Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.

uous process can be expressed by multiplying the dilution rate by the
product concentration (DP), it is easy to conclude that low cell concentra-
tions result in low product concentrations and low productivities, making
this process not very attractive for industrial applications.
9.4.4 Continuous cultivation with cell retention (perfusion)
Widely known as perfusion, this operation mode presents the highest
productivities and, at the same time, the highest operational complexity.
Since the innovating work on a spin-filter in 1969 (Himmelfarb et al.,
1969), the use of this operation mode has become more and more popular,
both at laboratory and industrial scales (Chu and Robinson, 2001).
(B)
Cell concentration (cells/mL)
0.0E 00⫹
8.0E 05⫹
1.6E 06⫹
2.4E 06⫹
3.2E 06⫹
Viable cells
0.6d
⫺1
0.7d
⫺1
0.8d
⫺1
(A)
F, S
A
F, S, X
v
0
5
10 15 20
Time (days)
Xv
S
P
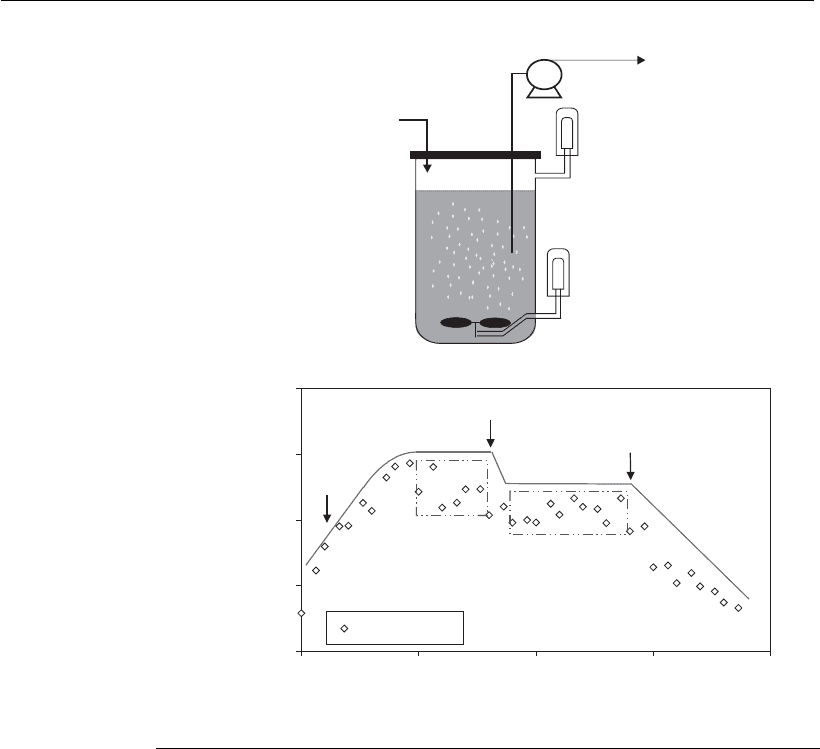
Figure 9.16
Continuous cultivation: (A) typical operational configuration; (B) cell
concentration as a function of dilution rate for a continuous culture of a myeloid
transfectoma producing a humanized monoclonal antibody (Center of Molecular
Immunology – CIM, Cuba). The different steady states are indicated by rectangles
and the line has been included to facilitate interpretation of the cell concentration
profile.
242 Animal Cell Technology
In this operation mode, it is possible to mitigate the major limitation of
continuous cultures, that is, the low productivity due to the loss of cells in
the bioreactor outlet. In perfusion, this issue is overcome by using a cell
retention device to maintain cells inside the bioreactor. Figure 9.17 shows
a scheme of a stirred-tank bioreactor operating in perfusion mode, as well
as the kinetic behavior of a perfusion run.
