Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


0014 The volume of ICF is fixed by the prevailing os-
motic pressure and the amount of solute that the cells
keep within them by metabolic activity. The kidney,
controlling the excretion of water under the influence
of ADH, fixes the osmotic pressure in the body, thus
setting the stage for the cells to maintain their appro-
priate volume of ICF. Fixing the osmotic pressure
throughout the body also determines the amount of
water to go with each unit of sodium in the ECF.
Hence, with osmotic pressure and the volume of ICF
stabilized by controlling the excretion of water, the
kidneys can also regulate the volume of ECF by
excreting or retaining salt.
See also: Body Composition; Hormones: Adrenal
Hormones; Potassium: Physiology; Renal Function and
Disorders: Kidney: Structure and Function; Sodium:
Physiology
Further Reading
Bray JJ, Cragg PA, Macknight ADC, Mills RG and Taylor
DW (1989) Lecture Notes on Human Physiology, 2nd
edn. Oxford: Blackwell Scientific.
Henderson LJ (1913) The Fitness of the Environment. New
York: Macmillan.
Robinson JR (1970) Water and life. World Reviews of
Nutrition and Dietetics 12: 172–207.
Robinson JR (1978) Water in the animal body. In: McIntyre
AK (ed.) Water: Plants, Planets and People, pp. 60–
70.Canberra: Australian Academy of Science.
Robinson JR (1988) Reflections on Renal Function, 2nd
edn. Oxford: Blackwell Scientific.
WATER ACTIVITY
Contents
Principles and Measurement
Effect on Food Stability
Principles and Measurement
Y H Roos, University College Cork, Cork, Ireland
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Water is the main component of most food materials.
The control of water content is often used to increase
food stability, e.g., by dehydration. It is also known
that high concentrations of sugars and salts may
increase food stability by affecting the availability
of water to microbial growth, although the water
content may remain high. It is obvious that the
water contents of different foods vary substantially,
and water content as such cannot be used as a meas-
ure of water availability or food stability. The water
availability for microbial growth is a function of the
vapor pressure of water in a food, which has led to the
development of the water activity concept. It has been
shown that food storage stability and microbial
safety, i.e., rates of chemical and enzymatic reactions
and microbial growth, can be controlled by water
activity in addition to other factors, such as pH and
temperature. Therefore, the development of methods
to control water activity of foods and water activity
measurement has received much attention in the food
industry. Furthermore, reference values for water ac-
tivity are used in legislation and regulations to insure
food safety. This article discusses the basic concepts
of water activity, its definition, temperature depend-
ence, measurement, relationship with water content,
and mathematical approaches to predict water
activity in food systems.
Water Activity
0002The water activity, a
w
, in a solution or a food is
derived from its chemical potential, m
w
. The chemical
potential, m, of a pure substance is given by its molar
Gibbs free energy, G
m
. The Gibbs free energy is pres-
sure-dependent, and it can be shown that the Gibbs
free energy of a perfect gas at a pressure, p, can be
related to its value, G*, at a standard pressure, p*.
Then, the Gibbs free energy will be given by eqn (1).
G ¼ G* þ nRT ln
p
p*
: ð1Þ
Assuming perfect behavior and taking into account
that for water vapor m
w0
¼ G
w
/n, eqn (2), where m
w
*is
WATER ACTIVITY/Principles and Measurement 6089
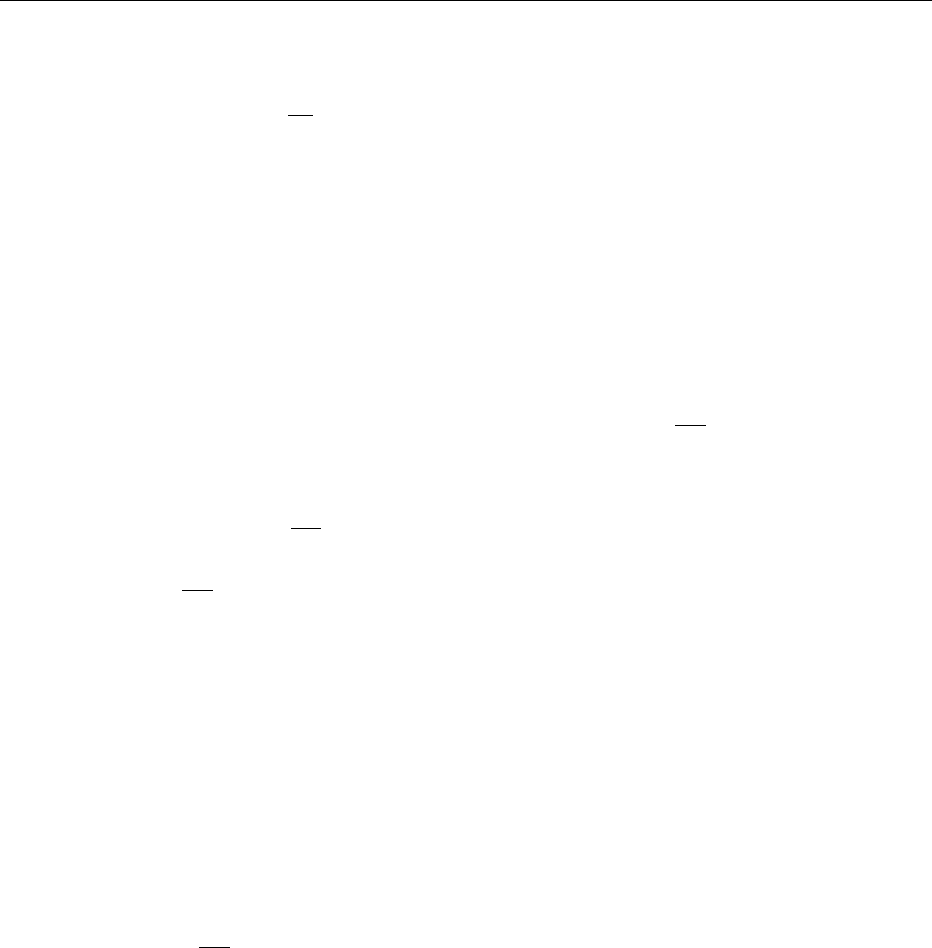
the chemical potential at the standard pressure, p
w
*, is
obtained.
w0
¼
w
* þ RT ln
p
w
p*
w
:ð2Þ
The vapor pressure of pure water can be taken as
p
w0
¼ p
w
/p
w
*. Thus, the chemical potential is ex-
pressed by eqn (3). If water exists in a solution, its
chemical potential will be m
w
and vapor pressure
p
w(liquid)
. At equilibrium, the chemical potential in
the liquid and vapor states is equal, and, therefore,
the chemical potential of water in a solution, m
w
,is
defined by eqn (4). Eqns (3) and (4) can be combined
to give eqn (5) and the chemical potential of liquid
water in the solution. The activity, in the case of
water, the water activity, a
w
, is defined by eqn (6).
w0
¼* þ RT lnp
w0
ð3Þ
w
¼* þ RT lnp
w
ð4Þ
w
¼
w0
þ RT ln
p
w
p
w0
ð5Þ
a
w
¼
p
w
p
w0
:ð6Þ
As defined by the preceding equations, the chemical
potential, vapor pressures, and water activity are all
temperature-dependent. Therefore, the water activity
is a measure of water availability at equilibrium at a
constant temperature, e.g., room temperature.
