Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.


directly through the mitochondrial membranes: they must first undergo
the three enzymatic reactions of the carnitine shuttle. The first reaction is
catalyzed by a family of isozymes (different isozymes for fatty acids
having short, intermediate, or long carbon chain) present in the outer
mitochondrial membrane, the acyl-CoA synthetases, which promote the
general reaction:
Fatty acid þ CoA þ ATP $ fatty acyl-CoA þ AMP þ PP
Thus, acyl-CoA synthetases catalyze the formation of a thioester
linkage between the fatty acid carboxyl group and the thiol group of
coenzyme A to yield fatty acyl-CoA, coupled to the cleavage of ATP to
form AMP (adenosine monophosphate) and PP (Pyrophosphate). Fatty
acyl-CoA molecules, like acetyl-CoA, are high energy compounds. Fatty
acyl-CoA esters formed at the cytosolic side of the outer mitochondrial
membrane can be transported into the mitochondria and oxidized to
produce ATP, or they can be used in the cytosol to synthesize membrane
lipids. The second reaction of fatty acyl-CoA oxidation is a transesterifica-
tion catalyzed by carnitine acyltransferase I, located in the outer mem-
brane. This enzyme catalyzes the attachment of the fatty acyl-CoA to the
hydroxyl group of carnitine to form fatty acyl-carnitine. The fatty acyl-
carnitine ester then enters the matrix by facilitated diffusion through the
acyl-carnitine/carnitine transporter in the inner mitochondrial membrane.
In the third and final step of the carnitine shuttle, the fatty acyl group is
enzymatically transferred from carnitine to intramitochondrial coenzyme
A by carnitine acyltransferase II. This isoenzyme, located on the inner face
of the inner mitochondrial membrane, regenerates fatty acyl-CoA and
releases it, along with free carnitine, into the matrix. Carnitine re-enters
the intermembrane space via the acyl-carnitine/carnitine transporter. The
carnitine-mediated entry process is the rate-limiting step for oxidation of
fatty acids in mitochondria and is a regulation point.
Mitochondrial oxidation of fatty acids takes place in three stages. In the
first stage (-oxidation), fatty acids undergo oxidative removal of succes-
sive two-carbon units in the form of acetyl-CoA, starting from the
carboxyl end of the fatty acyl chain. The fatty acid undergoes a number of
metabolic cycles sufficient for the total conversion to acetyl-CoA. In the
second stage, the acetyl groups are oxidized to CO
2
in the TCA cycle,
which also takes place in the mitochondrial matrix. The third stage consists
of oxidative phosphorylation, where the reduced electron carriers NADH
and FADH
2
donate electrons to O
2
with the concomitant phosphoryla-
tion of ADP to ATP. The energy released by fatty acid oxidation is thus
conserved as ATP.
Although the role of lipids in cell culture has been rather neglected, it is
recognized that lipids are important for cell proliferation in serum-free
media. In this respect the importance of serum albumin has been recog-
nized as a carrier of supplemented fatty acids and lipids. Fatty acids are
often included in serum-free media to replace the growth-promoting
properties of the lipid components of serum. However, the effect of
selective fatty acids on cell growth is variable: they can stimulate (Jager et
al., 1988; Rose and Connolly, 1990; Grammatikos et al., 1994), inhibit
92 Animal Cell Technology

(Calder et al., 1991), or have no effect on cell growth (Bailey and Dunbar,
1973; Spector et al., 1981; Cornwell and Morisaki, 1984).
The regulatory mechanism of cellular uptake of fatty acids appears to be
limited and so the composition of the intracellular lipids is likely to reflect
the availability of the fatty acids in the medium. This was shown for the
CC9C10 hybridoma (Butler et al., 1997) and for BHK and CHO cells
(Schmid et al., 1991). Thus, cells growing in serum-supplemented cultures
are likely to attain a fatty acid composition reflecting that of serum, in
which the predominant fatty acids are palmitic, stearic, oleic, and linoleic
acids at a ratio 2:1:3:1, respectively.
