Cooper L.N., Feldman D. (Eds.) BCS: 50 Years
Подождите немного. Документ загружается.

September 14, 2010 9:46 World Scientific Review Volume - 9.75in x 6.5in ch16
The Evolution of HTS: T
c
-Experiment Perspectives 411
or 0011-R]. In 1988, Siegrst et al. succeeded in stabilizing (Ca
0,85
Sr
0.15
)CuO
2
[0011-Ca]. Unfortunately, it was not superconducting and changing the
Ca/Sr-ratio away from 0.85/0.15 immediately triggered the collapse of the
crystal. Three years later, Goodenough et al. used the high pressure synthe-
sis technique and stabilized Sr
1−x
Nd
x
CuO
2
with a T
c
= 40 K at ambient.
21
Later, Takano et al.
22
demonstrated superconductivity up to ∼110 K in
(Sr
1−x
Ca
x
)
0.9
CuO
2
after it was prepared at 6 GPa. Unfortunately, high
resolution electron micrographs revealed that the superconducting sample
was loaded with defects of Ca- and Sr-vacancies, which may be one of the
superconducting members of the Sr- deficient series of Sr
n−1
Cu
n+1
O
2n
with
n = 3, 5, . . . , and not the infinite layer cuprate. As a result, the exact nature
of superconductivity in the defected CCO or 0011-Ca remains unclear.
3.2. The iron pnictides and chalcogenide superconductors
In the last 23 years, almost all major HTS systems were discovered as a
result of a conscientious search for novel superconductors with higher T
c
.
One exception is the discovery of F-doped LaFeAsO with a T
c
of 26 K by
Hideo Hosono et al.
23
of Tokyo Institute of Technology in early 2008 that
inaugurated the exciting iron-pnictide and chalcogenide HTS period. The
discovery was an outgrowth of the effort of Hosono et al. on the functionality
of novel layer transparent oxide semiconductors, such as LaCuOCh where
Ch = chalcogen. LaCuOCh consists of two subdtructures: one a narrow-
gap semiconducting slab (CuCh) and the other a wide-band insulating slab
(LaO), reminiscent of the multi-substructure of cuprate HTSs. Similar to the
cuprate HTS, modulation doping can be carried out by introducing charge
carriers to semiconducting slabs without defects through chemical doping
into the insulating slabs.
The announcement of the LaFeAsO discovery in early 2008 could not
have come at a better time. It has rejuvenated the field of HTS, boosted
the morale of scientists in the field and hence effectively issued a reprieve
of the “death sentence” proposed for HTS in 2006. While scientists con-
tinue to work on HTS, T
c
stopped rising after 1994 and the theory remains
unsettled. A feeling of pessimism began to creep in. The situation seems
to have been epitomized in the 2006 article by Andreas Barth of FIZ Karl-
sruhe and Werner Marx of MPI Stuttgart, Germany titled “Mapping High
Temperature Superconductors: A Scientometric Approach.”
24
Using scien-
tometric analysis of the time-dependence of the overall numbers of articles
and patents, and the time-variation of publications related to specific com-
pound subsets and subject categories, they sentenced HTS to die sometime
September 14, 2010 9:46 World Scientific Review Volume - 9.75in x 6.5in ch16
412 C. W. Chu
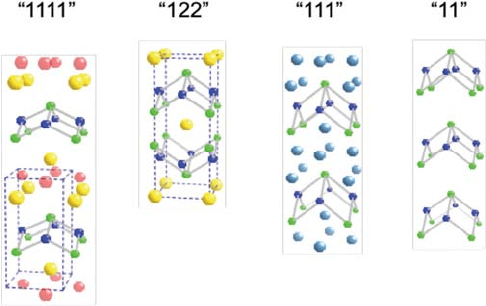
Fig. 12. The structures of Fe-pnictides and Fe-chalcogenides [1111], [122], [111] and [11].
Fig. 12. The structures of Fe-pnictides and Fe-chalcogenides [1111], [122], [111]
and [11].
between 2010 and 2015 by linear extrapolation. While experts in database
collection and research consider the analysis accurate and sound, many sci-
entists point out the pitfall of statistics in predicting the future of science.
