Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.

formation. The combination of a low incorporation of acetyl-CoA into
the cycle and the outflow of citrate depletes the metabolites of the TCA
cycle, which need to be replenished to satisfy requirements for energy
generation, since the ATP production by glycolysis is low. Replenishment
may occur at the level of Æ-ketoglutarate. The primary role of glutamine is
to supply this intermediate to keep the operation of the cycle. In this way,
glutamine is a major source of carbon and energy. Other amino acids and
lipids also participate in the supply of intermediates to the TCA cycle
through anaplerotic reactions (Figure 4.2).
In most media formulations for mammalian cell lines, glucose is in-
cluded at an initial concentration of 10–25 mM, which decreases to around
half the concentration level within the period of a batch culture. The
production of lactic acid may lower the pH of the culture and this may
decrease the cellular growth rate. However, if the culture is appropriately
buffered then the accumulated lactate levels typically found in a batch
culture should not cause any growth inhibition (Hassell et al., 1991).
As glucose is metabolized at a far greater rate than it is needed to
maintain viability, it is better to supplement it during the culture than to
Phosphoenolpyruvate
PYRUVATE
KINASE
PyruvateLactate
LACTATE DEHYDROGENASE
ALANINE AMINO
TRANSFERASE
Alanine
Oxalacetate
Malate
Citrate
PYRUVATE
CARBOXYLASE
MALIC
ENZYME II
PYRUVATE
DEHYDROGENASE
PHOSPHOENOL-
PYRUVATE
CARBOXYKINASE
GLUTAMATE
HYDROGENASE
GLUTAMINASE
TCA
CYTOSOL
MITOCHONDRIA
Acetyl CoA
α-ketoglutarate
Glutamate
Glutamane
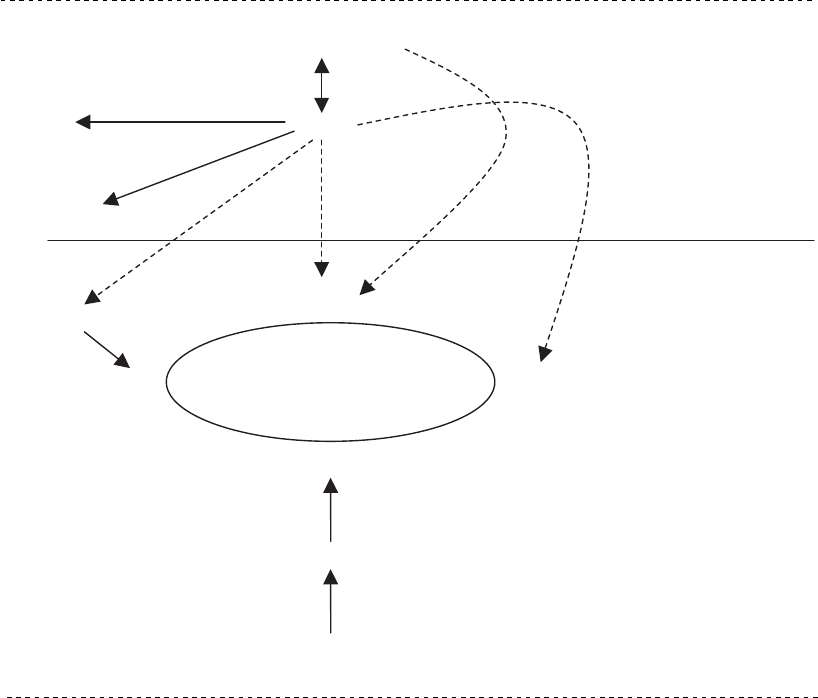
Figure 4.3
Enzymes that link glycolysis and the tricarboxylic acid (TCA) cycle. Dashed arrows indicate reduced
or suppressed enzymatic activity.