Over 90% of the fatty acid composition of the cells could be accounted
for by linoleic, palmitic, oleic, stearic, and arachidonic acids. Minimal
quantities of other fatty acids (C8:0, C10:0, C12:0, C14:0, C18:3) were also
determined but were less than 10 mol % in control cells and were de-
creased to less than 5 mol % after one passage of growth in either linoleic
or oleic acid (Butler et al., 1997).
Linoleic acid has been shown to enhance the proliferation of mouse
mammary epithelial cells by metabolism to arachidonic acid, which is a
precursor of prostaglandin E
2
(Bandyopadhyay et al., 1987). However, the
mechanism of growth promotion of the unsaturated fatty acids in culture
may be related to their importance in the synthesis of cellular membranes
(Rintoul et al., 1978; Rockwell et al., 1980), which may have a significant
effect on membrane fluidity (Calder et al., 1994).
Unsaturated fatty acids (linoleic or oleic acid) have been shown to be
essential for hybridomas in serum-free cultures, as they significantly
enhance the cell yield and monoclonal antibody (mAb) productivity
(Butler and Huzel, 1995; Butler et al., 1997). When Butler et al. (1999)
cultured the murine hybridoma CC9C10 in spinner flasks at high shear
rates to determine the effects of fatty acids, they obtained up to threefold
enhanced cell yields in 25 M linoleic acid or 50 M oleic acid compared
with fatty acid-free control cultures. The half-lives of viable cells were
2.38 and 3.63 h, respectively, for cultures containing 25 and 50 M linoleic
acid, compared with the control culture half-life of 1.97 h (Butler et al.,
1997). At a higher concentration (over 75 M), they found that cell yields
fall below the level of the control cultures. Hexanoic, lauric, margaric, and
stearic acids had no effects on cell growth over the concentration range
they tested. Arachidonic, linolenic, octanoic, and myristic acids caused a
concentration-dependent inhibition of cell growth. Palmitic and decanoic
acids enhanced cell growth marginally (9%) but significantly at 25 M.
Linoleic acid (25 M) enhanced growth more than oleic acid (25 M), but
an equimolar mixture of oleic and linoleic acid (25 M) stimulated growth
more than either fatty acid alone (Butler et al., 1999). The most likely
mechanism for growth enhancement in hybridoma cells is that the fatty
acids are required as components of phospholipids contained in mem-
branes. The effect of growth enhancement was reversible: when cells that
had been passaged continuously in the presence of fatty acids were re-
introduced into unsupplemented medium, the growth advantage over
control cultures was lost.
The effect of linoleic or oleic acid supplementation on mAbs production
has been studied. There was a significant initial enhancement of antibody
Cell metabolism and its control in culture 93

titers in fatty acid-supplemented cultures but this enhancement effect
gradually decreased to insignificance. Subsequent transfer of the cells to
fatty acid-free cultures resulted in a transitory state in which higher mAb
titers were re-established for cells previously grown in the presence of the
fatty acids. The observed enhancement of mAb titer was significantly
higher in cultures supplemented with linoleic acid or linoleic/oleic acid
mix than in those supplemented with oleic acid alone (Butler et al., 1997).
Partial inhibition of glycolysis by saturated fatty acids has been ob-
served in Ehrlich ascites tumor cells. Palmitate and acetate decreased
glutamate formation from glutamine (the first step in glutaminolysis) in
this cell line, suggesting the possible role of fatty acids as an alternative
energy source (Butler et al., 1999).
Butler et al. (1997) cultivated a murine B-lymphocyte cell line
(CC9C19) and a myeloma (SP2/0) in T-flasks in a serum-free medium
supplemented with linoleic acid. They determined that, in the presence
of linoleic acid, the glutamine consumption rate was significantly lower,
and this correlated with a significant decrease in the specific rate of
production of ammonia. The glucose consumption rate was slightly
lower but the specific rate of production of lactate increased signifi-
cantly. This can be explained by a change in the formation of glycolytic
end-products. The rate of production of alanine was lower in linoleic
acid-grown cells. Alanine is produced from pyruvate by transamination
with glutamate in response to the need for sequestration of excess
metabolic nitrogen. It is likely that the decreased glutamine utilization
in linoleic acid-grown cells would lower the level of intracellular
glutamate. This would in turn decrease the transamination reaction and
cause a greater proportion of pyruvate to be converted to lactate. These
changes were reversible on the removal of the fatty acids (Butler et al.,
1997, 1999).
