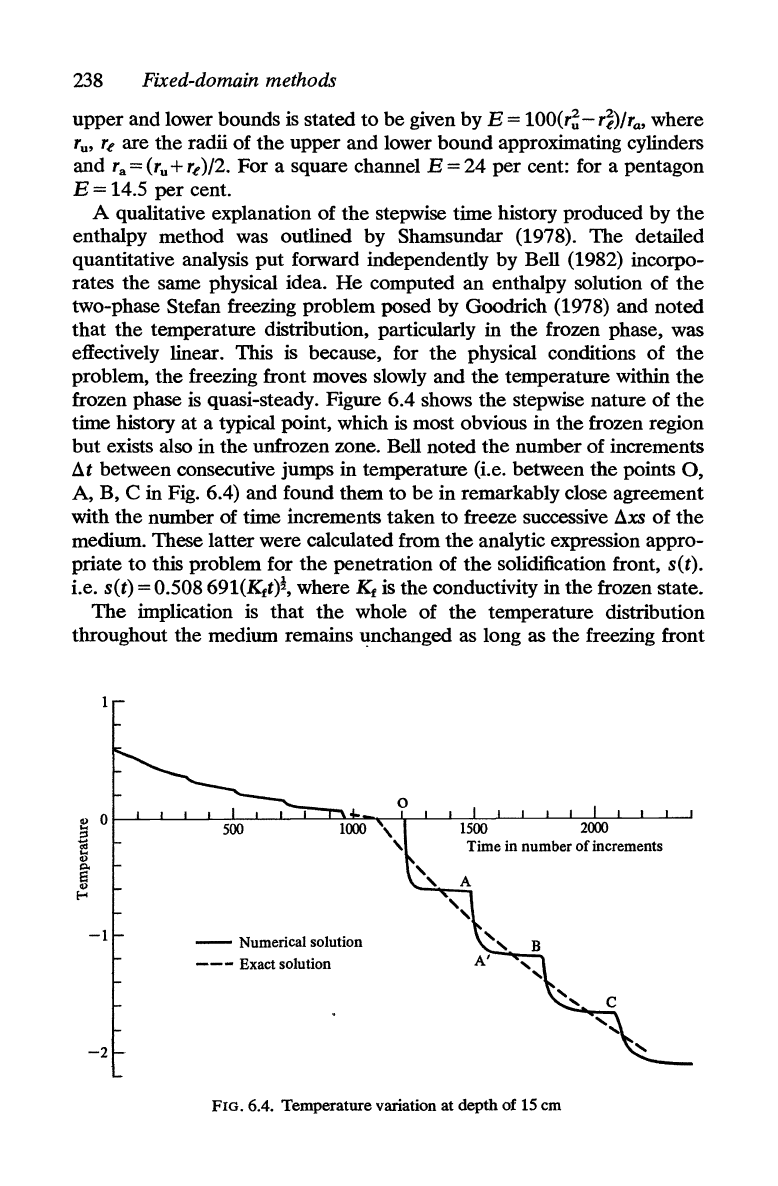
A qualitative explanation
of
the stepwise time history produced by
the
enthalpy method was outlined by Shamsundar (1978).
The
detailed
quantitative analysis
put
forward independently by Bell (1982) incorpo-
rates the same physical idea.
He
computed
an
enthalpy solution
of
the
two-phase Stefan freezing problem posed by Goodrich (1978) and noted
that
the temperature distribution, particularly in
the
frozen phase, was
effectively linear. This is because, for the physical conditions
of
the
problem,
the
freezing front moves slowly and the temperature within
the
frozen phase
is
quasi-steady. Figure 6.4 shows
the
stepwise nature of
the
time history at a typical point, which is most obvious in
the
frozen region
but exists also in the unfrozen zone. Bell noted the number
of
increments
at
between consecutive jumps in temperature (i.e. between the points
0,
A,
B, C in Fig. 6.4) and found them to
be
in remarkably close agreement
with the number of time increments taken
to
freeze successive
axs
of
the
medium. These latter were calculated from
the
analytic expression appro-
priate
to
this problem for
the
penetration of
the
solidification front, s(t).
i.e. s(t) = 0.508
691(Ktt)~,
where
Kt
is the conductivity in the frozen state.
The
implication is
that
the
whole of
the
temperature distribution
throughout the medium remains
~nchanged
as long as the freezing front
-1
- Numerical solution
---
Exact solution
-2
FIG.