82 Animal Cell Technology

increase the initial concentration. This will limit the accumulation of
lactate in the culture. Nevertheless, the cell concentration and the protein
productivity may be affected by glucose concentration. Meijer and van
Dijken (1995) have reported the effects of the glucose supply on growth
and metabolism of an SP2/0 derived recombinant myeloma cell line in a
chemostat culture with Iscove’s Modified Dulbecco’s Medium (IMDM)
medium. Lowering glucose concentration of the feed medium from 25.0 to
1.4 mM resulted in a decrease of steady-state viable cell concentration,
whereas viability remained above 90%. Sun and Zhang (2001a) have
reported the effects of the glucose concentration on batch cultures of
recombinant CHO (Chinese hamster ovary) cells. They demonstrated that
the accumulated concentration of erythropoietin (EPO) increased with the
increase of glucose concentration from 8.9 to 17.9 mM, and decreased with
its further increase from 17.9 to 49.6 mM. They concluded that there is an
optimal glucose concentration for the enhancement of EPO expression by
these recombinant CHO cells. They also demonstrated that the yield
coefficient of lactate from glucose increased with the initial glucose
concentrations from 8.9 up to 17.9 mM, and then kept constant above
17.9 mM.
An alternative is to substitute glucose by other hexoses, like galactose or
fructose, or by disaccharides, like maltose. Galactose is converted into
galactose-6-phosphate, a reaction catalyzed by hexokinase, and then it is
transformed into glucose-6-phosphate, an intermediate of glycolysis.
Fructose can also be phosphorylated to fructose-6-phosphate by hexoki-
nase. Fructose can also enter the glycolysis pathway at the level of
glyceraldehyde-3-phosphate, via the fructose-1-phosphate pathway. As
maltose is a disaccharide of glucose, it is slowly hydrolyzed into glucose
molecules and then transported into the cell and metabolized. The meta-
bolism of alternative carbohydrate sources provides different amounts of
energy. The flux through glycolysis may be slower due to a low affinity of
the hexokinase enzyme for galactose and fructose or due to the slow
hydrolysis of maltose. Thus, substitution of glucose with fructose, galac-
tose, or maltose may decrease the rate of production of lactic acid (Butler
and Christie, 2004).
The reduced glycolytic rate and formation of lactic acid may result in a
slower growth rate (Griffiths, 2000). Duval et al. (1992) cultured a mouse
hybridoma cell line (VO 208) in batch/fed-batch cultures in a medium
supplemented with fructose instead of glucose and demonstrated an in-
crease of the lifespan of the culture and an enhancement in the antibody
secretion. However, it has been reported that complete glucose substitution
by other hexoses can alter product glycosylation (Paredes et al., 1999).
Another strategy to improve the efficiency of central carbon metabolism
and to reduce lactate accumulation is metabolic engineering. Increasing
the flux of glucose into the TCA cycle can improve the efficiency of
glucose for energy metabolism by enhanced formation of ATP. Weide-
mann et al. (1994) have reported that the introduction of a cytosolic
pyruvate carboxylase derived from Saccharomyces cerevisiae into BHK-21
cells enabled cells to transfer glycolysis-derived pyruvate into malate,
which then entered the TCA cycle for complete oxidation. As a result,
higher yields of recombinant EPO were achieved by the BHK cells.
Cell metabolism and its control in culture 83

4.2.2 Glutamine
Glutamine is the major source of energy, carbon, and nitrogen for
mammalian cells. It plays two important functions: it acts as an energy
donor and plays a critical role in nitrogen metabolism, acting as a collec-
tion point for amino groups. For example, in the cytosol of hepatocytes,
amino groups from most amino acids are transferred to Æ-ketoglutarate to
form glutamate, which enters the mitochondria and gives up its amino
group to form ammonium. Excess ammonium generated in most tissues
other than the liver is converted to the amide nitrogen of glutamine.
Glutamine and glutamate, or both, are present in higher concentrations
than other amino acids in most tissues or biological fluids.
Glutamine is transported into the cytosol of the cell by means of two
kinds of transporters: Na
þ
-dependent and Na
þ
-independent. Once in the
cytosol, glutamine must be transported into the mitochondria, where it is
metabolized.