Scientific breakthroughs go beyond statistics. Many must remember that
superconductivity had also been sentenced to die in the late 1980s.
Because of the unusually rich Fe-content and the high T
c
of 26 K, the
discovery of F-doped LaFeAsO by Hosono et al. in early 2008 has attracted
worldwide attention. The research intensity on this and related materials was
unmatched except by that associated with YBCO. In the ensuing months,
four families of Fe-pnictide and chalcogenide superconductors with their re-
spective derivatives were discovered with a T
c
as high as 57 K (Fig. 12).
They share many similarities with the cuprate HTSs but differ in electronic
structures, e.g. single band for cuprates whereas multibands for Fe-pnictides.
The four families are doped FeAsRO [1111], where R = rare-earth; doped
AeFe
2
As
2
[122], where Ae = alkaline earth = Ca, Sr, Ba; undoped AFeAs
[111], with A = alkaline; and Fe(Se,Te) [11], as shown in Fig. 12. Distinct
gaps exist among the T
c
s of the four families with their maxima at 57, 38,
20 and 10 K, respectively. All Fe-pnictide and chalcogenide superconductors
display a layer structure that consists of the (FeAs)
2
-slabs, where Fe-ions
form a square planar sheet sandwiched between two As-layers. The pres-
ence or absence of the different layers separating the (FeAs)
2
-slabs in the
compounds differentiate them from one another.
I shall address the discovery of each of the four families of Fe-pnictide
and chalcognide systems. Summaries of other aspects of Fe-pnictide and
chalcogneide superconductors can be found in several review articles.
25
September 14, 2010 9:46 World Scientific Review Volume - 9.75in x 6.5in ch16
The Evolution of HTS: T
c
-Experiment Perspectives 413
3.2.1. The first Fe-pnictide superconductor family with a T
c
up to
57 K: doped RFeAsO, where R = rare-earth [R1111]
The discovery
23
of F-doped LaFeAsO [La1111] with a T
c
= 26 K in early
January 2008 by Hideo Hosono et al. of Tokyo Institute of Technology has
inaugurated the new Fe-pnictide superconductor era, demonstrating that su-
perconductivity with a relatively high T
c
can occur in a Fe-rich environment,
contrary to conventional wisdom, providing a new opportunity to explore the
relationship between HTS and magnetism and offering a possible new avenue
for high T
c
.
Hosono and colleagues had engaged extensively in modifying the func-
tionality of transparent novel oxide semiconductors with 2D layer structures
and with two separate or loosely coupled substructures, and gained exten-
sive knowledge on the chemistry, structure and electron-band manipulation
of inorganic solids. Realizing the importance of layer structure and magnetic
interaction in HTS cuprates, they investigated LaTPO and LaTAsO, where
T is a transition metal element. They detected superconductivity in com-
pounds LaFePO and LaNiPO when the number of d-electrons in T is even,
and magnetism in LaMnPO and LaCoPO when the number of d-electrons
in T is odd. The T
c
s observed in 2006 of LaNiPO and LaFePO were 3 K and
5–12 K, respectively. Unfortunately, the results did not attract much atten-
tion by the HTS community due to the low T
c
. The situation changed when
Hosono et al. observed superconductivity up to 26 K in F-doped LaFeAsO,
or La(O
1−x
F
x
)FeAs, and the results (Fig. 13) appeared in the March 19,
2008, issue of Journal of American Chemical Society in an article entitled
“Iron-Based Layered Superconductor La[O
1−x
F
x
]FeAs (x = 0.05–0.12) with
T
c
= 26 K.”