0003 The relative humidity, RH, of an atmosphere is
defined as the ratio of the quantity of water present
to the saturation quantity at the temperature of the
atmosphere, and it is also the ratio of the vapor
pressure of water present to that of saturated water
vapor at the same temperature, i.e., the relative vapor
pressure (RVP), as given by eqn (7).
RH ¼
p
w
p
w0
100% :ð7Þ
If a solution or a food is placed in an atmosphere, it
will lose or gain water until equilibrium is reached
and the chemical potential and, therefore, the water
activity in the atmosphere and the solution or food
have become equal. This has a great importance in a
w
measurements, because a
w
is often derived from the
equilibrium relative humidity (ERH) of the headspace
of a food system.
Raoult’s Law
000 4 The vapor pressure of ideal solutions is related to the
mole fraction of water, as defined by Raoult’s law.
Raoult’s law is based on the observation that
the partial vapor pressure of each component in a
solution is a function of its mole fraction and the
vapor pressure of a pure liquid at the same tempera-
ture. In the case of water, assuming that the solution
behaves as an ideal solution, Raoult’s law is given by
eqn (8). However, Raoult’s law assumes linearity, it
applies for ideal solutions, and often the vapor pres-
sure deviates from ideal behavior with increasing
solute concentration. For real solutions, an activity
coefficient is defined, and the water activity is related
to the mole fraction of water, x
w
, by eqn (9), where g
w
is the activity coefficient of water. Therefore, water
activity may also be considered as the ‘effective’ mole
fraction of water.
p ¼ x
w
p
w0
ð8Þ
a
w
¼
p
w
p
w0
¼
w
x
w
:ð9Þ
Raoult’s law is important in understanding the
boiling temperature elevation and freezing tempera-
ture depression of solutions. The freezing temperature
depression may also be measured to derive water
activity.
Water Activity Measurement
0005The water activity of simple, ideal solutions can be
derived from Raoult’s law by applying eqn (8) and
real solutions from eqn (9). Unfortunately, food
systems are very complex, the water activity is diffi-
cult to estimate, and it must be measured for most
foods. A simple, but rough, water activity estimation
is based on placing samples in several environments
(chambers) with various relative humidities and
estimating the water activity from a plot showing
weight gain or loss against RH.
0006The water activity of food materials can be meas-
ured using various systems to observe the vapor pres-
sure of water in a food sample or by measuring the
relative humidity of air in equilibrium with a food
sample. The measurement of the vapor pressure of
water can be carried out using a manometer and other
relatively simple laboratory equipment. Simple, com-
mercial instruments may apply hair hygrometry. Such
devices are not very accurate and require that appro-
priate time is allowed for the sample material in a
container to equilibrate with the measuring unit.
Various other commercial hygrometers have been ap-
plied in water activity measurements. For these meas-
urements, an appropriate amount of sample material
often needs to be placed in a closed container or jar at
a constant temperature. The sensor of the instrument
is placed in the headspace, and the relative humidity
at equilibrium is recorded and expressed as water
activity.
6090 WATER ACTIVITY/Principles and Measurement
0007 Several commercial water activity measurement
devices have been developed for rapid and accurate
water activity determinations. These devices use
either a chilled-mirror dew-point technology or meas-
ure relative humidity with sensors that detect electric
resistance or capacitance. The dew-point instruments
provide relatively rapid and accurate water activity
measurements. The main principle of such instru-
ments is based on cooling air at equilibrium water
vapor pressure with a food sample without changing
the water content and determination of the tempera-
ture at which water in the air saturates at a chilled-
mirror surface. The water activity of the sample is
given by the ratio of the saturation vapor pressure at
the dew-point temperature and the saturation vapor
pressure at the sample temperature. Instruments
using relative humidity sensors require equilibration
with the sample at the same temperature in a closed
container or jar, as described earlier for hygrometer
measurements.
Instrument Calibration
0008 Water activity measurements require adequate and
accurate instrument calibration. This is usually
carried out with the help of saturated salt solutions
or other substances with known water vapor pres-
sures at a given temperature. Different instruments
may require different specific calibration procedures,
but most instruments should be checked for correct
water activity readings. Saturated salt solutions are
probably the most common means of producing
atmospheres with known relative humidities or
water vapor pressures. Saturated salt solutions can
be prepared to give a wide range of water activities
in a number of temperatures. Although the solutions
are relatively easy to prepare, accurate, and safe,
appropriate procedures and safety precautions should
be followed. The water activities of common satur-
ated salt solutions are listed in Table 1. Other mater-
ials, which in various concentrations in solutions with
water can be used to adjust water activity, include
glycerol and sulfuric acid (H
2
SO
4
).
Water Sorption
0009It is often beneficial to establish water sorption
isotherms, which show the relationship between
water content and water activity for particular food
systems. Sorption isotherms can be obtained experi-
mentally by using dehydrated, for example freeze-
dried, samples of food solids. The samples should be
initially stored over a ‘zero’ relative humidity in a
hermetic container with CaSO
4
or P
2
O
5
. These
samples can be used to obtain the ‘anhydrous’ weight,
and the samples are then placed in hermetic contain-
ers, preferably evacuated desiccators at a constant
temperature over saturated salt solutions with
known water activities. The samples in each con-
tainer are weighed at intervals until subsequent
weighings level off, and the weight gain can be con-
sidered as equilibrium water content corresponding
tbl0001 Table 1 Water activities of selected saturated salt solutions at various temperatures
Salt Water activityat temperature (
C)
5 10 15 20253035404550
CsF 0.055 0.049 0.043 0.038 0.034 0.030 0.027 0.024 0.022 0.021
LiBr 0.074 0.071 0.069 0.066 0.064 0.062 0.060 0.058 0.057 0.055
ZnBr 0.087 0.085 0.082 0.079 0.078 0.076 0.076 0.075 0.076 0.077
LiCl 0.113 0.113 0.113 0.113 0.113 0.113 0.113 0.112 0.112 0.111
CaBr 0.216 0.202 0.185 0.165
LiI 0.217 0.206 0.196 0.186 0.176 0.166 0.156 0.146 0.135 0.124
CH
3
COOK 0.234 0.234 0.231 0.225 0.216
MgCl
2
0.336 0.335 0.333 0.331 0.328 0.324 0.321 0.316 0.311 0.305
NaI 0.424 0.418 0.409 0.397 0.382 0.362 0.347 0.329 0.310 0.292
K
2
CO
3
0.431 0.431 0.432 0.432 0.432 0.432
MgNO
3
0.589 0.574 0.559 0.544 0.529 0.514 0.499 0.482 0.469 0.454
NaBr 0.635 0.622 0.607 0.591 0.576 0.560 0.546 0.532 0.520 0.509
CoCl
2
0.649 0.618 0.586 0.555 0.526 0.500
KI 0.733 0.721 0.710 0.699 0.689 0.679 0.670 0.661 0.653 0.645
NaNO
2
0.786 0.775 0.765 0.754 0.743 0.731 0.721 0.710 0.700 0.690
NaCl 0.757 0.757 0.756 0.755 0.753 0.751 0.749 0.747 0.745 0.744
KBr 0.851 0.838 0.826 0.817 0.809 0.803 0.798 0.794 0.792 0.790
KCl 0.877 0.868 0.859 0.851 0.843 0.836 0.830 0.823 0.817 0.812
KNO
2
0.963 0.960 0.954 0.946 0.936 0.923 0.908 0.890 0.870 0.848
K
2
SO
4
0.985 0.982 0.979 0.976 0.973 0.970 0.967 0.964 0.961 0.958
WATER ACTIVITY/Principles and Measurement 6091
to the water activity of the particular salt solution.