The metabolic pathway of glutamine, called glutaminolysis, begins with
an enzymatic reaction catalyzed by glutaminase, which converts glutamine
into glutamate with ammonium removal. Then, glutamate is converted
into Æ-ketoglutarate by the action of the glutamate dehydrogenase, liberat-
ing another ammonium ion (Figure 4.4). This Æ-ketoglutarate enters
directly to the TCA cycle (Figure 4.2).
In culture media for mammalian cells, together with the carbohydrate
source, glutamine is the most abundant source of reduced carbon. Similar
to glucose, glutamine is also consumed by mammalian cells at a high rate,
particularly if the glutamine concentration is higher than the minimal
needs for maintaining cell viability. This leads to the unwanted accumula-
tion of ammonium ions, a toxic byproduct, in addition to an inefficient
use of glutamine.
Glutamine is normally included at a concentration of 1–5 mM, which is
a significantly higher concentration than that of any other amino acids.
Glutamine is an important precursor for the synthesis of purines, pyrimi-
dines, amino sugars, and asparagine. However, glutamine also has an
important role as substrate for the TCA cycle (Butler, 2004).
For some mammalian cells, glutamine is the main source of energy. It
was observed in an antibody-secreting murine hybridoma (CC9C10)
culture that, after 2 days of exponential growth in batch culture in a
medium containing 20 mM glucose and 2 mM glutamine, the glutamine
content of the medium was completely depleted, whereas the glucose
content was reduced to only 60% of the original concentration. Petch and
Butler (1994) demonstrated that glucose is normally metabolized via
GlutamateGlutamine
GLUTAMINASE
NADP
⫹
NH
4
⫹
GLUTAMATE
DEHYDROGENASE
NADPH
H⫹
⫹
HO
2
NH
4
⫹
α-ketoglutarate
Figure 4.4
Initiation of the glutamine metabolism pathway.
84 Animal Cell Technology

glycolysis (. 96%), the PPC (3.6%), and the TCA cycle (0.6%). Gluta-
mine, on the other hand, is partially oxidized via glutaminolysis to alanine
(55%), aspartate (3%), glutamate (4%), lactate (9%), and CO
2
(22%)
(Petch and Butler, 1994). Fitzpatrick et al. (1993) demonstrated that, in a
murine B-lymphocyte hybridoma (PQXB1/2) batch culture, glutamine
was completely depleted, but glucose was partially depleted to only 50%
of its original concentration when the cells reached a stationary phase
following exponential growth. This suggests that glutamine is the major
contributor of cellular energy. Vriezen et al. (1997) cultured two different
cell lines (MN12, a mouse–mouse hybridoma, and SP2/0-Ag14, a mouse
myeloma) in steady-state chemostat cultures fed with IMDM medium
with 5% serum and glutamine concentrations ranging from 0.5 to 4 mM.
The culture profiles showed that glutamine was the growth-limiting
substrate in this concentration range. Furthermore, they showed that
excess glutamine gives rise to high consumption rates: in glutamine-limited
cultures, the specific rates of ammonia and alanine production were low
compared with glutamine-rich cultures containing 4 mM glutamine in the
feed medium.
The preferential utilization of glutamine as an energy source is cell-
dependent. Maranga and Goochee (2006) have demonstrated that
PER.C6
TM
cells fall into a minor category of mammalian cell lines for
which glutamine plays a minor role in energy metabolism.
Although glutamine is used as a major substrate for the growth of
mammalian cells in culture, it suffers from some disadvantages. The meta-
bolic deamination of glutamine leads to ammonia, which accumulates in
the culture up to 2–4 mM and can be inhibitory to cell growth. The
problem of ammonia accumulation is made worse by the fact that
glutamine may decompose spontaneously to produce ammonia in the
culture medium at a rate of 0.1 mM per day at 378C. The extent of growth
inhibition by ammonia is greater at higher pH levels and is cell line-
dependent.
The accumulation of ammonia from glutamine can be decreased by a
continuous feed of a low concentration of glutamine into the culture.