There may be several mechanisms for these metabolic effects. Unsatu-
rated fatty acids have been shown to directly activate specific enzymes and
to induce DNA synthesis and cytokine release from lymphocytes (Karsten
et al., 1994). The induction of specific protein synthesis may produce the
reduction in glutamine metabolism. The increase in the robustness of the
fatty acid-grown hybridomas in agitated cultures could be explained by a
high incorporation of the available fatty acids into the cellular phospho-
lipids fraction, which is a major structural component of the outer
membrane of the cell (Butler et al., 1999).
Cell-protecting additives, such as Pluronic
1
F-68, are commonly in-
cluded as a media component to reduce cell damage in gas-sparged,
agitated cultures. Despite the value of Pluronic
1
F-68 in protecting cells,
there is a potential problem in that Pluronic
1
may have some cytosolic
effects: it may reduce the yield of the producer cell line in culture and
there is a possibility of complexation or co-purification with a cell
product. The replacement of Pluronic
1
with fatty acids, as well as being
positive for the half-lives and production of cultured cell lines, provides an
alternative method to protect producer cells in agitated cultures (Butler
et al., 1999).
The effect of the unsaturated fatty acids on protein secretion of cultured
cells can be dissociated from growth effects. Recombinant protein produc-
94 Animal Cell Technology

tivity from BHK cells seems to be stimulated by unsaturated fatty acids
independently of cell growth (Schmid et al., 1991).
4.3 Metabolic byproducts
4.3.1 Lactate
Cells in vivo convert a proportion of the pyruvate generated by glycolysis
into lactic acid, in a reaction catalyzed by the lactate dehydrogenase
enzyme. This lactic acid is largely secreted into the blood; some passes into
the liver, where it is reoxidized to pyruvate and either further metabolized
to CO
2
aerobically or converted to glucose. Much lactate is metabolized
to CO
2
by the heart, which is perfused by blood and can continue aerobic
metabolism at times when exercising skeletal muscles secrete lactate. In the
case of mammalian cells in culture, the lactic acid is accumulated in the
medium. Lactate generation requires the use of pH control methods to
avoid the direct negative effects of medium acidification on cell growth.
Generally, lactate concentrations below 20 mM are considered not to
show any negative effects (Table 4.1). There are several actions that can be
performed in a culture process to reduce lactate accumulation, and these
have already been mentioned.
Lao and Toth (1997) have studied the effects of lactate concentration on
the metabolism of a CHO cell line producing a recombinant glycoprotein,
cultivated in batch mode with an initial concentration of sodium lactate of
60 mM. They demonstrated a reduction of glucose and glutamine con-
sumption (decreased by 20%) and ammonia and alanine production (de-
creased by 64% and 70%, respectively), while productivity was not
affected. They showed that cultures with added lactate have no lactate
production and they hypothesized that inhibition of lactate dehydrogenase
was the cause of this decreased lactate production. Inhibition of lactate
dehydrogenase prevents the regeneration of NADH to NAD
þ
coupled
with the pyruvate/lactate conversion, which leads to an accumulation of
NADH. This excess of NADH inhibits glycolysis in the cytosol. In-
creased efficiency of the malate–aspartate shuttle to transport NADH
across mitochondrial membrane would be one of the responses from the
cells to retain the glycolytic rate. The lactate inhibition of glycolysis also
leads to a lower concentration of pyruvate, which may be the cause of a
decrease in glutamine consumption. Due to the diminished energy produc-
tion from lower glycolytic and glutaminolytic rates, with the diversion of
Table 4.1 Influence of different lactate concentrations on cell growth at
constant pH (Wagner, 1997)
Lactate (mM) Growth Productivity Reference
, 20 No effect No effect Wagner et al. 1988
Miller et al. 1988
20–40 No effect Inhibition Glacken et al. 1988
40–60 Slight inhibition Inhibition Glacken et al. 1988
60 Inhibition Inhibition Glacken 1988
Cell metabolism and its control in culture 95

more energy into maintenance of the ion gradient to counter the effects of
a hypertonic environment, growth was inhibited under high concentra-
tions of lactate.