The equilibration times may vary depending on
the container design, water activity, and sample
material among other factors. However, equilibration
in evacuated desiccators is rapid and often occurs
in a few hours and should not take more than a
week. Equilibration in nonevacuated containers
may take substantially longer and is more subject
to errors caused by nonequilibrium conditions and
water transfer between the container and external
atmosphere.
0010 Food materials exhibit two basic types of sorption
isotherms reflecting their either amorphous or crys-
talline state. Most foods are noncrystalline or par-
tially crystalline systems and have a sigmoid shaped
sorption isotherm. Crystalline materials, e.g., crystal-
line sugars, show a low water sorption until solubil-
ization, and other changes occurring at high water
activities result in a substantial increase in sorbed
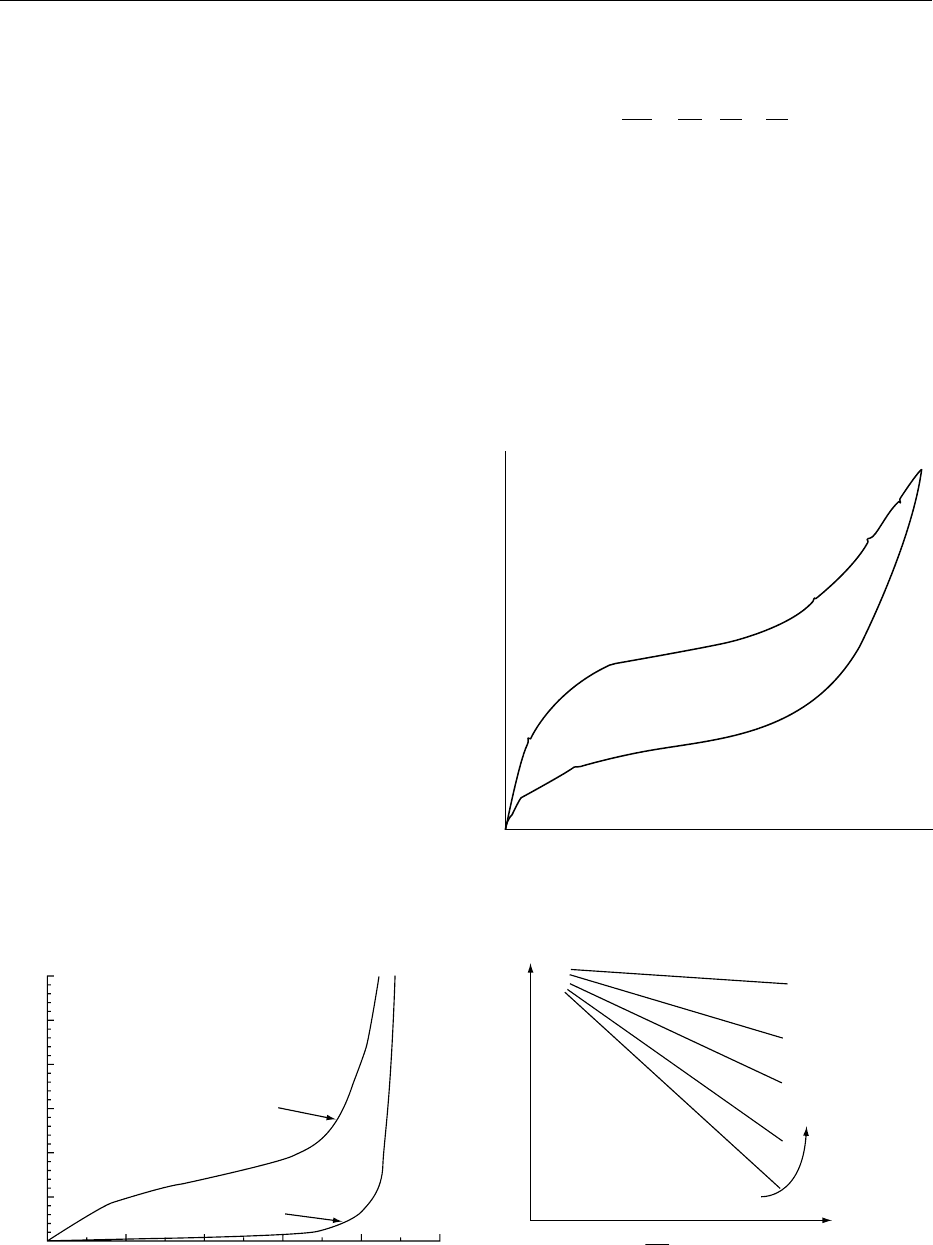
water (Figure 1). Food materials also have a hysteresis
between desorption and adsorption isotherms, i.e.,
the water content in desorption at the same water
activity is higher than in adsorption (Figure 2). The
hysteresis results most likely from retention of water
in capillaries and structural differences. Sorption iso-
therms are extremely useful in practical applications,
as only water content needs to be known to derive
water activity. Furthermore, changes in storage rela-
tive humidity of low moisture foods can be related to
corresponding changes in water content.