Equations 8 and 9, previously shown for the substrate and product mass
balances in continuous cultures, can also be applied to this cultivation
mode. However, due to cell retention, the equation that expresses the cell
mass balance is altered, when compared with the mass balance of cells in a
continuous culture (Equation 11):
0 1020304050
(A)
F, S
Cell retention
device
F, S, Xvα
(B)
Viable cells Product
Time (days)
Product concentration (cells/mL)
Product concentration (mg/L)
0.0E 00⫹
1.0E 07⫹
2.0E 07⫹
3.0E 07⫹
4.0E 07⫹
0
20
40
60
80
100
120
Xv
S
P
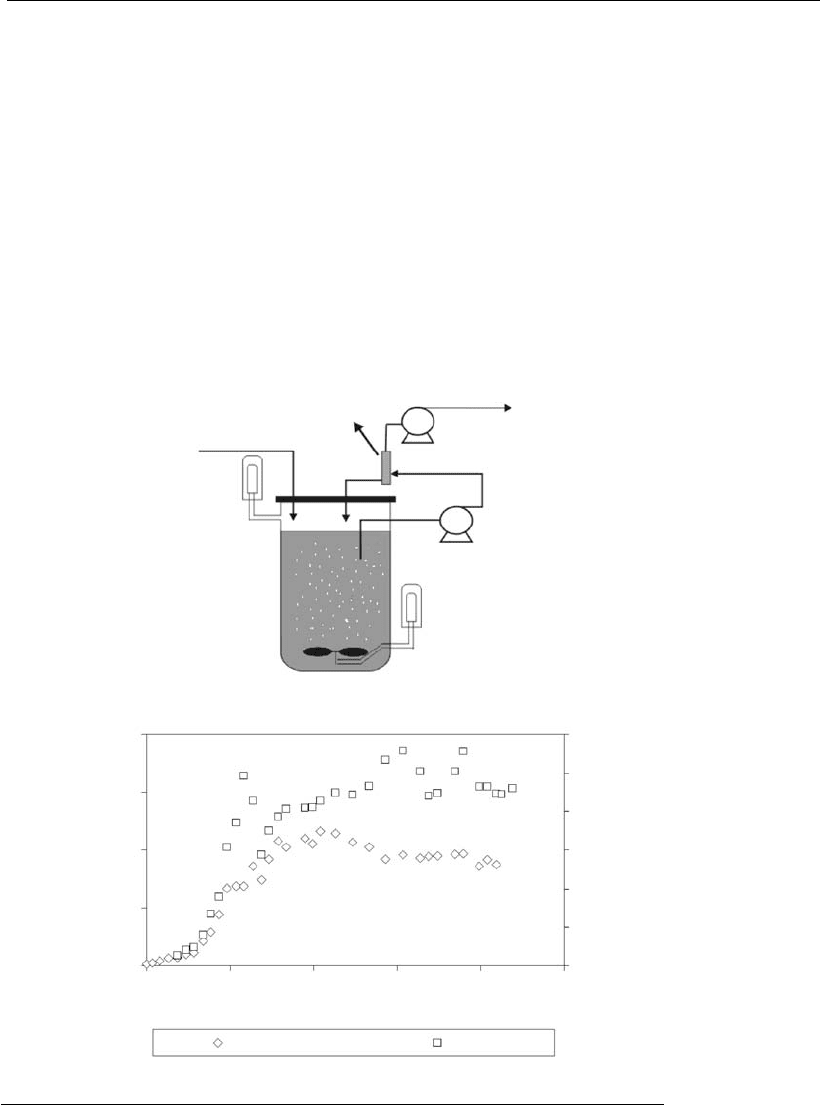
Figure 9.17
Perfusion culture: (A) t ypical operational configuration, using an external cell
retention device; (B) concentration of viable cells and product in a perfusion
bioreactor culture of a myelo id t ransfectoma producing a humanized mono clonal
antibody (Center of Molecular Immunology – CIM, Cu ba).
Bioreac tors for animal cells 243
dX
v
dt
¼ X
v
ÆDX
v
(11)
In Equation 11, Æ is the cell passage factor, defined as the ratio between
cell concentration in the perfusate and in the bioreactor (Equation 12).