Ljunggren and Ha
¨
ggstro
¨
m (1992) cultured the murine myeloma cell line
Sp2/0-Ag 14 in an ordinary batch culture and in a glutamine-limited fed-
batch culture. In batch culture, the overflow metabolism of glutamine led
to excess production of ammonium and the amino acids alanine, proline,
ornithine, asparagine, glutamate, serine, and glycine. In the fed-batch
culture, glutamine limitation halved the cellular ammonium production
and reduced the ratio of ammonium/glutamine. The excess production of
alanine, proline, and ornithine was reduced by a factor of 2–6, while
asparagine was not produced at all. They demonstrated that essential
amino acids were used more efficiently in the fed-batch culture as judged
by the increase in the cellular yield coefficients in the range of 1.3–2.6
times for 7 of the 11 amino acids consumed. Altogether, this leads to a
more efficient use of the energy sources glucose and glutamine, as revealed
by an increase in the cellular yield coefficient for glucose by 70% and for
glutamine by 61%, as well as a reduction in the generation of ammonium.
Maranga and Goochee (2006) have reported that, when PER.C6
TM
cells
were cultured in suspension in a 2-liter serum-free bioreactor at a con-
Cell metabolism and its control in culture 85

trolled glutamine concentration of 0.25 mM, the consumption rate of
glutamine and the production rate of ammonium were reduced by
approximately 30%, with respect to the same culture with a higher
glutamine concentration. Furthermore, the consumption rate of alanine
and the consumption rate of non-essential amino acids were reduced by
85% and 50%, respectively. The fed-batch control of glutamine also
reduced the overall accumulation of ammonium ion by approximately
50% by minimizing the spontaneous thermal degradation of glutamine.
In order to reduce the ammonium production, substitution of glutamine
by an alternative substrate such as glutamate may be possible. However, if
glutamate is used, a period of adaptation is required during which the
activity of glutamine synthetase and the rate of transport of glutamate
both increase, since glutamate has a low efficiency transport system. The
cell yield increases when ammonia accumulation is decreased following
culture supplementation with glutamate rather than glutamine. However,
some cell lines fail to adapt to growth in glutamate (McDermott and
Butler, 1993). The cell line HL-60 was adapted to growth in glutamine-
deficient medium by stepwise glutamine deprivation. The successful
adaptation of different cell lines to non-ammoniagenic medium has been
described by Hassell and Butler (1990). They replaced glutamine by either
glutamate or Æ-ketoglutarate. A mole to mole substitution of glutamine by
glutamate was successful for a McCoy cell line and led to normal growth
rates after approximately 10 days. Cell yield was increased by 17%,
ammonia accumulation was reduced by 70%, and glucose consumption
and lactate production both decreased by more than 70%. A BHK and a
Vero cell line had to be slowly adapted from an initially high glutamate
concentration. The MDCK cell line could not be adapted to growth on
glutamate. The authors proposed that the glutamate uptake, and not the
glutamine synthetase activity, is responsible for the ability of a given cell
line to grow in a glutamine-free medium (McDermott and Butler, 1993).
An alternative to cell adaptation is metabolic engineering. Cells trans-
formed with the glutamine synthetase gene can grow in media supplemen-
ted with glutamate instead of glutamine, with a direct elimination/
reduction of ammonia generation, either by glutamine decomposition or
metabolism (Paredes et al., 1999). Bell et al. (1992, 1995) successfully
obtained a murine hybridoma cell line that could grow in the complete
absence of glutamine by transformation with the glutamine synthetase
gene. Ammonia concentrations in the medium of batch cultures of these
cells were below detection levels. This cell line could not be adapted to
glutamine-free growth even in the presence of elevated levels of glutamate,
so metabolic engineering was the unique alternative.