On the other hand, Cruz et al. (2000) reported that 28 mM lactate
reduced the growth of a BHK cell line by 50%, and that lactate was
consumed at concentrations above 30 mM. Increased concentrations of
lactate reduced cell growth and specific ammonia production but increased
specific glutamine and glucose consumption. The effect of lactate was at
least partially due to an increase of osmolarity. An increase in lactate from
0 to 60 mM induced a 40% reduction in specific productivity of a
recombinant fusion protein secreted by the BHK cells in both stationary
and stirred cultures.
The effects of elevated lactic acid concentration on the cell cycle kinetics
of hybridoma cell growth and antibody production in batch culture were
studied by Kromenaker and Srienc (1994). When 33 mM lactic acid was
initially present, the specific growth rate was reduced by 37% and the cell-
specific antibody production rate increased by a factor of 2.6 relative to a
control culture with no additional lactic acid.
4.3.2 Ammonia
Ammonia (NH
3
) and the ammonium ion (NH
4
þ
) are highly toxic to
mammalian cells. In vivo, ammonium is secreted by the cells and trans-
ported to the mitochondria of hepatocytes, where it is converted into urea
via the urea cycle. Urea production occurs almost exclusively in the liver
and is the fate of most of the ammonium channeled there. The urea passes
into the bloodstream and thus to the kidneys and is excreted into the
urine. Mammalian cells in culture secrete ammonium into the culture
medium, where its concentration increases gradually because there is no
ammonium recycling pathway (Newland et al., 1990).
As previously stated, ammonium in cell culture medium is the product
of glutamine metabolism and its spontaneous decomposition at 378C.
Negrotti et al. (1989) showed that the half-life for glutamine at 378C and
pH 7.2 is only 7 days. The effects of ammonium on cell metabolism are
observed from concentrations as low as 2 mM, which is easily reached in
culture systems (Table 4.2). For example, the spontaneous decomposition
of glutamine can result in 0.1 mM ammonia per day (Butler and Spier,
1984). Anchorage-dependent cells grown on microcarriers produce be-
tween 2 and 3 mM ammonia after growth in a batch culture (Butler et al.,
Table 4.2 Effect of different medium concentrations of ammonium on
growth and productivity (Wagner, 1997)
Ammonium (mM) Growth Productivity Reference
, 2 No effect No effect Glacken 1988
2–5 Inhibition No effect Glacken et al. 1988
McQueen and Bailey
1990
Glacken 1988
5 Inhibition Inhibition Glacken 1988
96 Animal Cell Technology

1983; Butler and Spier, 1984), and hybridomas in batch suspension culture
produce between 4.5 and 5.5 mM (Reuveny et al., 1986).
Ammonium can either perturb the intracellular or intra-organelle pH
and electrochemical gradients, or directly interact with enzymes. The toxic
effects can be classified into two possibilities, as described below.
(i) Perturbation of intracellular pH and electrochemical gradients.