Æ ¼
X
v,PERFUSATE
X
v
(12)
Cell retention may be total or partial. A total cell retention would result
in Æ ¼ 0, whereas a value of 1 would mean no retention of cells.
Wash-out conditions in perfusion cultures are different due to the
retention of cells. In the same way as Equation 10 was obtained, Equation
13 can be derived from Equation 11:
MAX
¼ Æ D
washout
(13)
For low Æ-values (between 0 and 0.2), the dilution rate may be increased
up to values that are much higher than the maximum specific growth rate,
without establishing a wash-out condition. The increase in the dilution
rate that can be employed results in a greater availability of nutrients and,
consequently, in an increase of cell and product concentrations. Thus, the
perfusion mode allows a high-cell-density culture to be maintained for a
long period, at a high perfusate flow rate and an elevated product
concentration. This results in productivities that are 1–2 orders of magni-
tude higher than those obtained in continuous processes. In fact, the
performance of perfusion cultures is superior to that of all other operation
modes (Bibila and Robinson, 1995).
The operation of cultures in perfusion mode is possible for almost all
existing bioreactor types. Heterogeneous bioreactors are usually operated
in perfusion mode, and homogeneous bioreactors can be if a solid–liquid
separation device (cell retention device) is used (see Chapter 11).
As indicated for other modes, it is necessary to maintain the inoculum
concentration in the range of 0.1 3 10
6
to 0.4 3 10
6
cells mL
–1
,to
minimize the adaptation lag phase at the beginning of a cultivation process.
The profiles for cell growth and product formation will depend on the
feeding strategy adopted, on the cell line characteristics, and on the
performance of the cell retention device.
In the case of homogeneous bioreactors, the maximum cell concentra-
tion in perfusion cultures can attain 10
7
–10
8
cells mL
–1
. When this opera-
tion mode is used in heterogeneous bioreactors, cell concentration in the
cell compartment can approach the packing limit of tissues, which is in the
order of 10
9
cells mL
–1
. Product concentrations reported for these pro-
cesses vary considerably, but are most commonly in the range of 100–
500 mg L
–1
. Culture duration can be in the range of several days up to
several months (Bo
¨
deker, 1994).
The feeding strategy adopted in a perfusion process is of great impor-
tance. The ideal approach, that is, the one that results in the highest
productivity, depends on the relationship between growth kinetics and
product formation kinetics. For cell lines that present growth-associated
production, it is recommended to feed culture medium at an increasing
dilution rate to allow maximum cell growth. However, if product forma-
244 Animal Cell Technology

tion by the cells is not associated or inversely associated with growth
kinetics, then the dilution rate should be increased up to a given value and
then remain constant, resulting in a steady state of the main process
parameters (Figueredo, 2002).
An example of the first strategy is the so-called cell-specific perfusion
rate (Ozturk, 1996), which allows maintenance of cells in a constant
environment by exponentially increasing the feed rate according to the cell
concentration profile (Chico and Ja
¨
ger, 2000). A negative aspect of this
strategy is that very high cell concentrations are achieved rapidly, and this
may negatively affect the cell separation device (Deo et al., 1996), and thus
limit the culture duration. This negative aspect can be minimized by
establishing a cell bleed stream for a controlled removal of cells from the
bioreactor. This allows maintenance of a high cell viability, combined with
a stable and high cell concentration, at a level that is compatible with good
performance of the cell separation device. Furthermore, the high dilution
rates used in this case result in short residence times of the product inside
the bioreactor, which can be relevant in the case of products that are very
unstable or that can be easily degraded under culture conditions.
Another feeding strategy, which is sometimes called ‘‘stationary strat-
egy’’, aims at extending the culture duration for months (Bo
¨
deker, 1994).
However, the use of this type of strategy results in a considerable decrease
in cell growth rate (Figueredo, 2002) and in an accumulation of dead cells,
causing the release of cell debris and substances potentially dangerous to
product integrity. To diminish this problem, cell purges are periodically
carried out in order to maintain a more constant and controlled cell
environment (Kempken et al., 1991).