Another strategy for the reduction of ammonia accumulation can be the
use of glutamine-containing dipeptides, which hydrolyze slowly in the
culture (Butler and Christie, 2004). The supplementation of a glutamine-
free medium with dipeptides containing glutamine allows a reduction in
the rate of ammonium generation, but this requires the presence of
dipeptidases, which may be produced by the cells and may be released into
the medium. Christie and Butler (1994) grew a murine hybridoma
(CC9C10) in media containing the dipeptides alanyl-glutamine (Ala-Gln)
or glycyl-glutamine (Gly-Gln) as a substitute for glutamine. They
86 Animal Cell Technology

obtained high cell yields in the presence of 6 mM Ala-Gln or 20 mM
Gly-Gln, with the final cell yield in Gly-Gln 14% higher than in Gln. The
higher concentration of Gly-Gln was necessary for cell growth because of
the presence of a peptidase (in the cytosolic fraction of the cells) with a
lower affinity for Gly-Gln. Monoclonal antibody productivity was com-
parable in Gln, Ala-Gln, or Gly-Gln. Substrate utilization and metabolism
was affected by the presence of the dipeptides, particularly with Gly-Gln.
The specific consumption rates of glucose and six amino acids were
reduced and the accumulation of ammonia and lactate was significantly
lower.
The replacement of glutamine (2 mM) by pyruvate (10 mM) supported
cell growth of several adherent cell lines (MDCK, BHK-21, CHO-K1) in
serum-containing and serum-free media, without adaptation for at least 19
passages and with no reduction in growth rate. Even at very low levels of
pyruvate (1 mM), MDCK cells grew to confluence without glutamine or
accumulation of ammonia. Also glucose uptake was reduced, which
resulted in lower lactate production. Amino acid profiles from the cell
growth phase for pyruvate medium showed a reduced uptake of serine,
cystine, and methionine, an increased uptake of leucine and isoleucine, and
a higher release of glycine compared with glutamine medium (Genzel
et al., 2005).
4.2.3 Amino acids
Amino acids are a class of biomolecules that make a significant contribu-
tion to the generation of metabolic energy. The fraction of metabolic
energy obtained from amino acids varies greatly with the type of cell and
with metabolic conditions.
The transport of amino acids into mammalian cells can be regulated by
nutritional, hormonal, or other environmental factors or by changes with-
in cells like transformation. The intracellular or extracellular concentration
of amino acids probably has the most profound influence on the efficiency
or capacity of transport into animal cells.
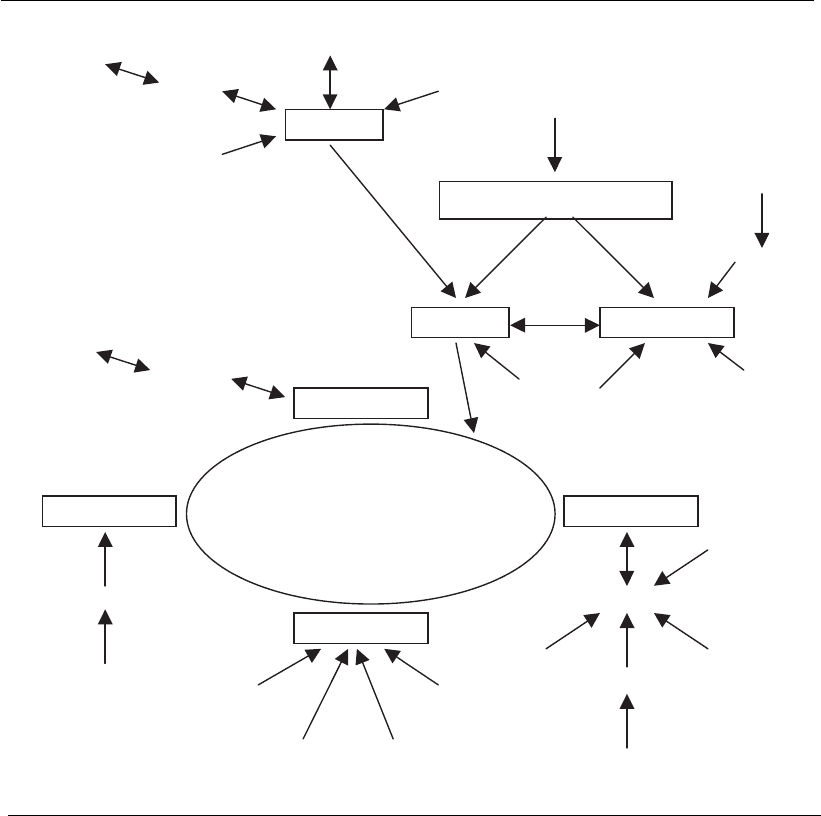
The TCA cycle functions as a major route for the synthesis and
oxidation of most amino acids and is the primary route through which
amino acid carbon flows in the synthesis of other small molecules (Figure
4.5).