At the physiological pH of 7.1–7.5, which is the pH at which most
mammalian cell cultures are maintained, only about 1% of the total
concentration of ammonia/ammonium is present as ammonia (NH
3
),
the rest being ammonium (NH
4
þ
). Ammonia is a small, uncharged,
lipophilic molecule, which readily diffuses across cellular mem-
branes. The small percentage of ammonia present in the extra- and
intracellular aqueous phases will diffuse across the membranes, thus
rapidly equilibrating any transmembrane gradient of ammonia. Am-
monium is produced, in the ionic form, inside the mitochondria
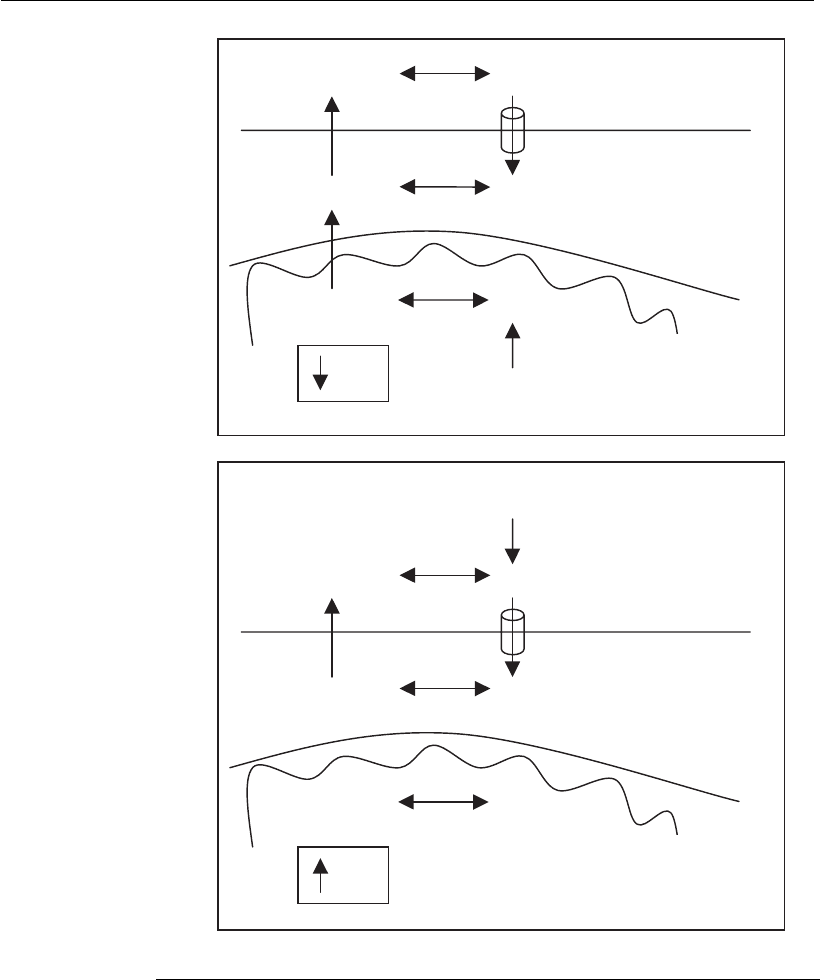
(Figure 4.6A) by the action of glutaminase and glutamate dehydro-
genase. The inner mitochondrial membrane is extremely imperme-
able to ions; however, ammonia readily passes from the
mitochondrial matrix into the cytoplasm. This ammonia outflow
from the mitochondria leads to a decrease of the pH in the matrix,
since a proton is left behind. Ammonia can diffuse out of the
cytoplasm into the environment; however, it can be transported back
as ammonium by carrier proteins. The consequence of such a cycle
is an acidification of the cytoplasm and the mitochondrial matrix,
and an alkalinization of the environment. Ammonia/ammonium
derived from glutamine decomposition or added externally (Figure
4.6B) will transiently increase the pH of the cytoplasm due to rapid
diffusion of ammonia into the cell. This alkalinization is followed by
an acidification due to transport of ammonium by carrier proteins.
Diffusion of ammonia into the mitochondria and other organelles
leads to an increase of the pH inside these compartments. The result
is an alkalinization of the cellular environment and of the inside of
the organelles, mitochondria included, and an acidification of the
cytoplasm. Thus, it is very important to realize that the physiological
consequences of adding extracellular ammonium to the medium are
very different to those resulting from ammonium produced intracel-
lularly.
(ii) Ammonium and enzymatic reactions. Ammonia or ammonium can
participate in enzyme reactions and displace equilibria or interact
with regulatory sites of enzymes. The key enzyme of the glycolytic
pathway, phosphofructokinase, as well as Æ-ketoglutarate dehydro-
genase, an enzyme of the TCA cycle, have been reported to be
activated by ammonium (Uyeda and Racker, 1965; Parmeggiani et al.,
1966). Thus, elevated ammonium concentrations could lead to a high
rate of glycolysis and lactate production and a reduced TCA cycle
activity. Other enzymes affected by ammonium concentration are
those involved in the glycosylation of proteins. Gramatikos et al.
(1998) have reported that the intracellular content of UDP-N-acetyl-
hexosamines (UDP-GNAc) is substantially elevated under high am-
Cell metabolism and its control in culture 97
monium concentrations in the medium. This may lead to an increase
in the antennarity of the N-linked oligosaccharides of glycoproteins,
or a decrease in terminal sialylation (Yang and Butler, 2002) (see
Chapter 6).