23
The parent compound LaOFeAs [La1111] is a member of the well-known
equiatomic quaternary layered compounds ROTPn where R = La, Nd,
Sm, Gd; T = Mn, Fe, Co, Ni, Cu; and Pn = P, As, Sb. They exhibit
a tetragonal layer structure of the ZrCuSiAs type with a space group of
P4/nmm. LaOFeAs consists of (Fe
2
As
2
)-layers sandwiched by the (LaO)-
layers. Each (Fe
2
As
2
)-layer contains a squared-planar Fe-sheet sandwiched
by two As-sheets; and each (LaO)-layer comprises an O-sheet sandwiched
between two La-sheets. Similar to the cuprates, the (Fe
2
As
2
)-layers form the
active block where the charge carriers flow, while the (LaO)-layers consti-
tute the charge-reservoir block that inject charge carriers into the former
without degrading the integrity of the (Fe
2
As
2
)-layers. But different from
the cuprates: the divalent Fe is tetrahedrally coordinated with four As-ions
in LaOFeAs, whereas the divalent Cu forms the fourfold square plane in the
September 14, 2010 9:46 World Scientific Review Volume - 9.75in x 6.5in ch16
414 C. W. Chu
(a) Electrical resistivity (F) versus temperature (T) for undoped
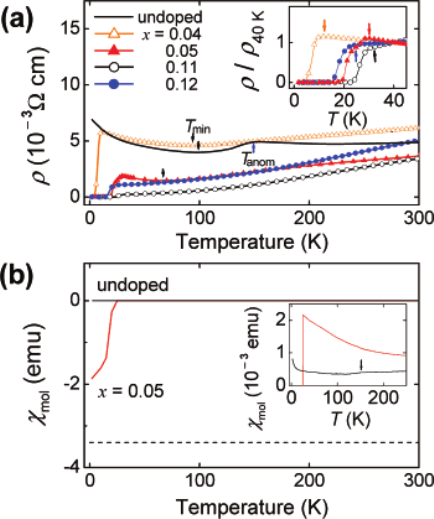
Fig. 13. ρ(T ) and χ(T ) of LaFeAs(O
1−x
F
x
) [1111] shows a T
c
= 26 K in 2008 by
Hosono et al. [Y. Kamihara et al., J. Am. Chem. Soc. 130, 3296 (2008)].
cuprate HTSs; LaOFeAs is a semimetal and undergoes a crystallographic
transition at ∼155 K from tetragonal to orthorhombic symmetry followed by
an antiferromagnetic transition [or a spin-density-wave (SDW) transition] at
T
N
∼ 145 K. Accompanying these transitions, anomalies in resistivity, mag-
netic susceptibility, specific heat, thermoelectric power, Moessbauer, µSR
and NMR measurements have been observed. LaOFeAs becomes supercon-
ducting when charge carriers are introduced via electron-doping by partial
substitution of O by F or by O-reduction to suppress the T
N
.
The electron-doped La(O,F)FeAs [La1111] displays a T
c
as high as 26 K.
23
The discovery has generated great excitement because 1111 is a new material
compound system with a large concentration of magnetic Fe that may lead
to higher temperature and provide a basis for unraveling the origin of HTS.
In the ensuing few weeks after the report, the T
c
was raised by replacing
La with rare-earth elements of smaller radii. Pressure was also later found
to raise the T
c
of La(O,F)FeAs to 43 K. Development parallel to that for
the cuprates seemed to be taking place with some thinking that another
September 14, 2010 9:46 World Scientific Review Volume - 9.75in x 6.5in ch16
The Evolution of HTS: T
c
-Experiment Perspectives 415
1987 “cuprate miracle” might be in the offing. Euphoria permeated the
community and once again, the sky was considered the only limit to T
c
.
Soon after the discovery of the 26 K superconductor of the doped La1111,
the doped Sm(O
1−x
F
x
)FeAs [Sm1111] was found to have a T
c
of 43 K, then
highest among the non-cuprate superconductors. We examined the pressure
effect on the T
c
of Sm1111 with different x to determine whether the T
c
of Sm1111 in particular and R1111 in general can be further enhanced by
pressure or doping. The results show that pressure can either enhance or
suppress the T
c
, depending on the doping x, similar to the cuprate R123.