Although in most cases perfusion operation presents several economic
advantages, its industrial use is not as widespread as that of fed-batch
cultures. This is due mainly to false ideas of the technological and
operational difficulties and an underestimation of the productivity gains of
this operation mode. A critical evaluation of the characteristics of perfu-
sion operation indicates that the disadvantages have usually been empha-
sized or even exaggerated (Kadouri and Spier, 1997).
The design of bioreactors for perfusion operation is more sophisticated,
which makes the equipment more expensive. However, the productivity
increases obtained by perfusion operation allow the use of much more
compact systems than those operated under batch or fed-batch mode. In
this way, perfusion bioreactors can be up to 10-fold smaller for a given
production scale (Bibila and Robinson, 1995), decreasing the costs not
only of the bioreactors themselves, but also of storage tanks and down-
stream processing equipment.
As regards validation, perfusion bioreactors, which are more complex
and of more recent use, have been classified in the past as difficult to
validate (Kadouri and Spier, 1997). The longer cultivation runs result in
longer validation processes when compared with discontinuous processes.
However, the growing number of approved biopharmaceutical production
processes based on perfusion mode suggests a change in attitude by the
regulatory agencies (Chu and Robinson, 2001).
In summary, the main technological features of this operation mode are:
Bioreac tors for animal cells 245

(i) more complex operation than in the case of other operation modes;
(ii) higher contamination risk, since it is an open system that operates
continuously for long periods of time;
(iii) higher volumetric productivity;
(iv) short residence time of product in the bioreactor (ideal for the
production of labile molecules);
(v) process optimization basically through manipulation of medium
composition, feeding strategy, and controlled cell removal (cell bleed-
ing);
(vi) scales generally up to 2000 L.
9.5 Aeration and agitation
Aeration and agitation are two important operations in animal cell culture.
Oxygen has a low solubility in aqueous media, with a saturation concen-
tration of approximately 7 mg L
–1
at 378C. This implies that the oxygen
must be provided continuously to the cultures, to ensure that the dissolved
oxygen levels in the culture medium remain at an adequate level. The mass
balance for oxygen in the liquid phase can be written as:
dDO
ðÞ
dt
¼ k
L
aDO
DO
ðÞ
q
O2
X
v
(14)
Where:
DO
¼ concentration of dissolved oxygen at saturation (in equilibrium
with the gas phase);
DO ¼ dissolved oxygen concentration;
k
L
¼ global oxygen transfer coefficient;
a ¼ gas–liquid interfacial area per reactor volume;
q
O2
¼ oxygen specific consumption rate.
This mass balance concerns the liquid phase, since oxygen must be
dissolved in order to be used by the cells. Due to the difficulty in measur-
ing the interfacial area (a), especially when oxygenation is carried out by
bubble aeration, it is common to use the product of k
L
times a (k
L
a),
known as the volumetric oxygen transfer coefficient, as the relevant
parameter.
The dissolved oxygen concentration at equilibrium follows Henry’s
Law (Equation 15), with the constant H
O2
being a function of tempera-
ture. Since the air contains 21% of oxygen, the partial pressure P
O2
when
using pressurized air is fivefold lower than when using pure oxygen. Thus,
DO* is higher for pure oxygen injection, resulting in a larger oxygen
transfer rate to the liquid medium.
DO
¼ H
O
2
P
O
2
(15)
Where:
P
O2
¼ oxygen partial pressure;
H
O2
¼ Henry’s constant.
Different studies have established an optimal dissolved oxygen concen-
tration for animal cell culture in the range of 20–50% of air saturation
246 Animal Cell Technology

(Butler, 2004). Too high oxygen concentrations lead to the formation of
free radicals in the medium, which may cause oxidative damage to cells.
The DO concentrations are monitored with previously calibrated electro-
des, whereby the 100% value is adjusted after saturation of culture
medium with air, at the cultivation temperature (usually 378C). The
oxygen transfer system must be designed to meet the demands of the
culture, avoiding situations of limitation or excess of dissolved oxygen.