6.4. Temperature variation at depth of
15
cm

Enthalpy method
239
remains within
the
spatial element
ax
associated with a particular mesh
point. A change in temperature only occurs when the front moves from
the
locality of one mesh point
to
the
next. This is exactly
the
behaviour to
be
expected from
the
quasi-steady temperature profile. Thus,
if
the
interval (m
-1)
ax';;;x
.;;;(m
+1)
ax
is changing from liquid
to
solid, the
enthalpy computed
at
the
point m
ax
lies between 0 and L, and the
temperature
at
m
ax
stays
at
zero. Thus, there is a region with fixed
boundaries, namely x
= 0, where
the
temperature is
-10°C
in Goodrich's
example,
and
x = m
ax
where
the
temperature is O°C.
The
analytical
expression for the temperature in this subsidiary heat-Bow problem
(Carslaw and Jaeger 1959, p. 100) has
the
form
(
x)
f .
mrx
(-n
2
7T
2
K
f
t')
-10
1---:-
+
£."
An
sm~exp
2(A)2
'
m
~x
n=l
m
~x
m
~x
where t' is measured from
the
instant the temperature
at
x = m
ax
becomes zero.
The
series represents
the
transient
part
of
the
solution.
The
term
-10(1-
x/m
ax)
is
the
ultimate steady state achieved theoreti-
cally after infinite time
but
for this problem the transient effects are very
short lived.
The
intervals
of
time over which transient
heat
Bow
occurs
are
the
short periods
of
rapid temperature drop in Fig. 6.4, e.g. between
the
points A and
N.
The
near-constant-temperature plateaux, e.g. be-
tween
A'
and B, indicate
the
period
of
the
steady-state
Bow.
Bell also showed, with
the
aid
of
the
analytical expression,
that
the
time-scale
of
the
transient effects increases in comparison. with
the
time
taken
for an element
to
freeze, as m increases. This is confirmed in Fig.
6.4.
There
is an implication for
the
linear interpolation which Voller and
Cross
(1981a) used as described above
to
determine
the
time
at
which the
enthalpy is
1L.
It
is
that
the accuracy will deteriorate when a point well
below the surface freezes, because
the
time increments for
the
element
to
freeze will
be
larger, and non-linear transient effects more significant.
6.2.6. Body heating
There
is an important class
of
problems in which
the
heat
needed
to
produce melting comes from
heat
sources distributed throughout the
volume of
the
material
rather
than
by entering through
the
outer
bound-
aries.
An
example is joule heating associated with electric currents within
the
medium. In such cases
the
melting does
not
necessarily take place
across a surface as in the classical Stefan problem. Instead, there may
be
an
extended region the whole
of
which is
at
melting temperature, and the
solid and liquid phases coexist.
This volume of material is called a 'mushy'
region, a
term
used by Tien
and
Geiger (1967) in relation
to
alloys. The
physical nature of a mushy region has been discussed more generally in