The observation of the same sign of dT
c
/dP and dT
c
/dx suggests that both
pressure and doping have a similar effect on superconductivity and therefore
on the SDW-state. Given the gross similarity of the R1111 to the cuprate
R123, we therefore proposed that members of R1111 are expected to have a
similar maximum T
c
of ∼ 50s K,
26
independent of R, as was confirmed by
later theoretical calculations. Unfortunately, the prediction remains correct
to date.
Following the observation of superconductivity in La(O
1−x
F
x
)FeAs, the
T
c
of R(O
1−x
F
x
)FeAs has been raised from 26 K to 41 K, 52 K, 52 K,
55 K, 36 K, 46 K and 45 K for R = Ce, Pr, Nd, Sm, Gd, Tb and Dy,
respectively. Electron doping via O-reduction in RO
1−x
FeAs results in
similar T
c
as through F-doping. Partial replacement of elements in other
sites of R1111 can also induce superconductivity with high T
c
, for exam-
ple, in (La
1−x
Th
x
)OFeAs (T
c
≤ 30.3 K), (La
1−x
Sr
x
)OFeAs (T
c
≤ 25 K),
LaO(Fe
1−x
Co
x
)As (T
c
≤ 22 K), and (A
1−x
R
x
)FeAsF where A = Ca, Sr
(T
c
≤ 50 K), accompanied by suppression of the SDW-state. It is evident
that doping the O-site gives a higher T
c
in R1111. High pressure studies up
to 30 GPa on the T
c
of many of these compounds, superconducting or not,
have been carried out.
27
The T
c
shows an initial rapid rise to a maximum
< 57 K followed by a slow decrease at higher pressure, but for some, their T
c
does not display the initial increase, depending on the detailed doping state
and pressure homogeneity. For those non-superconducting R1111, pressure
turns them superconducting while suppressing the SDW-state. It is clearly
evident that the suppression of the SDW-state by doping and pressure is
critical to the appearance of superconductivity, although some experiments
do show the coexistence of the superconducting and the SDW-states in the
intermediate region. We therefore propose that defects externally introduced
such as by irradiation should suppress the SDW and induce superconduc-
tivity in R1111. In spite of the great progress made on R1111, no T
c
higher
than 60 K has been realized.
September 14, 2010 9:46 World Scientific Review Volume - 9.75in x 6.5in ch16
416 C. W. Chu
3.2.2. The second Fe-pnictide oxygen-free superconducting family
with a T
c
up to 38 K: doped AFe
2
As
2
, where A = Ba, Sr,
Ca [A122]
The discovery
28
of K-doped BaFe
2
As
2
[Ba122] with a T
c
up to 38 K in late
May 2008 by Marianne Rotter et al. of Ludwig-Maximilians-University of
Muenchen demonstrated that two (Fe
2
As
2
)-layers per unit cell is achievable,
a simple elemental A-layer can serve as the charge reservoir and oxygen is
not critical for superconductivity in Fe-pnictides, giving hope that higher T
c
in Fe-pnictides may be possible due to their material flexibility.
After the discovery of the 26 K-superconductivity in R1111, it was soon
recognized that the (Fe
2
As
2
)-layer is crucial for the high T
c
in Fe-pnictides.
In the hope of raising T
c
by increasing the (Fe
2
As
2
)-layers per unit cell, it is
rather natural to examine the well-known AFe
2
As
2
[A122], which contains
two (Fe
2
As
2
)-layers per unit cell. The compound family was first synthe-
sized in the early 1980s, with A = the alkaline earth Ba, Sr, or Ca. The
undoped A122 crystallizes in the tetragonal layer structure of the ThCr
2
Si
2
-
type of the I4/mmm space group with the (Fe
2
As
2
)-(A)-(Fe
2
As
2
)-(A) layer-
stacking. They are semi-metallic. Upon cooling, they undergo a tetragonal
to orthorhombic structural transition at T
o
= 122 K, 205 K and 170 K for
A = Ba, Sr and Ca, respectively, similar to R1111. These structural tran-
sitions coincide with the antiferromagnetic transitions at T
N
, indicative of
the onset of the SDW-state in contrast to the R1111 where T
o
> T
N
. From
valence consideration, A122 and R1111 are similar. Doping and pressure are
therefore expected to induce superconductivity in undoped A122.