This is accomplished by supplying air, pure oxygen, or mixtures of both
gases to the culture. Another aim of aeration is to allow dissolved carbon
dioxide originating from cell respiration to be removed in the gas exhaust
stream, to avoid toxic levels (Gray et al., 1996). The removal of carbon
dioxide is known as ventilation.
Different aeration methods have been employed in animal cell culture:
(i) surface aeration;
(ii) aeration through membrane devices or gas-permeable tubing;
(iii) bubble aeration.
The first two methods are widely used just for laboratory-scale applica-
tions, since there are limitations for their use at larger scales. In surface
aeration, the oxygen transfer rate is related to the liquid surface area (gas–
liquid interface). When the culture scale is increased, maintaining constant
geometric proportions, the volume increases with the third power of a
characteristic linear dimension of the system, whereas the surface area
increases with its square.
A recent option is that found in wave bioreactors, where the generation
of waves increases oxygen transfer by augmenting both the interfacial area
(a) and the global transfer coefficient k
L
(Singh, 1999). Therefore, these
reactors are already available commercially at volumes up to 500 L.
The second method uses devices that can be positioned either inside the
bioreactor or in external recirculation loops. This method allows aeration
in the absence of bubbles, minimizing the negative effects that these can
exert on the cells, as will be discussed later in this chapter (Lehmann et al.,
1987). The aeration devices can be based either on membranes or on non-
porous tubes made of oxygen-permeable materials, such as silicon, or on
porous devices made of materials that are impermeable to oxygen, such as
polypropylene. This method also has limitations regarding scale-up, and
its use has been reported just for bioreactors up to 150 L in volume
(Lehmann et al., 1987). Its use at a larger scale becomes unfeasible, due to
the operational difficulties associated with assembling, cleaning, and
sterilizing systems that contain hundreds of meters of tubes or membranes.
In heterogeneous bioreactors, external aeration devices are most com-
monly employed, since it is easy to recirculate a cell-free or almost cell-
free stream through an independent oxygenation system. Due to the low
solubility of oxygen in aqueous media, the recirculation speed usually
needs to be very high, reaching up to 100 vvd (recirculation volume per
reactor volume per day).
The aeration method most widely used in animal cell cultures is bubble
aeration, just as in microbial fermentations. This method is simple and
consists of bubbling a gas stream directly into the culture medium, using a
Bioreactors for animal cells 247
sinter sparger, orifice sparger or jet-flow device. Sinter spargers are made
of porous stainless steel and can generate bubbles with diameters in the
order of hundreds of micrometers. Orifice-type and jet-flow spargers
generate bubbles 1 mm in diameter or larger (Puleo et al., 2004). In the
bioreactors, the spargers are generally positioned below the impellers, to
promote a homogeneous distribution of bubbles inside the culture vessel.
Although simple, the use of bubble aeration in animal cell cultivation
requires the solution of certain technological challenges for its implemen-
tation, since one of the most characteristic features of animal cells is their
low resistance to mechanical stresses. Cell membranes have a mechanical
resistance that is much lower than that of microbial cell walls. According
to Castilho and Anspach (2003), shear stresses in the order of 60 Pa are
critical for cell death.