0011 Water activity and water sorption isotherms are
temperature-dependent. The temperature dependence
of water activity follows the well-known Clausius–
Clapeyron relationship (eqn (10)), where a
w1
and
a
w2
refer to water activity at absolute temperatures
T
1
and T
2
, respectively, Q
s
is the heat of sorption,
and R is the gas constant (8.3144 J K
1
mol
1
). A
schematic representation of the temperature depend-
ence of water activity is shown in Figure 3.
ln
a
w2
a
w1
¼
Q
s
R
1
T
1
1
T
2
: ð10Þ
In general, the water activity of a food with a constant
water content increases with increasing temperature,
while the water sorption decreases with increasing
temperature at the same water activity. This is
observed from sorption isotherms determined for
the same food at different temperatures (Figure 4).
Understanding the temperature dependence of water
sorption is extremely important, for example, in esti-
mating the effects of temperature fluctuations on
water activity changes inside a food package. The
0.0
0
5
10
15
20
25
30
0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0
Water activity
Water content (g per 100 g)
Typical food system
Crystalline material
fig0001 Figure 1 Hypothetical sorption isotherms typical of food
systems and crystalline materials, such as sugar crystals.
Water activity
Adsorption
Desorption
Water content
fig0002Figure 2 Hysteresis between water desorption and adsorption
typical of foods.
1
T
(K
−1
)
In a
w
m
5
m
4
m
3
m
2
m
1
Increasing water content
fig0003Figure 3 Temperature and water content dependence of water
activity. The temperature dependence of water activity increases
with decreasing water content.
6092 WATER ACTIVITY/Principles and Measurement

temperature change under normal storage conditions
may result in a water activity shift exceeding 0.1 a
w
.
Water Sorption Models
0012 A number of mathematical models have been used to
fit experimental water sorption data of food mater-
ials. The most common models are the Brunauer–
Emmett–Teller (BET) model (eqn (11)) and the
extended Guggenheim–Anderson–De Boer (GAB)
model (eqn (12)). These models originate from stud-
ies of sorption of gases on solid surfaces. The BET
model relates the water content (m) to water activity
using two parameters, m
0
and C, defined as the
monolayer value and a constant related to the excess
enthalpy of sorption, respectively. The monolayer
value gives the amount of water sorbed initially to
first available sorption sites, and it is often found to
correspond to the water content of low moisture food
systems with improved stability. The BET sorption
model can be written to the form of eqn (12), which
gives a linear relationship between a
w
/(1 a
w
m) and
a
w
. Thus, experimental water activity and water con-
tent values obtained from sorption studies can be
used to derive the constants m
0
and C. The
BET equation, however, fits experimental data only
between 0 and 0.5a
w
.
m
m
0
¼
Ca
w
1 a
w
ðÞ1 þ C 1ðÞa
w
½
ð11 Þ
a
w
1 a
w
ðÞm
¼
1
m
0
C
þ
C 1
m
0
C
a
w
ð12 Þ
m
m
0
¼
KC
0
a
w
1 Ka
w
ðÞ1 þ C
0
1ðÞKa
w
½
ð13Þ
a
w
m
¼
K
m
0
1
C
0
1
a
2
w
þ
1
m
0
1
2
C
0
a
w
þ
1
m
0
KC
0
:
ð14Þ
The GAB model (eqn (13)) has been shown to fit
experimental sorption data for most food materials
over the entire a
w
range. Experimental data may also
be used to derive the three parameters, the constants
C
0
and K, and the monolayer value, m
0
, of the GAB
model. This can be carried out, for example, by using
nonlinear regression or rearranging the equation to a
form of a second-order polynomial and plotting
experimental values of a
w
/m against a
w
, as suggested
by eqn (14). However, the BET and GAB monolayer
values are not equal, and neither of these values can
be considered as a water content giving maximum
stability in a low-moisture state.
Water Activity in Food System
0013Water is the main component of most fresh foods, and
their water activities are high and close to 1. The
water activity has increasing importance in concen-
trated foods, dehydrated foods or systems that con-
tain salts, sugars, and other compounds reducing
water activity to provide stability, for example highly
salted meats and fish or confectionery and candies.
Therefore, it is often of great interest in food product
development to estimate effects of food composition
and recipe components on end-product water activ-
ity. It is also important in packaging of mixtures of
different foods, such as dry ingredients, to understand
how water activity in a sealed package with several
components may change owing to differences in com-
ponent water activities. Furthermore, water activity
in different parts of foods may differ and result in
time-dependent water migration until equilibrium is
attained. This is often the case in bakery products,
which may have a high water activity filling in a lower
water activity matrix. However, it is important to
note that lipids and fat are not water-soluble, and
water activity is mainly affected by water-soluble
and water-miscible food components only. Therefore,
high-fat products with relatively small amounts of
water, such as butter and margarine, may have a
relatively high water activity.
0014The water activity of dilute sugar and salt solutions
can be calculated using Raoult’s law (eqn (8)) and for
more concentrated solutions when the activity coeffi-
cient is known using eqn (9), as discussed above.
Many food systems, however, deviate from ideal
Water activity
Water content (g per 100 g of solids)
Temperature increases
Constant water content
Temperature decreases
Constant water activity
fig0004 Figure 4 Schematic representation of water sorption iso-
therms determined at different temperatures.
WATER ACTIVITY/Principles and Measurement 6093

behavior, and Raoult’s law cannot be used. Then,
either sorption isotherms or other water activity cal-
culation methods must be followed to estimate water
activity in food product development. Several equa-
tions are available for predicting water activity of a
food product based on the product composition.
These include the Norrish equation, which extends
the use of mole fractions of food components with
additional coefficients to calculate water activity,
and the Grover equation, which uses different em-
pirical constants for the various components in
a polynomial. The Ross equation (eqn (15)) gives
probably the most accurate water activity predictions
for complex solutions. The equation is relatively
simple and does not require the use of empirical
constants.
a
w
¼ a
w1
a
w2
...a
wi
:ð15Þ
Mixtures of two or more ingredients or food com-
ponents with different water activities in a sealed
package will exchange water until the water activity
of the components becomes the same. The water
content of the components initially and after equili-
bration may differ significantly, owing to differences
in sorption properties. There are several approaches,
which can be applied to estimate the final water
activity of a food mixture in a sealed package. These
methods are based on knowledge of the sorption
isotherms and their use to predict the equilibrium
water activity. A simple approach is to establish a
sorption isotherm for the mixture and predict water
activity using the total water content of the ingredi-
ents. Another approach assumes that the sorption
isotherms of the components over the applied water
activity range follow a straight line. This leads to
eqn (16), which can be used to predict the equilibrium
water activity, a
w
. The method is also useful in esti-
mating an appropriate ratio of food components to
keep the final, equilibrium water activity at an accept-
able level.
a
w
¼
a
w1
b
1
w
w
þ a
w2
b
2
w
2
...þ a
wi
b
i
w
i
b
1
w
1
þ b
2
w
2
...þ b
i
w
i
, ð16Þ
where a
w1
, a
w2
...a
wi
, b
1
, b
2
...b
i
,andw
1
, w
2
...w
i
refer to the initial component 1, 2 ...i water activity,
slope of the sorption isotherm, and weight of dry
solids, respectively.