Realizing the existence of the iso-structure compound KFe
2
As
2
, Rot-
ter et al.
28
hole-doped BaFe
2
As
2
to induce superconductivity in Ba122 by
suppressing the SDW-state. They succeeded in achieving a T
c
of 38 K in
(Ba
1−x
K
x
)Fe
2
As
2
[(Ba
1−x
K
x
)122] when x = 0.4 in late May 2008 and pub-
lished the results in the September 5, 2008, issue of Physical Review Let-
ters in an article entitled “Superconductivity at 38 K in the iron arsenide
(Ba
1−x
K
x
)Fe
2
As
2
” (Fig. 14). At about the same time, the iso-electronic
and iso-structural (Sr
1−x
K
x
)Fe
2
As
2
[(Sr
1−x
K
x
)122] was independently found
by us to be superconducting with a maximum T
c
= 38 K at x = 0.4–
0.5, similar to (Ba
1−x
K
x
)122. At the same time, we found that undoped
A
0
Fe
2
As
2
with A
0
= K, Rb and Cs are superconducting at 3.7 K, 2.8 K and
2.6 K, respectively, as expected based on the valence count of the elements
in A
0
122. A complete T
c
-x phase diagram of (Sr
1−x
K
x
)Fe
2
As
2
, the first
for Fe-pnictide superconductors, was therefore constructed and a systematic
study was made. It displays the possible coexistence of superconductivity
September 14, 2010 9:46 World Scientific Review Volume - 9.75in x 6.5in ch16
The Evolution of HTS: T
c
-Experiment Perspectives 417
0 50 100 150 200 250 300
0.0
0.2
0.4
0.6
0.8
1.0
1.2
BaFe
2
As
2
(Ba
0.6
K
0.4
)Fe
2
As
2
KFe
2
As
2
Resistance (m1cm)
Temperature (K)
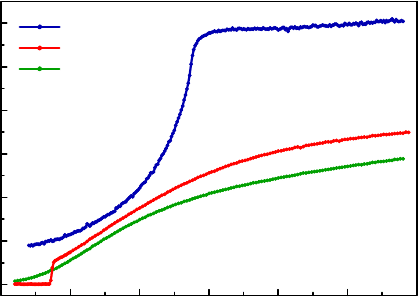
Fig. 14. ρ(T ) of (Ba
0.6
K
0.4
)Fe
2
As
2
[122] shows a T
c
up to 38 K in 2008 by Rotter
et al. [M. Rotter et al., Phys. Rev. Lett. 101, 107006 (2008)].
and SDW at 40.15 < x < 0.35. It also showed that dT
c
/dP and dT
c
/dx have
the same sign, similar to cuprate HTSs, lending support to the previously
suggested T
c
-limit of ∼50s K for Sm1111 and R1111.
Various doping and high pressure experiments similar to those made on
R1111 were carried out on A122 and were observed to generate grossly sim-
ilar, although qualitatively different, results, leading to the same conclusion
that superconductivity competes against the SDW and becomes a robust
ground state when SDW is suppressed either completely or partially by dop-
ing or pressure. Unfortunately, no effort using high pressure to date has been
able to raise the T
c
of A122 to above 38 K, the highest attained by doping.
The lower T
c
of A122 than that of R1111 may signal the need to look for a
new avenue to higher T
c
rather than increasing the (Fe
2
As
2
)-layers per unit
cell. This leaves a distinct T
c
-gap between R1111 and A122 for reasons that
remain unknown.