It is reported that bubble disengagement and bursting at the liquid
surface is a major cause of cell death in animal cell cultures (Kunas and
Papoutsakis, 1990; Butler, 2004). The cell membrane is slightly hydropho-
bic under culture conditions, and therefore cells tend to adhere to bubbles
ascending inside a bioreactor. When these reach the liquid surface, the film
of fluid that surrounds the bubble begins to be drained by gravity,
decreasing in thickness. The bubble bursts at the moment the mechanical
resistance of this film is not sufficient to resist the pressure inside the
bubble. The explosion of a bubble causes large velocity gradients in the
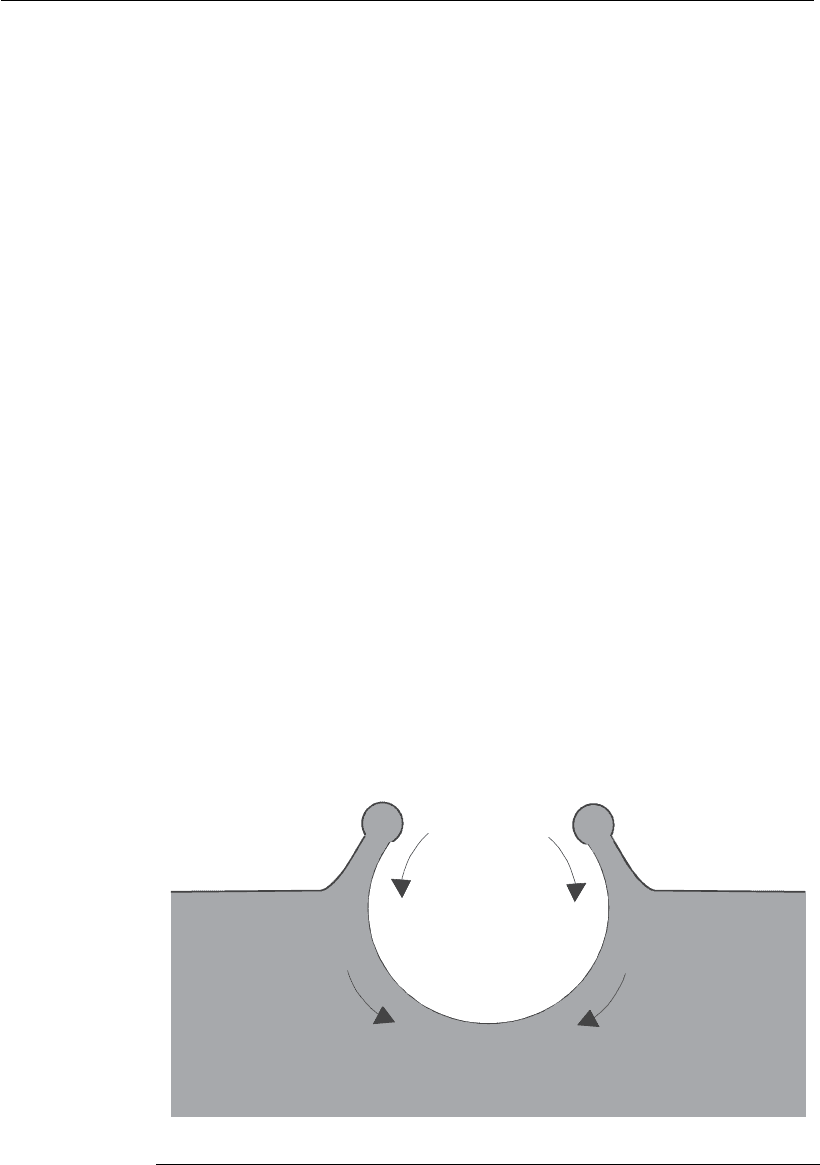
liquid below the bubble. Figure 9.18 shows that the presence of a bubble
creates a cavity in the liquid and, at the moment of bubble bursting, this
cavity is quickly filled with liquid, generating high velocity gradients
within short distances, resulting in shear stress levels that are sufficiently
high to destroy the cells found in this region (Meier et al., 1999).
In general, to minimize damage to the cells, very small bubbles should
be avoided in animal cell cultures, since the interfacial area of the bubbles
Cavity created by
the bubble
Retroceding
film
Liquid
Flow below the
bubble cavity
Air
Interface
Figure 9.18
Dynamics of bubble explosion (adapted from Wu and Goosen, 1995).
248 Animal Cell Technology

is intrinsically related to the kinetics of cell death in bioreactors (Tramper
et al., 1988; Wu and Goosen, 1995). Small bubbles provide a greater total
interfacial area than large bubbles. Therefore, small bubbles are able to
carry more cells to the top gas–liquid interface, where cell damage occurs
due to bubble explosion. On the other hand, small bubbles can transfer
oxygen more efficiently, and this allows the use of much lower aeration
rates. Thus, all these factors should be evaluated when determining the
most adequate operational conditions.