240
Fixed-domain methods
o
H
K
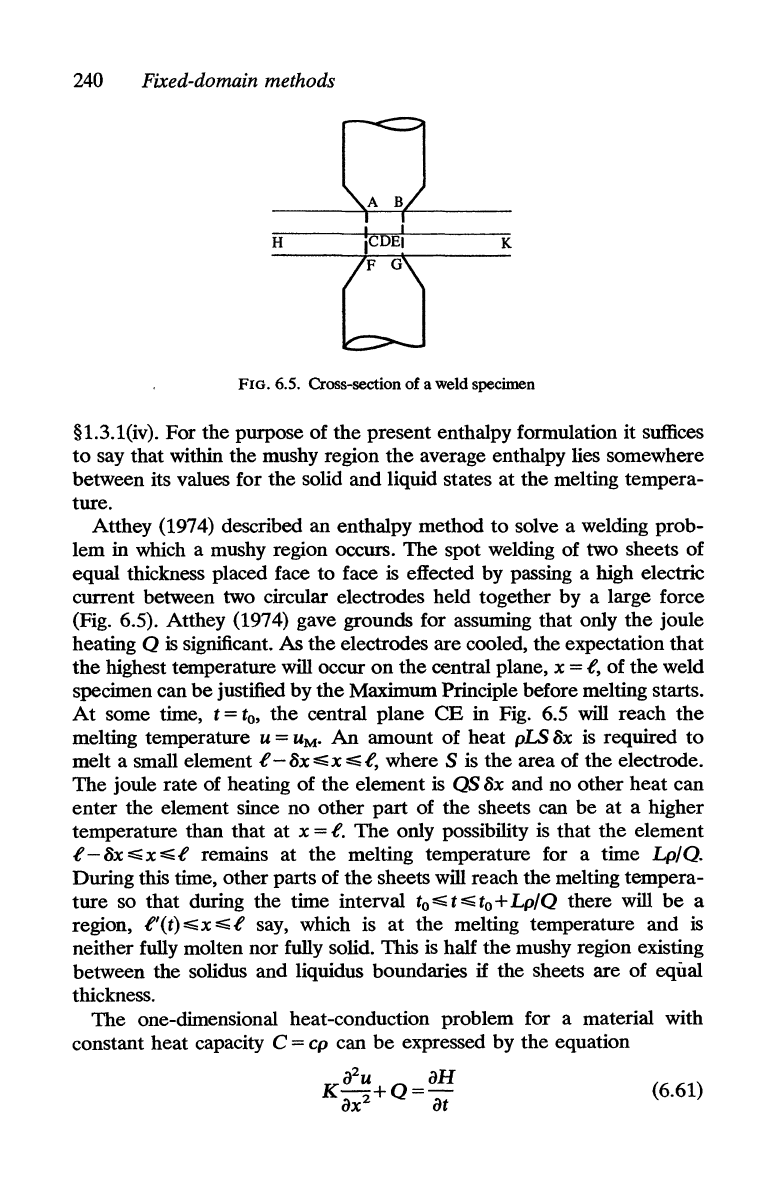
FIG. 6.5. Cross-section
of
a weld specimen
§1.3.1(iv). For the purpose of
the
present enthalpy formulation it suffices
to
say that within the mushy region
the
average enthalpy lies somewhere
between its values for the solid and liquid states at
the
melting tempera-
ture.
Atthey (1974) described an enthalpy method
to
solve a welding prob-
lem in which a mushy region occurs.
The
spot welding of two sheets of
equal thickness placed face to face
is
effected by passing a high electric
current between two circular electrodes held together by a large force
(Fig. 6.5). Atthey (1974) gave grounds for assuming that only
the
joule
heating
Q
is
significant. As
the
electrodes are cooled, the expectation
that
the
highest temperature will occur
on
the
central plane, x = t, of
the
weld
specimen can
be
justified by
the
Maximum Principle before melting starts.
At
some time, t =
to,
the
central plane
CE
in Fig. 6.5 will reach
the
melting temperature U =
UM.
An
amount
of
heat
pLS 8x
is
required
to
melt a small element t - 8x
~
x
~
t,
where S is
the
area
of
the electrode.
The
joule
rate
of heating
of
the
element
is
OS 8x and no
other
heat
can
enter
the element since no
other
part
of the sheets can
be
at
a higher
temperature than
that
at x = t.
The
only possibility is
that
the
element
t-8x~x~t
remains at the melting temperature for a time Lp/Q.
During this time, other parts of
the
sheets will reach the melting tempera-
ture
so
that
during the time interval
to~t"'"'to+Lp/Q
there will
be
a
region,
t'(t)~x"'"'t
say, which is
at
the melting temperature and
is
neither fully molten nor fully solid. This
is
half
the
mushy region existing
between the solidus and liquidus boundaries
if
the sheets are of equal
thickness.
The
one-dimensional heat-conduction problem for a material with
constant heat capacity C
=
cp
can
be
expressed by
the
equation
K;Pu
+Q=aH
(6.61)
ax
2
at
Enthalpy method
241
where u =
u(H)
is
defined by
u=H/C,
H~CuM'
}
u = u
M
,
CuM<H
<
CuM
+ Lp, (6.62)
u=(H-L)/C,
H~CuM+Lp.
The
body-heating
term
Q(u)
is
taken
to
be a given piecewise linear
function of temperature only and other boundary conditions are, for
example,
au/ax
=au,
x=O; au/ax
=0,
x=t.
(6.63)
An
initial condition
H(x,
0) = ho(x)
is
given. Atthey (1974) approximated
(6.61) by the explicit finite-difference scheme
(6.64)
where..\
= 8t/(8x)2 and
the
conditions (6.63) become, as in (6.22), (6.23),
uo=
u~(2-a
8x)/(2+a
8x), (6.65)
where E
8x
= t. The initial condition
is
simply
H:!.
= ho(m 8x). Proceeding
as
in
§§6.2.1, 2, Atthey (1974) showed that the finite-difference solution
converges
to
a unique weak solution analogous
to
(6.13) modified to
include
the
body-heating term and
the
derivative boundary conditions
(6.63).