3.2.3. The third family of Fe-pnictide superconductors of the simplest
structure with a T
c
∼ 23 K without doping and 33 K with
doping: undoped A
0
FeAs where A
0
= Li, Na [A
0
111]
The discovery
29
of superconductivity in LiFeAs [Li111] without doping (or
in the absence of SDW) with a T
c
up to 18 K by Joshua Tapp et al. of the
University of Houston and independently by X. C. Wang et al. of the Physics
Institute in Beijing in July 2008 showed that superconductivity occurs in the
September 14, 2010 9:46 World Scientific Review Volume - 9.75in x 6.5in ch16
418 C. W. Chu
10 20 30
-1.0
-0.5
0.0
0
2
4
6
8
15 16 17 18 19
4 o
T
(
K
)
ZFC
FC
T
c
(K)
H (Tesla)
51030
50
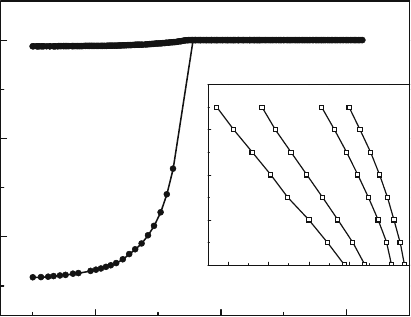
Fig. 15. χ(T ) of LiFeAs [111] shows a T
c
∼ 18 K in 2008 by Tapp et al. [J. H. Tapp
et al., Phys. Rev. B 78, 060505(R) (2008); and X. C. Wang et al., Solid State
Comm. 148, 538 (2008)].
simplest structure of Fe-pnictides with only the (Fe
2
As
2
)-layers separated by
the nominal double layers of Li, demonstrating further the important role
of the (Fe
2
As
2
)-layers in superconductivity of Fe-pnictides but at the same
time raising questions concerning the role of magnetism in superconductivity
in Li111.
The reason is not known for the lower T
c
of A122 than of R1111 which has
two (Fe
2
As
2
)-layers per unit cell compared with that of R1111 which pos-
sesses only one (Fe
2
As
2
)-layer per unit cell, in contrast to cuprate HTSs
where T
c
increases with the number of (CuO
2
)-layers per unit cell. A
Fe-pnictide superconductor with a simpler structure might help reveal the
underlying reason. Li111 crystallizes in a tetragonal structure of the Cu
2
Sb
type of the P4/nmm space group. It has a structure similar to R1111
after replacing the (LaO)-layer in R1111 by the simpler (Li)-layer. Li111 was
found
29
superconducting with a T
c
of 18 K and the results appeared in the
August 18, 2008, issue of Physical Review B in an article entitled “LiFeAs:
An Intrinsic FeAs-based superconductor with T
c
= 18 K” (Fig. 15).
The Li111 examined was found to be stoichiometric with Li:Fe:As =
1:1:1 within the resolutions of XRD and neutron analyses. It is rather
unusual to find that Li111 becomes superconducting at a relatively high
temperature but without external doping or any sign of SDW as in undoped
R1111 and A122, in spite of their similarities in structure and valence count.
Unlike A
0
122 with A
0
= alkaline, Li111 should not be a metal let alone a
September 14, 2010 9:46 World Scientific Review Volume - 9.75in x 6.5in ch16
The Evolution of HTS: T
c
-Experiment Perspectives 419
superconductor based on a valence consideration. The negative Seebeck co-
efficient detected seems to suggest that the Li111 samples investigated are
effectively electron-doped, implying that the samples may be Li-rich, in con-
trast to XRD and neutron analyses. An alternative scenario is that the
superconductivity in stoichiometric Li111 is caused by chemical pressure as-
sociated with the very small ionic radius of Li, which promotes the electron
population in the bands of the compound and suppresses the SDW. This
seems to be consistent with the large negative pressure effect on the T
c
of
Li111, putting the undoped compound in its overdoped region.
Later, the isostructural and isoelectronic NaFeAs [Na111] were success-
fully prepared and studied. Similar to Li111, the undoped Na111 was found
superconducting with a T
c
∼ 23 K. While the stoichiometric issue remains,
its T
c
increases rapidly to 31 K with pressure up to 3 GPa. Chemical pressure
induced by partial replacement of As by P with a smaller ionic radius was
found to enhance the T
c
to 33 K.