0015 The role of water activity as a food stability param-
eter has been well recognized. Water activities can be
measured with commercially available equipment,
and sorption isotherms are available for most food
components. Furthermore, computer software for
water activity calculations and establishment of sorp-
tion isotherms has been developed and used widely in
the food industry.
See also: Water: Structures, Properties, and
Determination; Physiology; Water Activity: Effect on Food
Stability
Further Reading
Barbosa-Ca
´
novas GV and Welti-Chanes J (eds) (1995) Food
Preservation by Moisture Control: Fundamentals and
Applications. Lancaster, PA: Technomic.
Bell LN and Labuza TP (2000) Moisture Sorption. Practical
Aspects of Isotherm Measurement and Use. St. Paul,
MN: American Association of Cereal Chemists.
Fontana AJ (2000) Understanding the importance of water
activity in food. Cereal Foods World 45: 7–10.
Iglesias HA and Chirife J (1982) Handbook of Food Iso-
therms. New York: Academic Press.
Jouppila K and Roos YH (1997) Water sorption isotherms
of dehydrated milk products: Applicability of linear and
nonlinear regression analysis in modeling. International
Journal of Food Science and Technology 32: 459–471.
Labuza TP (1968) Sorption phenomena in foods. Food
Technology 22: 263–265, 268, 270, 272.
Labuza TP (1980) The effect of water activity on reaction
kinetics of food deterioration. Food Technology 34(4):
36–41, 59.
Rockland LB and Stewart GF (eds) (1981) Water Activity:
Influences on Food Quality. New York: Academic Press.
Roos YH (1995) Phase Transitions in Foods. San Diego,
CA: Academic Press.
Roos YH (2000) Water activity and plasticization. In: Eskin
NAM and Robinson DS (eds) Food Shelf Life Stability.
Boca Raton, FL: CRC Press.
Roos YH, Leslie RB and Lillford PJ (eds) (1999) Water
Management in the Design and Distribution of Quality
Foods. Lancaster, PA: Technomic.
Seow CC, Teng TT and Quah CH (eds) (1988) Food Preser-
vation by Moisture Control. London: Elsevier.
Simatos D and Multon JL (eds) (1985) Properties of Water
in Foods. Dordrecht: Martinus Nijhoff.
Slade L and Levine H (1991) Beyond water activity: Recent
advances based on an alternative approach to the assess-
ment of food quality and safety. Critical Reviews in Food
Science and Nutrition 30: 115.
Effect on Food Stability
Y H Roos, University College Cork, Cork, Ireland
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001Water activity, together with pH and temperature, is
often an important factor in controlling food stability.
These three parameters all, but not alone, influence
microbial activity, rates of chemical and enzymatic
reactions, and changes in food texture. Water activity,
6094 WATER ACTIVITY/Effect on Food Stability
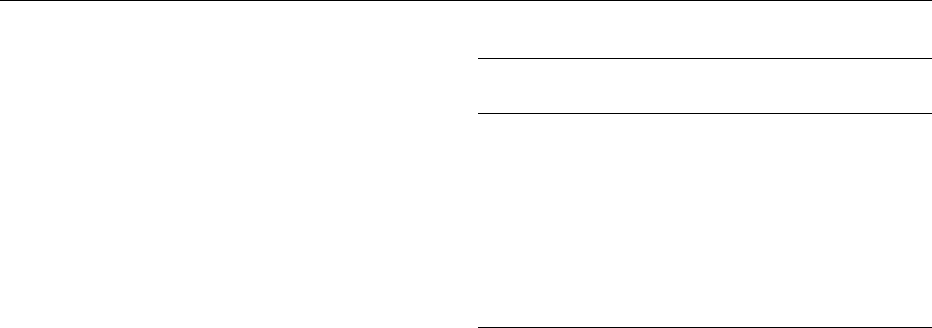
however, has a significant role in controlling texture
formation and textural changes in intermediate-
moisture foods (IMF) and low-moisture foods. In
such foods, removal or sometimes sorption of water
results in concentration changes of food solids and
thereby has an effect on reaction rates. Enhanced
reaction rates resulting from water removal may be
desired, as in baking to provide color and flavor, or
they may be detrimental as in production of many
food powders, such as dairy powders. That makes the
time–temperature–water or water activity control in
food production and storage extremely important.
0002 In some cases, the maximum food stability at low
water contents is achieved at a water activity cor-
responding to the Brunauer–Emmett–Teller (BET)
monolayer water content. Furthermore, water affects
the physical state and water plasticization, i.e.,
softening of water miscible food solids. As a plasti-
cizer, water controls the glass transition of food com-
ponents, such as carbohydrates and proteins. The
glass transition is a well-known property of all inor-
ganic and organic amorphous materials. The glass
transition occurs over a temperature range, which is
often referred to with the glass transition tempera-
ture, T
g
. The physico-chemical properties of amorph-
ous materials change considerably over the glass
transition, as the transformation includes a change
between the liquid-like and solid-like material states.
There are also established and possible relationships
between the physical state, water plasticization,
and rates of diffusion-controlled reactions, such as
nonenzymatic browning, enzymatic reactions, and
oxidative changes.
0003 In general, water activity provides valuable infor-
mation of the effects of water content on water
availability and the physical state of food solids.
Such information can be described using sorption
isotherms and state diagrams. They provide critical
values for water content, water activity, and tempera-
ture, which are important in the characterization of
food behavior in processing and in establishing cri-
teria for packaging requirements and appropriate
storage conditions. These areas and the role of
water activity in controlling food stability are
discussed in the present article.
Water Activity and Food Stability
0004 Most fresh foods are high-moisture materials, and
their shelf-life is reduced by enzymatic changes, the
growth of microorganisms, and mechanical damage.
High-moisture foods have an a
w
of 0.90–0.999, and
they often contain more than 50% (w/w) water
(Table 1). These foods include fresh meats and sea-
food, various dairy products, fruits, vegetables, and
beverages. Most bacteria, molds, and yeasts are likely
to grow in high-moisture foods. However, the types of
spoilage microorganisms and the growth of various
species are highly dependent on pH, temperature, and
water activity.
0005IMF have a water activity within the range of 0.60–
0.90a
w
, and their water contents normally vary
between 10 and 50% (w/w) (Table 1). These foods
include many traditional low-moisture foods, such as
grains, nuts, and dehydrated fruits, but also a number
of processed foods or foods designed and manufac-
tured to have a known composition to provide stabil-
ity. Such foods may have particular applications and
requirements for stability, e.g., when used as fillings
in bakery products or confectionery. Although micro-
bial spoilage is prevented below 0.60a
w
, and many
microbes do not grow in IMF, their stability and shelf-
life are reduced by deleterious changes, such as struc-
tural transformations, enzymatic changes, browning
reactions, and oxidation, depending on a
w
, pH, and
temperature. The rates of such changes are often at
least to some extent affected by the physical state of
the materials and the extent of water plasticization
of water-miscible solids.