Amino acids are a vital constituent of all cell culture media. They are
normally added as defined components to cell culture medium. The
importance of amino acids in synthetic media for in vitro growth of
mammalian cells has long been recognized, as both a nitrogen donor and a
carbon source. Studies on the rates of amino acid uptake have shown
generally that glutamine is the most rapidly consumed, followed by lysine,
leucine, and isoleucine (Roberts et al., 1976). The nutritional requirement
for a certain metabolite, however, may also be influenced by the cell
population density. For example, serine, cystine, glutamine, and asparagine
have been shown to be required at low, but not at high, cell densities
(Eagle and Piez, 1962). This occurs in situations when the metabolite is
utilized in amounts that exceed the biosynthetic capacity of the cell. The
critical population density occurs at the minimum effective intracellular
Cell metabolism and its control in culture 87
level, before the cells die of the specific deficiency. At high cell densities,
however, the cell culture medium may require supplementation with extra
amino acids, to prevent their depletion (Doyle and Griffiths, 1998). The
uptake of leucine, isoleucine, and methionine is closely related to the cell’s
position in the cell cycle and is regulated by growth factors (such as
insulin) in mammalian cells (Doverskog et al., 1997).
Higher eukaryotic cells have lost the ability to synthesize a number of
amino acids. These amino acids are generally called essential amino acids,
while those that can be synthesized are called non-essential. However, this
nomenclature is very misleading for two reasons. First, some of the non-
essential amino acids are in fact very essential in that they are required for
synthesis of nucleotides (glycine, aspartate, and glutamine). The reason
why the ability to synthesize these amino acids has been retained may well
be that they are indispensable. Secondly, the capability to synthesize them
Pyruvate
Glycine
Serine
Alanine
Cystine
Threonine
Lysine
Acetyl CoA Acetoacetate
3-hydroxy 3-methylglutaryl-CoA-
Oxalocetate
Fumarate
Succinyl CoA
TCA
Leucine
Phenylalanine
Tyrosine
Tryptophan
Asparagine
Aspartate
Tyrosine
Phenylalanine
Valine
Methionine Isoleucine
Threonine
α-ketoglutarate
Proline
Histidine
Glutamate
Glutamine
Ornithine
Arginine
Figure 4.5
Points at which amino acids enter or exit the tricarboxylic acid (TCA) cycle in mammalian cells.
88 Animal Cell Technology

may be conditional depending on the nutritional situation, the prolifera-
tive status, and cell line-specific properties (Doverskog et al., 1997). The
early work of Eagle (Eagle, 1955) demonstrated a need for 12 amino acids
to support the proliferation of strain L mouse fibroblasts in medium
containing 0.25–2% dialyzed horse serum. Cells would die within 1–
3 days in the absence of any one of the 12 amino acids. Glutamine was
later added to this list (Eagle, 1959), which also includes arginine, cystine,
histidine, isoleucine, leucine, lysine, phenylalanine, methionine, threonine,
tryptophan, tyrosine, and valine.
The rates at which these and other amino acids are utilized or produced
can vary dramatically between cell lines. The relative concentration of
amino acids and serum in the culture medium and other conditions of the
culture environment will also influence the rates of utilization or produc-
tion of specific amino acids.
Amino acids, whose carbon skeleton can be synthesized de novo in
mammalian cells, include serine, glycine, alanine, aspartate, asparagine,
and in principle glutamate and glutamine. The biosynthesis of amino acids
is closely linked to the central intermediary metabolism, occurring directly
from intermediates of glycolysis or TCA, in one or a few steps. The key
enzymes involved are transaminases (Meister, 1955). Although most cells
possess glutamate dehydrogenase, it is doubtful if there is any significant
net synthesis of glutamate through this enzyme in cultured cells. The
allosteric regulation of glutamate dehydrogenase involving up-regulation
by GDP (guanosine 5’-diphosphate) and ADP, and inhibition of enzyme
activity by GTP and ATP indicates that it has a catabolic function, that is,
the enzyme is activated when cells need amino acids for energy production
(Mehler, 1982). Glutamine, proline and ornithine are all synthesized from
glutamate (Doverskog et al., 1997).