A
B
NH H
3
⫹
⫹
NH H
3
⫹
⫹
NH
4
⫹
Glutamine
metabolism
pH
diffusion
CYTOPLASM
EXTRACELLULAR
SPACE
MITOCHONDRIA
diffusion
A
pH
diffusion
CYTOPLASM
MITOCHONDRIA
diffusion
EXTRACELLULAR
SPACE
Glutamine decomposition
Ammonium addition
B
active transport
NH
4
⫹
NH H
3
⫹
⫹
NH H
3
⫹
⫹
NH
4
⫹
NH
4
⫹
active transport
NH H
3
⫹
⫹
NH
4
⫹
NH H
3
⫹
⫹
NH
4
⫹
Figure 4.6
Effects of ammoniu m on mammalian cell cult ures. (A) Effects of ammonium
generated by glutamine metabolism. (B) Effects of ammonium generated by
glutamine decomposition or by ammonium addition in the culture medium.
98 Animal Cell Technology

The toxic effects produced by ammonium are: (i) enzymatic inhibition
(in glycolysis, TCA cycle, glutaminolysis, and PPC); (ii) perturbation of
the transcellular ionic gradient; (iii) intracellular pH modification; (iv)
increase of UDP-GNAc synthesis, with concomitant glycolysis modifica-
tion and affecting the quality of the recombinant protein; and (v) increase
in the alanine secretion.
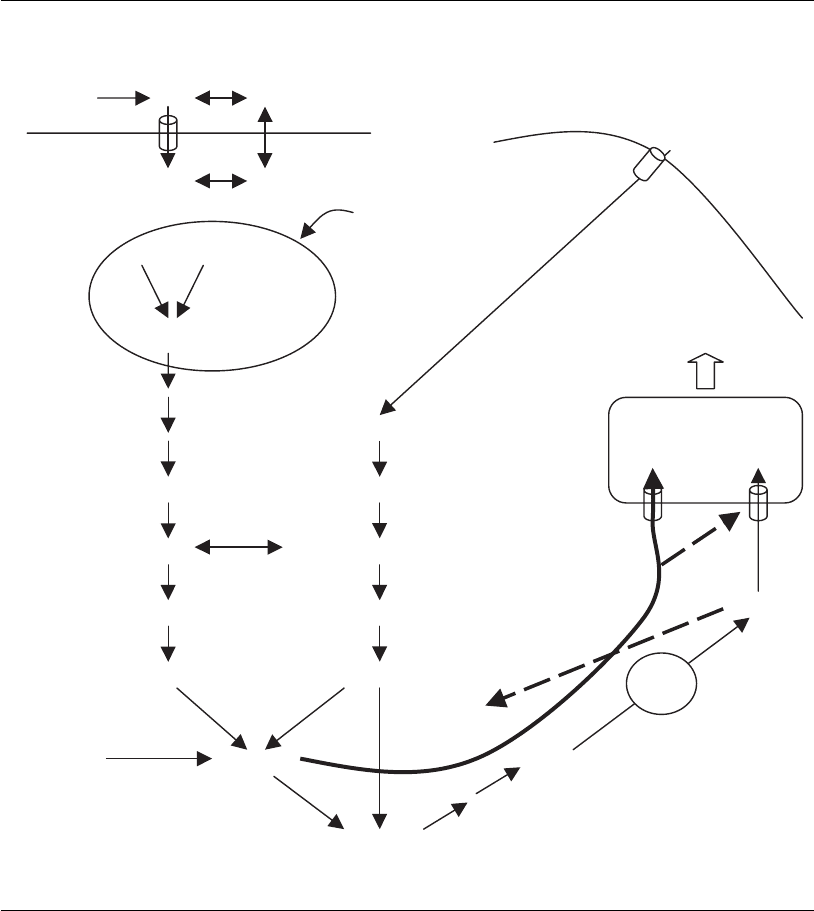
As shown schematically in Figure 4.7, an increase of the UDP-GNAc
concentration in the cytoplasm leads to elevated transport of UDP-GNAc
into the Golgi vesicle, by means of the sugar nucleotide transport system
I. This higher concentration of UDP-GNAc, however, inhibits the sugar
nucleotide transport system II that is responsible for CMP-NANA import
to the Golgi, and a higher CMP-NANA concentration inhibits in turn, via
feedback inhibition, the UDP-N-acetylglucosaminyl epimerase that cata-
lyzes the first step of UDP-NANA synthesis. This block, together with
the inhibition of the transport system, reduces CMP-NANA concentra-
tion, resulting in a decrease of sialylation.