0006Low-moisture foods obviously have the lowest
water contents, often below 10% (w/w), and their
water activity is lower than 0.6a
w
(Table 1). Such
foods are not subject to microbial spoilage. Their
shelf-life, however, is often limited by chemical and
textural changes, particularly browning and other
changes in color and flavor as well as oxidation.
These foods may be exceptionally hygroscopic and
exhibit water sorption from their surroundings.
Water sorption is reduced by the use of protective
packaging, but sorption occurs during storage as a
result of permeation or damage of protective pack-
aging. Many low-moisture foods have a solid appear-
ance, or they have a crispy, solid texture. Water
sorption in such foods may lead to stickiness, struc-
tural changes, such as loss of crispness and sogginess,
tbl0001Table 1 Examples of water activity ranges of various foods
Food systems Wateractivity
range
Fresh meat, fish, vegetables, <40% (w/w)
sucrose, <7% (w/w) salt
>0.95
Bread, cooked sausages, medium aged cheese 0.90–0.95
Salami, old cheese, >65% sucrose, >15% salt 0.87–0.90
Dried beef, sweet condensed milk, cereals with
15% (w/w) water
<0.86
Jam, marmalade, old salami, >26% (w/w) salt 0.80–0.87
Flour, cereals, nuts 0.75–0.80
Caramels, honey, toffee 0.60–0.75
Breakfast cereals, snack foods, food powders 0.20–0.60
WATER ACTIVITY/Effect on Food Stability 6095

and coincident increases in the rates of browning and
enzymatic changes. A number of products contain
amorphous components, for example, lactose in
dairy powders and sucrose in many bakery products
and confectionery. Such amorphous components may
crystallize during water sorption, as water activity
increases. It is important to notice that low-moisture
foods are often glassy materials with T
g
values above
their normal storage temperature. Water sorption and
associated plasticization may decrease the T
g
, and the
glass transition may occur over the range of the con-
current increase in water activity.
Stability Control
Microbial Stability
0007 Microbial growth requires a minimum a
w
, in addition
to pH, temperature, and other appropriate conditions
that are important for the growth of bacteria, molds,
and yeasts. The water activity of high-moisture foods,
especially processed foods, can be manipulated to
some extent by the addition of salts and sugars or
other ingredients, which are known to reduce water
activity. Such compositional alterations are highly
advantageous in product development and food
safety control. For many products, the effects of com-
positional changes on water activity can be predicted
on the basis of composition and confirmed by meas-
uring water activity of the final product. This allows
an understanding of storage requirements and estima-
tion of the product shelf-life in various storage condi-
tions. In high-moisture foods, the main role of water
activity control is to govern and reduce the risk of
the growth of pathogenic and spoilage bacteria.
Examples of water activity limits for the growth of
selected microorganisms are given in Table 2.
0008 The lowest water activity limit for microbial
growth of 0.60a
w
allows the growth of xerophilic
yeasts. Above this limiting water activity, IMFs have
an increasing possibility for the growth of various
microorganisms with increasing water activity. How-
ever, the water activities of IMFs are such that patho-
genic bacteria are unable to grow, but there is a
possibility for the growth of molds and yeast. The
growth of these microorganisms must be controlled
by careful adjustment of product water activity, use of
protective packaging to avoid contamination, and
selection of appropriate humectants, pH control, and
use of antimicrobial agents.
0009 Microbial stability is an obvious, and often the
most important, criterion in food preservation. The
a
w
limits for growth of various microorganisms,
as shown in Figure 1, are well established and suc-
cessfully used in food product development and
manufacturing as well as control of product safety.
Furthermore, in high-moisture foods and several IMF
products, water activity is relatively constant and
dependent on composition, especially solids content,
and the type of water-soluble components.
Chemical and Enzymatic Stability
0010The chemical stability of high-moisture foods is not
significantly affected by water activity, as the micro-
bial quality is the main determinant of product shelf-
life. The role of water activity in determining the
chemical and enzymatic stability of IMF and low
moisture foods is more important. As the water activ-
ity and water content decrease, the concentration of
reactants in the water phase of foods becomes obvi-
ously increased. Therefore, rates of several reactions
may increase with decreasing water activity. The rela-
tive rate of deteriorative changes in intermediate and
low moisture foods is traditionally related to water
content and a
w
, as shown by the ‘Food stability map’
in Figure 2. Early studies applying nuclear magnetic
resonance spectroscopy found ‘mobilization points’
(water activity allowing reactant mobility) for solutes
in low-moisture food matrices. The mobilization
tbl0002Table 2 Water activity (a
w
) limits for the growth of selected
pathogenic and spoilage microorganisms
Microorganism Minimum a
w
Bacteria
Bacillus cereus 0.930
Bacillus subtilis 0.900
Campylobacter jejuni 0.990
Clostridium botulinum 0.940
Clostridium perfringens 0.945
Escherichia coli 0.935
Halobacterium halobium 0.750
Lactobacillus plantarum 0.940
Listeria monocytogenes 0.920
Salmonella spp. 0.940
Shigella spp. 0.960
Staphylococcus aureus 0.860
Vibrio parahaemolyticus 0.936
Yersinia enterocolitica 0.960
Molds
Aspergillus candidus 0.750
Aspergillus flavus 0.780
Aspergillus niger 0.770
Erotum echinulatum 0.620
Penicillium citrinum 0.800
Penicillium expansum 0.830
Penicillium patulum 0.810