The amino acids that can be synthesized by a cell depend upon the
strain-specific profile of biosynthetic enzymes. For example, BHK and
CHO cells are capable of glutamine synthesis (Street et al, 1993; Neer-
mann and Wagner, 1996), while hybridoma and myeloma cells that do not
possess glutamine synthetase are not (Bebbington et al., 1992). Another
example of a strain-specific difference is the ability to synthesize glycine.
Sf9 insect cells and certain CHO cell mutants are reported to be partial
glycine auxotrophs (Appling, 1991; Tremblay et al., 1992; Chasin et al.,
1994). The explanation involves the localization of serine hydroxymethyl-
transferase. This enzyme, which converts serine to glycine and tetrahydro-
folate-bound single-carbon units, is present both in the cytoplasm and
mitochondria. The mitochondrial isoenzyme activity may be absent in
these partial auxotrophs, which are self-supporting in single-carbon units
through the cytoplasm enzyme activity, but they need glycine from the
medium for protein synthesis. In contrast to mammalian cells, insect cells
are much more flexible in their amino acid metabolism (Ferrance et al.,
1993), some cell lines being capable of synthesizing many more amino
acids (Mitsuhashi, 1982), including glutamine, glutamate, and aspartate
simultaneously (O
¨
hman et al., 1996).
The capability of synthesis of a certain amino acid may be conditional,
and depends on the availability of carbon precursors and nitrogen donors.
For example, a glutamine-free medium for mammalian cells may have to
Cell metabolism and its control in culture 89

be supplemented with aspartate and/or asparagine (Bebbington et al.,
1992) to supply intracellular precursors for glutamine biosynthesis and as
a source of aspartate and asparagine, the synthesis of which may become
limited in a glutamine-free medium. Another example of conditional
biosynthesis is the formation of cystine (from methionine, the cystathio-
nine pathway) in Sf9 cells, which appears to be regulated in relation to the
proliferative status of the cells (Doverskog et al., 1997).
It has been recognized that certain cells have a specific requirement for
an amino acid, for example, serine for lymphoblastoid cells (Birch and
Hopkins, 1977). This may be due either to the inability of the cells to make
an amino acid, or because the amino acid is decomposed in the medium.
The concentration of amino acids usually limits the maximum cell concen-
tration attainable, influences cell survival and growth rate, and can affect
the synthesis of certain proteins. A too low concentration of an amino acid
can result in rapid depletion from the medium, and is thus ‘‘limiting,’’
whereas a too high concentration can be inhibitory.
Branched chain amino acids are consumed particularly rapidly by a
number of cell lines including MDCK cells (Butler and Thilly, 1982),
human fibroblasts (Lambert and Pirt, 1975), mouse myeloma cells
(Roberts et al., 1976), and BHK cells (Arathoon and Telling, 1982). Miller
et al. (1989) found that serine and branched amino acids were more
extensively oxidized by hybridoma cells when the specific glutamine
utilization rate was low. The sulfur-containing amino acids, methionine
and cystine, are also rapidly consumed by cells in culture (Lambert and
Pirt, 1975; Butler and Thilly, 1982). It is suggested that a function of
glutamine uptake and glutamate formation is to allow cystine uptake by
glutamate exchange into the culture medium (Bannai and Ishii, 1988).
Certain amino acids often accumulate in the culture medium during
batch growth with surplus glucose and glutamine (Griffiths, 1971; Lanks
and Li, 1988; Duval et al., 1991; Ljunggren and Ha
¨
ggstro
¨
m, 1992). How-
ever, in glutamine-limited fed-batch cultures of myeloma cells the over-
flow of asparagine, ornithine, proline, alanine, and glutamate is
considerably less than in batch cultures. The same situation exists in
hybridoma cells (Ljunggren and Ha
¨
ggstro
¨
m, 1995). These results can be
explained by the involvement of glutamine and glutamine-derived gluta-
mate, both in the nitrogen transfer reactions and in providing carbon
precursors. The results also highlight the close coupling between the
amino acid and energy metabolism (Doverskog et al., 1997).