It was reported by Yang and Butler (2002) that ammonia caused an
increase in the antennarity and a decrease in the sialylation of glycans of
recombinant EPO in CHO cells. Gawlitzek et al. (1998) have studied the
final carbohydrate structure of a human interleukin (IL)-2 mutant glyco-
protein, named IL-Mu6. Furthermore, Valley et al. (1999) demonstrated
that about 60–80% of N-acetylated sugars in N-glycan structures con-
tained
15
N, provided by
15
NH
4
Cl used as a supplement in the culture
medium. This indicates that ammonium is used as a building block during
synthesis of the carbohydrate structures expressed in cultivated mamma-
lian cells. Gawlitzek et al. (2000) cultured a BHK-21 recombinant clone in
a continuously perfused double membrane bioreactor. In the presence of
15 mM exogenously added NH
4
Cl, a significant and reproducible increase
in tri- and tetra-antennary oligosaccharides (45% of total) was detected in
the secretion product. They also studied the effect of ammonium on the
glycosylation pattern of the recombinant immunoadhesin tumor necrosis
factor-IgG (TNFR-IgG) produced by CHO cells. They demonstrated that
as ammonium increased from 1 to 15 mM, a concomitant decrease of up to
40% was observed in terminal galactosylation and sialylation of the
molecule. Gawlitzek et al. (1999) cultivated recombinant CHO cells
producing an immunoadhesin glycoprotein (GP1-IgG) under controlled
conditions in the presence of different ammonium N-Glycans synthesized
in the presence of
15
NH
4
Cl revealed an N-glycan-dependent increase in
mass to charge ratio of 2.5–4.8 Da. A 60–70% of the total nitrogen
contained in the monosaccharides was
15
N, which suggested that
15
NH
4
þ
was incorporated into GlcNAc and N-acetylneuraminic acid as proposed
earlier (Ryll et al., 1994). Studies of secreted EPO from CHO recombinant
cells during cultivation in a medium supplemented with NH
4
Cl revealed
that the presence of ammonium caused a significant increase in the
heterogeneity of the glycoforms, as shown by the isoelectric point, which
increased from a range of 4.06–4.67 in the control culture to 4.18–6.05,
when ammonium was added to the culture medium. A shift in molecular
weight, from 33 000–39 000 in the control culture to 27 000–37 000 in the
ammonium-supplemented medium, was further evidence for the increased
heterogeneity. Cell growth was inhibited above a culture concentration of
Cell metabolism and its control in culture 99
Glutamine NH
3
active transport
URIDILATE
KINASE
URIDINE
KINASE
NUCLEOSIDE
DIPHOSPHATE KINASE
CO
2
Carbamoyl
phosphate
CARBAMOYL
PHOSPHATE
SYNTHETASE I
OMP
UMP
UDP
UTP
UDP-GlcNAcUDP-GalNAc
UDP- -ACETYL-D-
GLUCOSAMINE 4-EPIMERASE
N
Urd
M
a
nNA
c6
P
NeuNAc
CMP-NANA
Glc 6-P-
Fruc 6-P-
GlcN 6-P-
GlcNAc 6-P-
GlcNAc 1-P-
MITOCHONDRIA
CYTOSOL
ACETYL GLUCOSAMINE
PHOSPHOMUTASE
GLUCOSE-6-PHOSPHATE
ACETYLASE
GLUCOSAMINE 6-
PHOSPHATE DEAMINASE
PHOSPHOFRUCTO
ISOMERASE
HEXOKINASE
UDP- -ACETYL-
GLUCOSAMINYL
EPIMERASE
N
TRANS-GOLGI
SI S II
NUCLEUS
PROTEIN OF INTEREST
EXTRACELLULAR
SPACE
EXTRACELLULAR SPACE (glutamine decomposition)
OR MITOCHONDRIA (glutamine metabolism)
NH
4
⫹
diffusion
Glucose
NH
3
NH
4
⫹
NH
4
⫹
Figure 4.7
Effects of increased ammonium concentrations on the glycosylation of proteins. OMP, orotidine
monophosphate; UMP, uridine monophosphate; UDP, uridine diphosphate; UTP, uridine
triphosphate; UDP-GlcNAC, UDP-N-acetylglucosamine; UDP-GalNAc, UDP-N-acetylgalactosamine;
ManNAc6P, mannose -N-acetyl-6-phosphate; NeuNAc, N-acetylsiali c acid; CMP-NANA, cytosine
monophosphate-N-acetylneuraminic acid; S I, sugar nucleoside transport system I; S II, sugar
nucleoside transport system II; Glc-6-P, glucose-6-phosphate; Fruc-6-P, fructose-6-phosphate;
GlcN-6-P, glucosamine-6-phosphate; GlcNAc-6-P , N-acetylglucosamine-6-phosphate; GlcNAc-1-P,
N-acetylglucosamine-1-phosphate. The dashed arrows indicate the influence of the increased
ammonium concentration.