Rhizopus nigricans 0.930
Xeromyces bisporus 0.610
Yeasts
Saccharomyces bailii 0.800
Saccharomyces cerevisiae 0.900
Saccharomyces rouxii 0.620
6096 WATER ACTIVITY/Effect on Food Stability
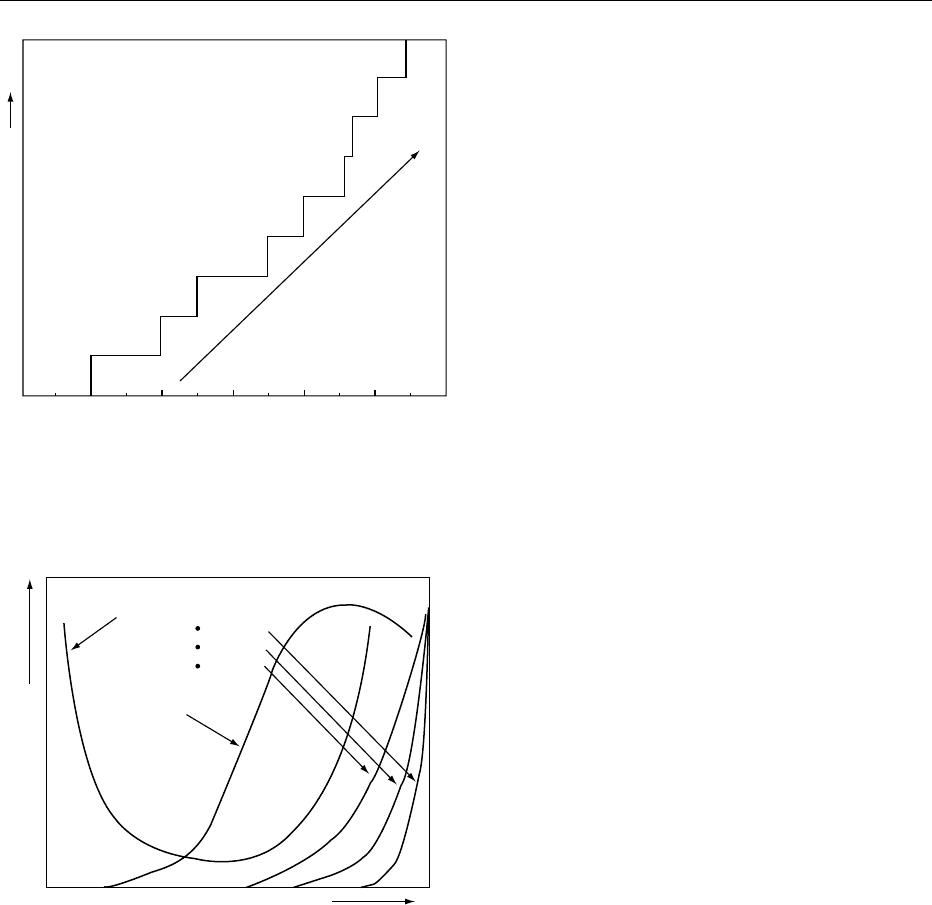
point was peculiar to the system, and the level of
hydration needed to achieve mobility was solute-
dependent. No solute mobilization occurred below
the BET monolayer value, and, for example, experi-
mental data of nonenzymatic browning reaction rates
suggested that browning initiated at the mobilization
point. An increase in the reaction rate was apparent
with increasing a
w
, and the rate maximum occurred at
the water activity corresponding to a hydration level
allowing complete mobilization. However, several
other factors, including the glass transition, are
important in controlling changes occurring in inter-
mediate and low moisture foods.
0011Enzyme activity in low-moisture foods is often re-
lated to hydration. At low water activities enzymatic
activity is generally not observed, as water cannot
enhance diffusion of substrates to enzyme molecules.
Such water activity dependence applies to hydrolases
and oxidases, unless the substrates are nonaqueous
liquids, allowing changes to occur fairly independ-
ently of water activity. It seems that not only water
activity but also the ability of water to give a known
mobility to enzymes and substrates is important in
controlling enzyme activity. The amount of water
needed increases with increasing molecular size
owing to impaired diffusion, in particular for
enzymes, which are active in the water phase of
foods. Therefore, for example, lipase activity is not
necessarily related to the mobility provided by
water.
0012Lipids exist often in a separate phase apart from the
water phase. At low water activities and water con-
tents, lipids become more accessible to the atmos-
pheric oxygen, and oxidation rates may increase
(Figure 2). The rate of oxidation, however, decreases
rapidly with increasing water activity, as the lipids
may become protected from atmospheric oxygen
owing to the formation of protective aqueous layers
in the food microstructure. In several low-moisture
foods, lipids, flavors, and other oxygen sensitive com-
pounds are entrapped within the amorphous struc-
ture. Such encapsulated compounds also exhibit
reduced rates of oxidation as compared with ‘free’
lipids.
0013Traditional shelf-life predictions of low-moisture
foods were based on the information of rates of de-
teriorative changes and loss of nutrients at various
temperatures and water contents. An increase in
water content above the BET monolayer value at a
constant storage temperature often results in rapid
deterioration as reaction rates increase at intermedi-
ate water contents. The main factors affecting reac-
tion rates in low-moisture food materials, however,
can be controlled by a number of factors, which
include food composition and the type of the reac-
tion, temperature, pressure, water content, and pH.
In concentrated food systems, changes in viscosity
and relaxation times in the vicinity of the glass transi-
tion may also affect diffusion and thereby contribute
to reaction rates and product shelf-life.
Water Plasticization
0014Intermediate and low moisture foods often exist in an
amorphous (noncrystalline, no defined structure
of molecular arrangement), elastic, ‘rubbery,’ or
‘leathery’ state or in a solid-like, glassy state. The
amorphous state is typical of liquids and remains in
molds
Water activity01
Relative rate
Nonenzymatic browning
Growth of:
bacteria
yeasts
Oxidation
fig0002 Figure 2 Food stability map describing the relationship
between water activity and food stability.
Xerophilic molds
Osmophilic yeasts
No
growth
Number of growing microbes
Most halophilic bacteria
Most molds, no growth of pathogenic
bacteria
Staphylococcus aureus may grow
Most yeasts, mycotoxin-producing molds,
spoilage often by molds and yeasts
Most cocci, lactobacilli, some molds, Salmonella,
lactic acid bacteria are major spoilage flora
Most bacteria, some yeasts,
pathogenic and spoilage organisms
a
w
0.91−0.95
0.80−0.87
0.75−0.80
0.65−0.75
0.60−0.65
<0.65
0.87−0.90
>0.95
>0.86
Growth
0.4 0.5 0.6 0.7 0.8 0.9 1.0
Water activity
fig0001 Figure 1 Growth of various microorganisms at different water
activity conditions.
WATER ACTIVITY/Effect on Food Stability 6097
many biological materials owing to complicated
chemical composition, rapid removal of solvent
water, or quench cooling of a liquid melt in processes
not allowing component crystallization. For example,
many sugars typical of foods do not crystallize in
dehydration, but remain in a supercooled, liquid
state after removal of the solvent water. Glassy,
solid-like foods may suffer the glass transition as a
result of water sorption, which may be observed from
rapid changes in structure and appearance, and
enhanced rates of chemical and enzymatic reactions.
0015 The glass transitions of food components have a
wide range of temperatures depending on the com-
ponent itself and also on its molecular weight. In
general, high-molecular-weight food components,
such as carbohydrate polymers and proteins, have
glass transition temperatures at very high
temperatures well above 100
C. Low-molecular-
weight components may have very low glass transi-
tion temperatures, for example, the glass transition of
amorphous water occurs at around 135
C, whereas
many anhydrous sugars have a T
g
between 0 and
100
C. The T
g
is often measured using differential
scanning calorimetry, and it can be observed with a
number of other thermal and spectroscopic tech-
niques. These include dielectric analysis, dynamic
mechanical analysis, electron spin resonance spec-
troscopy, infra-red and Raman spectroscopy, and
nuclear magnetic resonance spectroscopy. Examples
of glass transition temperatures of food components
are given in Table 3.