One of the determinants of cell growth and survival in cultures of Sf9
cells is the transport of amino acids across the plasma membrane. Uptake
of cystine increases as a function of the cystine concentration in the
medium. However, the increased cystine uptake does not lead to an
increase in the final cell concentration or in the growth rate. On the
contrary, there appears to be a negative influence on cell physiology as
more of the energy-yielding substrates, glucose, glutamine, and glutamate
are consumed at the higher cystine concentration. This is another example
of less tight regulation of metabolism in animal cells (Doverskog et al.,
1997).
Alanine, an end product of glutamine metabolism in many mammalian
cells (McKeenhan, 1986), is also formed by Sf9 cells. In a normal batch
90 Animal Cell Technology

culture of Sf9 cells with excess glucose and glutamine, alanine is the only
overflow metabolite that accumulates to significant concentrations. The
use of substrate-limited fed-batch cultures revealed some interesting
features of Sf9 metabolism. During glucose limitation alanine formation
was totally depressed, while instead ammonium formation was triggered.
Glutamine limitation decreased alanine formation somewhat without
provoking ammonium formation, and during simultaneous glucose and
glutamine limitation, very little overflow metabolism occurred. These
results indicate that in Sf9 cells, glucose-derived pyruvate is the carbon
precursor for alanine and that glutamine provides the nitrogen. As in
hybridoma (Ljunggren and Ha
¨
ggstro
¨
m, 1995) and myeloma cells (Ljung-
grenn and Haggstrom 1992), the energy metabolism of Sf9 insect cells
becomes more efficient during substrate limitation (O
¨
hman et al., 1996).
Consequently, quantitative amino acid data are important to optimize
the formulation of cell culture media. The concentration of many amino
acids can be determined and the data used to calculate the rate of
utilization or assimilation of the individual amino acids (Doyle and
Griffiths, 1998).
4.2.4 Lipids
The oxidation of long-chain fatty acids to acetyl-CoA is a central energy-
yielding pathway in many organisms and tissues. In mammalian heart and
liver, for example, it provides as much as 80% of the energetic needs under
all physiological conditions (Lodish et al., 2003).
Lipids have several important functions in animal cells, which include
serving as structural components of membranes and as a stored source of
metabolic fuel (Griner et al., 1993). Eukaryotic cell membranes are
composed of a complex array of proteins, phospholipids, sphingolipids,
and cholesterol. The relative proportions and fatty acid composition of
these components dictate the physical properties of membranes, such as
fluidity, surface potential, microdomain structure, and permeability. This
in turn regulates the localization and activity of membrane-associated
proteins. Assembly of membranes necessitates the coordinate synthesis
and catabolism of phospholipids, sterols, and sphingolipids to create the
unique properties of a given cellular membrane. This must be an extremely
complex process that requires coordination of multiple biosynthetic and
degradative enzymes and lipid transport activities.
Saturated fatty acids or unsaturated fatty acids, such as oleic acid (18:1,
n-9), can be synthesized by normal mammalian cells that posses elongation
and desaturation enzymes (Rosenthal, 1987). However, the polyunsatu-
rated fatty acids of the n-3 and n-6 group, such as linoleic acid (18:2, n-6)
or linolenic acid (18:3, n-3), are essential nutrients for animals because they
are precursors for the synthesis of eicosanoid hormones such as prosta-
glandins (Needleman et al., 1986).
The enzymes of fatty acid oxidation in mammalian cells are located in
the mitochondrial matrix. The fatty acids with chain lengths of 12 or fewer
carbons enter the mitochondria without the help of membrane transpor-
ters. Those with 14 or more carbons, which constitute the majority of fatty
acids obtained in the diet or released from adipose tissue, cannot pass
Cell metabolism and its control in culture 91