100 Animal Cell Technology

5mMNH
4
Cl, whereas the specific production of EPO increased with the
addition of NH
4
Cl above this level. At 10 mM NH
4
Cl, the final cell
density after 4 days in culture was significantly lower but the final yield of
EPO was significantly higher. This appeared to be due to continued
protein production after cell growth had ceased. The metabolic effects of
added NH
4
Cl included higher specific consumption rates of glucose and
glutamine and an increased rate of production of alanine, glycine, and
glutamate (Yang and Butler, 2000).
Sun and Zhang (2001b) showed that an increase of the initial ammonia
concentration resulted in decreased cellular yields with respect to glucose,
glutamine, and other consumed amino acids. In a batch culture with an
initial ammonia concentration of 5.66 mM, the cellular yields from glucose
and glutamine reduced by 78% and 74%, respectively, compared to that
with ammonia at an initial concentration of 0.21 mM. The yields of cells
with respect to other consumed amino acids also decreased by 50–70%.
The metabolic pathways were altered in the cultures with the higher
ammonia concentrations. The glucose consumption was more prone to
form lactate. For glutamine metabolism, the reaction of glutamate to Æ-
ketoglutarate catalyzed by the glutamate dehydrogenase was inhibited by
ammonia, and that by the glutamate amino transferase was facilitated.
However, the yields of glutamate to glutamine decreased with the increase
of ammonia concentrations, showing that the reaction of glutamate to Æ-
ketoglutarate was inhibited by ammonia as a whole. Lao and Toth (1997)
reported a decrease in alanine production. While aspartate consumption
increased sixfold, glutamate changed from consumption to low-level
production and higher consumption again with added ammonium concen-
trations. Cells consumed more aspartate and glutamine at high ammonium
concentrations. There was also a decrease in TCA intermediates, for
example, malate (Lao and Toth, 1997).
Chen et al. (2005) investigated the addition of amino acids to the growth
media for CHO cell cultures as a means of mitigating the negative effects
of ammonium. Threonine, proline, and glycine additions enhanced CHO
cell growth and recombinant protein levels. Furthermore, the addition of
these amino acids positively impacted important metabolic parameters,
including glucose consumption, lactate production, glutamine utilization,
and final ammonium levels. Additionally, threonine, proline, and glycine
increased the level of Æ-2,3-linked sialic acid, galactose--1,4-N-acetylglu-
cosamine, and Æ-2,6-linked sialic acid residues in recombinant tissue
plasminogen activator (tPA). Thus, these amino acids can be used to
mitigate some of the toxic effects of ammonium on cell growth, recombi-
nant protein productivity, and protein quality.
4.4 Factors affecting cell metabolism
There are several factors that have a large influence on metabolic path-
ways. Among the external factors, the most important are the dissolved
oxygen and carbon dioxide concentration, the pH, and the temperature of
the culture. These factors are discussed in Chapter 2, but here their effects
on metabolic pathways are described.
Cell metabolism and its control in culture 101