30
It is very likely that the T
c
of P -doped
Na111 may be further raised by pressure, similar to Hg12(n-1)n.
3.2.4. The first layer Fe-chalcogenide without intervening layers with
a T
c
of 12 K at ambient and 27 K at 1.5 GPa: FeSe [11]
The discovery
31
of superconductivity in FeSe [11] with a T
c
of 12 K by Maw-
Keun Wu et al. of the Physics Institute in Taipei in 2008 and the subsequent
rise of its T
c
to 27 K under pressure by Yoshikazu Mizuguchi et al. of the
National Institute for Material Science at Tsukuba in 2008 demonstrated
that superconductivity with a relatively high T
c
in Fe-chalcogenides is asso-
ciated with the (Fe
2
Se
2
)-layer, analogous to Fe-pnictides with the (Fe
2
As
2
)-
layer and that As is important but not indispensable for superconductivity at
high temperature. It offers a simple material system for model calculations
to identify the crucial parameters in the occurrence of superconductivity in
both the Fe-pnictides and Fe-chaogenides.
Because of the toxicity issue of As and the significance of (Fe
2
As
2
)-layers
in superconductivity in Fe-pnictide superconductors, Wu et al. of the Insti-
tute of Physics at Taipei decided to investigate the α-phase of FeSe which
possesses the tetragonal PbO-type structure of the P4/nmm space group and
consists of the (Fe
2
Se
2
)-layers. They
31
synthesized and discovered in June
2008 the Se-deficient α-FeSe
1−x
to be superconducting although at 12 K,
the first layer Fe-chalcogenide superconductor. The results appeared in the
September 23, 2008, issue of Proceedings of the National Academy of Sci-
ences in an article entitled “Superconductivity in the PbO-type Structure
α-FeSe” (Fig. 16).
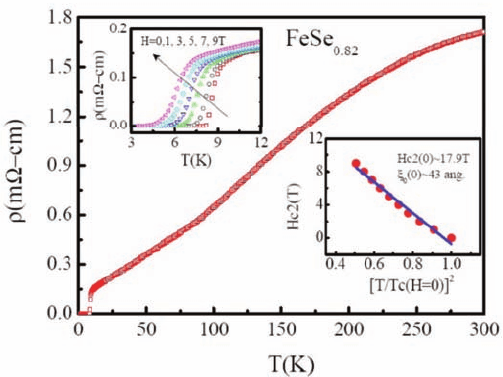
September 14, 2010 9:46 World Scientific Review Volume - 9.75in x 6.5in ch16
420 C. W. Chu
!
Fig. 16. ρ(T ) of FeSe
0.82
[111] shows a T
c
∼ 9 K in 2008 by Wu et al. [F. C. Hsu
et al., Proc. Natl. Acad. Sci. USA 105, 14262 (2008)].
Soon afterward, high pressure was found to raise the T
c
to 27 K at
1.48 GPa,
32
exceeding the ambient T
c
of La1111. Partial replacement of
Se with Te or S makes the synthesis easier but enhances the T
c
at ambient
only slightly.
3.3. Heavy fermion superconductors
For a long time, physicists have been intrigued by the interaction of mag-
netism with superconductivity. Matthias introduced the rare-earth ele-
ments to a superconductor to probe the coupling between superconductivity
and magnetism and to explore the possible coexistence of the two. This
demonstrated the importance of the exchange interaction between the 4f
electrons in the suppression of superconductivity with Ce as an exception
and, by extrapolation, proposed the possible coexistence of ferromagnetism
and superconductivity in the small doping region. The exceptionally large
T
c
-suppression by Ce was later attributed to the Kondo effect of Ce-ions. The
interaction results from the strong hybridization of its magnetic 4f-electrons
with the conduction electrons and thus suppresses superconductivity.
However, at temperatures below the characteristic Kondo temperature, the
depairing effect is reduced by Kondo-screening, which is responsible for the
observation of e.g. reentrant superconductivity in Ce
1−x
La
x
Al
2
. The ques-
tion concerning the possible coexistence of ferromagnetism and superconduc-