0016Carbohydrates and proteins in foods are water-
miscible components, and they are plasticized by
water. Water plasticization of the amorphous struc-
ture results in a decrease in T
g
. The decrease in T
g
is
substantial even at low water contents, and at high
water contents, the T
g
approaches that of amorphous
water. Therefore, at a constant temperature, e.g.,
room temperature or a typical storage temperature
of intermediate- and low-moisture foods, a water
activity or water content range can be established,
which corresponds to the glass transition of the ma-
terial. The relationships between glass transition,
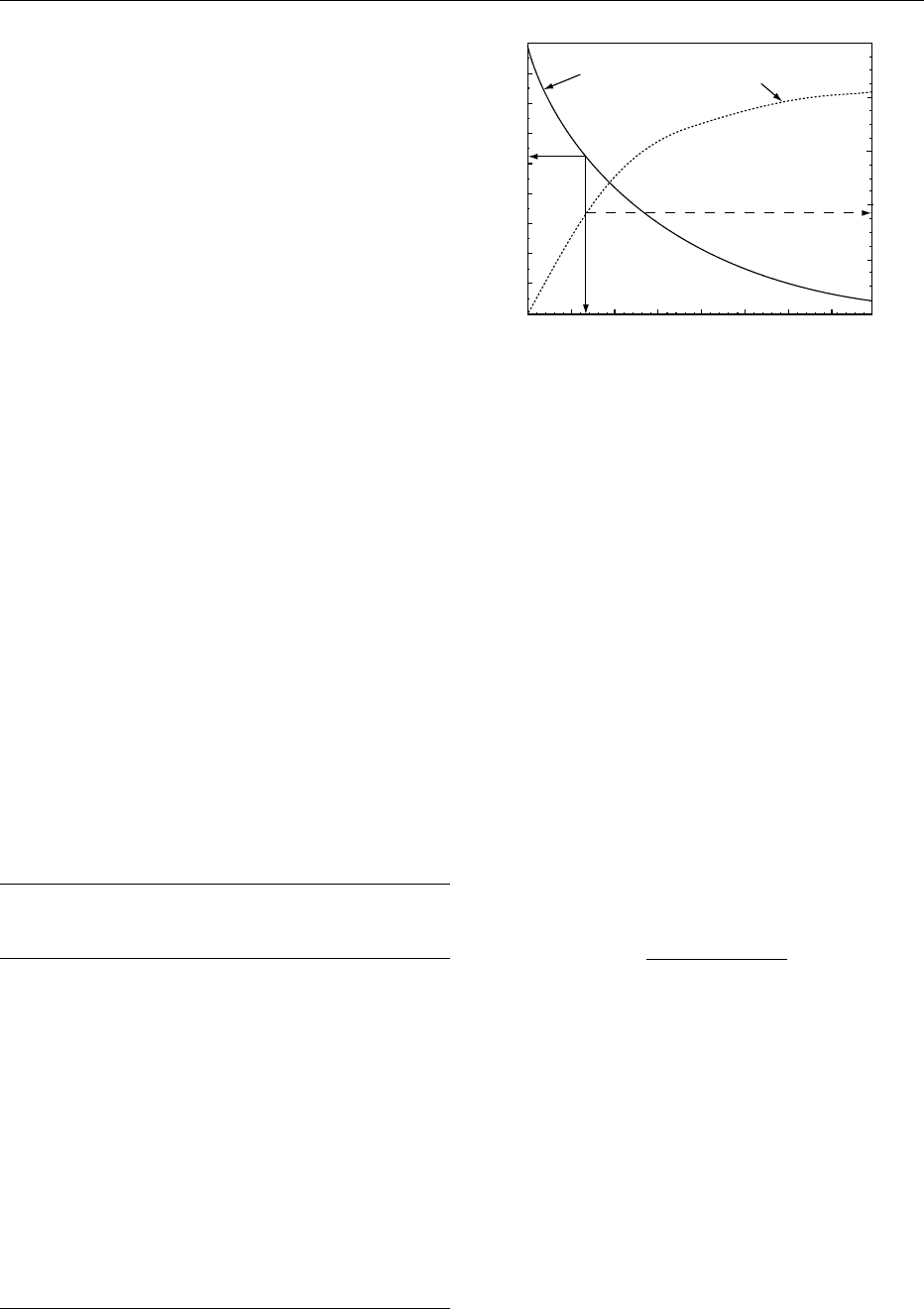
water activity, and water content are described in
Figure 3. The water activity and water content,
which correspond to the T
g
depression to the storage
temperature can be defined as ‘critical water activity’
and ‘critical water content,’ respectively.
T
g
¼
w
1
T
g1
þ kw
2
T
g2
w
1
þ kw
2
, ð1Þ
where w
1
and w
2
and T
g1
and T
g2
are the weight
fraction and glass transition temperature of solids
(1) and water (2), respectively.
0017The relationship between the glass transition tem-
perature depression and water content is often pre-
dicted with the Gordon–Taylor relationship (eqn (1)).
The model can be fitted to experimental T
g
and water
content data and used together with water sorption
data to establish relationships between the T
g
and
water sorption properties, as shown for amorphous
lactose in Figure 3. Such predictions are useful in food
product development, because they allow estimation
of food stability in terms of the physical state and
water activity.
tbl0003 Table 3 Examplesofmeltingandglasstransitiontemperatures
offoodcomponents
FoodcomponentMeltingtemperatureof
crystallinesolids
(DSC onset,
C)
Glasstransition
temperature
(DSC onset,
C)
Carbohydrates
Fructose1085
Galactose16330
Glucose14331
Lactose 223 101
Maltose 160 87
Sorbitol 85 9
Starch
a
na 250
Sucrose 173 62
Trehalose 215 100
Xylitol 89 29
Proteins
a
Gelatin na 207
Gliadin na 179
Glutenin na 189
Myoglobin na 149
Ovalbumin na 157
a
Estimated values.
Glass transition
Water activity
Critical water content
Critical
water activity
Water activity (at 25 ⬚C)
1.0
0.8
0.6
0.4
0.2
0.0
0 5 10 15 20 25 30 35 40
Water content (g per 100 g of solids)
100
80
60
40
20
0
−20
−40
−60
−80
Glass transition (⬚C)
fig0003Figure 3 Relationships between glass transition temperature,
water activity, and water content for amorphous lactose with
critical values for water content and water activity at room
temperature.
6098 WATER ACTIVITY/Effect on Food Stability