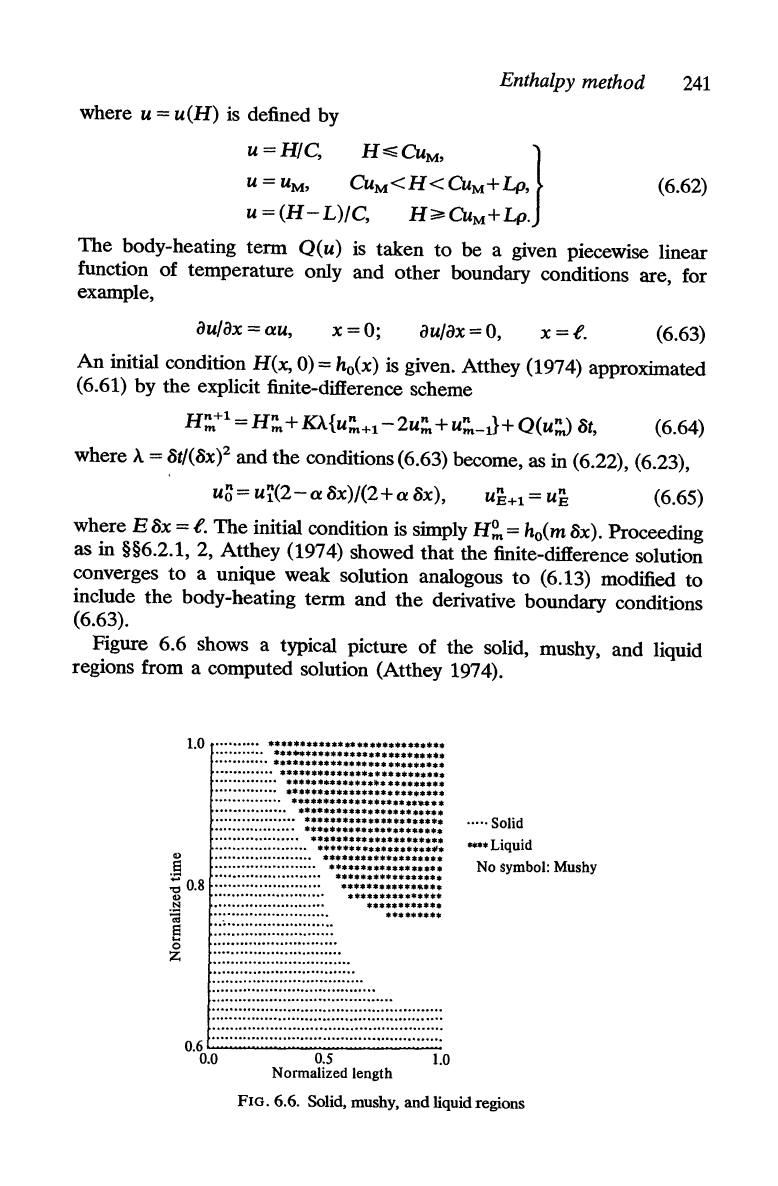
Figure 6.6 shows a typical picture of the solid, mushy, and liquid
regions from a computed solution (Atthey 1974).
"
S
1.0
.......... -*.* •••• *
•••••••
*.****.*
••••
............ *
••
_._
••••
-
.......
-
••••••••
............ *.*****
•••
******** *
••
* •• -
••
..............
.._
••••
*
•••••••
* •• *
.......
.
.............. • *
.......
_
•••
-."'
••
-
•••••••
..............
..
•••• -••••
_*
..............
..
............
0..
•
._
•••••
* *.*.* *
........
*'
•
........... u...
..
............
_*
•••••••• _ •
..................
..............
**.........
.
....
Solid
..................
..*
...
"'
..........
"'._.-
................... .
............
-
..........
"'.
..................... *
........
**
••••••••••
...................... **
••••
*
••••
*
••
*.**.
.......................
..**
••
**
••
*.**.***
..·.Liquid
No
symbol: Mushy
.............. .......... * ... *
..........
*.*.
........................ •
••
*.*
•••••
**
•••
......................... • ••• ****
••
"'*
•••
:;
0.8
"
N
~
S
o
z
.........................
.*
•••
*.**
••
*
........................... *.*."'*.* •
..
-.
.......................
..
................................
..................................
......................................
~
.......................................
.
....................................................
....................................................
....................................................
0.6
L-..
___
~:-::-
____
-:-
0.0 0.5
1.0
Normalized length
FIG. 6.6. Solid, mushy, and liquid regions