The addition of surfactants allows a modification of the kinetics of non-
specific cell adhesion to bubbles. When substances such as methyl cellu-
lose or Pluronic
1
F68 are added to the culture, the time needed for cell
adhesion to occur is increased (Meier et al., 1999). In this way, the number
of cells adhered to a bubble at the moment of its explosion is lower by
several orders of magnitude. The non-ionic surfactant Pluronic
1
F68 is so
far the best option, since it efficiently protects cells from bubble damage
without significantly affecting oxygen transfer. However, its presence may
be undesirable for certain stages of protein purification.
Foam formation is another problem that occurs in bubble-aerated
bioreactors, especially in the presence of serum-containing culture media
or at high protein concentrations (Butler, 2004). An uncontrolled accumu-
lation of foam in the upper part of the equipment can occur, resulting
eventually in severe problems due to blockage of exhaust gas filters. As a
consequence, gas transfer through the surface is seriously affected. To
decrease the negative effects of foam, different approaches can be adopted:
(a) chemical antifoams; (b) foam traps; (c) bubble-free aeration; or (d) low
aeration rates using pure oxygen.
The use of silicon-based antifoams is common in industry (van Bonarius
et al., 1993). However, they should be used with care, since these
substances can be toxic to the cell above certain concentrations. Further-
more, chemical antifoams can pose problems for the chromatographic
purification of the product. Foam traps, which are devices mounted in the
upper part of bioreactors to break the foam, have been used successfully at
small and intermediate scales, but are not widely used on a large scale. On
the other hand, low aeration rates using pure oxygen effectively lead to a
significant decrease or even complete elimination of foam, but may result
in CO
2
accumulation in the medium, which is harmful to the cells (Gray
et al., 1996).
Both aeration and ventilation are enhanced by mechanical agitation of
the cell suspension. However, agitation also has other functions in a
bioreactor: to maintain cells in suspension (in the case of homogeneous
bioreactors or suspended microcarriers), as well as to homogenize the
fluid. This homogenization is important to avoid the appearance of dead
zones and of nutrient, metabolite, and temperature gradients, as well as to
promote the transfer of heat and of the different chemical species, includ-
ing oxygen, in order not to limit the performance of the biological system.
When stirred-tank bioreactors started to be used for animal cell
cultivation, many problems related to deleterious effects of agitation on
cell viability were observed. However, it was noted that the use of large
impellers rotating at low speeds could minimize mechanical damage to
the cells. The most widely used impeller types are marine (Chisti, 1993)
Bioreac tors for animal cells 249

and inclined-blade impellers (Michaels et al., 1996). On a small scale,
stirrers with oscillating membranes (Lehmann et al., 1987) have also
been proposed. However, these and other similar systems have limited
efficiency and are difficult to scale-up. Currently, marine propellers
continue to be the most widely used impeller type, but also radial-flow
impellers, such as turbines, or combinations of different types are
utilized (Krahe, 2003).
The flow pattern in a stirred bioreactor with baffles is extremely
complex and, generally, turbulent under normal operational conditions
(Brucato et al., 1998). Higher shear rates and, consequently, higher energy
dissipation rates occur near smaller turbulent eddies, that rotate at higher
velocities. The smallest size an eddy can reach under given flow conditions
is known as microscale of turbulence and can be estimated through
Kolmogorov’s equation (Joshi et al., 1996):
º ¼
3
0:25
(18)
Where:
º ¼ Kolmogorov eddy length (microscale of turbulence);
¼ kinematic viscosity;
¼ energy dissipation per unit mass.
Several authors have proposed that, when the microscale of turbulence
is of the same order or smaller than the cell diameter, the energy dissipa-
tion due to the eddy action on the cell surface can destroy the cells (Kunas
and Papoutsakis, 1990). In the case of larger eddies, cells can be accom-
modated in their interior, moving at the same velocity as the eddies. Thus,
no velocity gradients are formed that could damage the cells. However,
other authors state that adopting Kolmogorov’s theory to explain cell
damage is inadequate, mainly due to the evidence that the dominating
deleterious effects on cells are due to bubble explosion at the gas–liquid
interface (Kioukia et al., 1992). Furthermore, it has been demonstrated
that in the absence of a gas phase inside the bioreactor, cells are more
resistant to high rotation speeds than when bubbles are present (Kunas
and Papoutsakis, 1990).
9.6 Scale-up
To meet the demand for a product in the market, two approaches are
possible; process intensification by increasing process productivity, or
increasing the production scale. The latter approach may be achieved by
increasing the number of bioreactors of a given size or by using bioreac-
tors of larger volume. From the economic viewpoint, generally the second
option is more adequate, since equipment costs usually increase with scale
to the power of 0.6 (Rouf et al., 2000). Beyond that, operation and
maintenance costs increase with the number of pieces of equipment,
indicating that for scale-up an increase in the bioreactor volume is more
advantageous than using several smaller units.
The aim of scaling-up is to reproduce in production scale the conditions
optimized at laboratory and/or pilot scale. Most of the general principles
250 Animal Cell Technology

adopted for the scale-up of microbial fermentation processes are applicable
to animal cell culture. Usually the factors that can influence volumetric
productivity are investigated when changing the scale in order to identify
those that are most critical. Once they are identified, similar criteria for
these factors are established for the different scales. The most commonly
used criteria concern the peripheral rotation velocity of the impeller and
the gas flow rate, in the case of bubble-aeration processes.
Before scaling-up a process established at a small scale, it is important
that it is appropriately characterized and optimized. First, a cell line with
desirable production, growth, and genetic stability should be selected. This
selection is usually carried out in multiwell plates and stationary flasks
(Castillo et al., 2005). An adaptation to suspension growth is usually
desirable. Then, the master and working cell banks are prepared, to
cryopreserve the selected cell line. Subsequently, process development is
initiated. Studies on the behavior of the cell line are carried out in
laboratory bioreactors that should have the same configuration as those to
be used at a production scale. Under these conditions, the kinetic charac-
teristics, production pattern, oxygen requirements, and shear resistance of
the cell line are determined (Vı´ctores, 2005). After that, a mode of
operation should be chosen and the operational variables that maximize
volumetric productivity should be determined. Finally, studies aimed at
process scale-up should then be carried out.
The stirred-tank bioreactor is an example of a reactor that is scaled-up
by direct increase of its volume. One of the critical parameters that should
be evaluated at different scales is the rotation speed of the impeller.
Different criteria can be employed:
(i) to maintain constant the power per fluid volume dissipated by the
impeller;
(ii) to keep constant the peripheral velocity of the impeller;
(iii) to maintain constant the volumetric oxygen transfer coefficient (k
L
a).
Each of these criteria will give different results, so that they should be
carefully evaluated, preferably from experimental results. For the aeration
parameters, it is important to evaluate which is the most relevant phenom-
enon; the capacity of bubbles for oxygen transfer or their negative effect
on the cells (Chisti, 2000). At very large scales, the ventilation is also a
very important issue, mainly if low aeration rates with pure oxygen are
used (Gray et al., 1996).
Since in animal cell culture processes the effects of mechanical stress are
much more relevant than in microbial fermentations (Chisti, 1993), it is
quite common to adopt scale-up criteria that are associated with cell
damage (Joshi et al., 1996), such as constant peripheral impeller velocity,
constant aeration rate, and constant integrated shear stress (Croughan et
al., 1987).
When bioreactors coupled to cell retention devices are used, it is also
necessary to evaluate the scale-up of the cell separation equipment. In the
case of the spin-filter (see Chapter 11), parameters such as filter rotation
velocity and the ratio of filtration area to bioreactor working volume are
particularly relevant (Deo et al., 1996).
Bioreac tors for animal cells 251
