Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


Rico AG (ed.) (1986) Drug Residues in Animals. London:
Academic Press.
Woodward KN (1991) The licensing of veterinary medi-
cinal products in the United Kingdom – the work of
the Veterinary Medicines Directorate. Biologist 38:
105–108.
Residue Determination
A A Bergwerff and J Schloesser, Utrecht University,
Utrecht, The Netherlands
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Residue Determination
0001 Farming of husbandry animals, as well as aquatic
animals, is associated with veterinary application
of pharmaceuticals, with the aim of influencing
growth, weight gain, fat/protein distribution, milk
production, stress, microbial infections, inflamma-
tion reactions etc. (See Antibiotics and Drugs:
Uses in Food Production). This administration of
pharmaceuticals results in so-called residues in the
treated animal, including its edible parts, and prod-
ucts such as muscle (meat), organs, eggs, honey,
and milk.
0002 In some cases, residues are persistent, and concen-
trations may be high enough to affect the health or
well-being of the consumer. In the case of direct car-
cinogenic substances, in theory, a single molecule may
cause malignancies in the consumer. The veterinary
use of such substances, when xenobiotic, is of course
unacceptable. Veterinary medicinal products that
have the ability to accumulate in the animal are
banned world-wide as well. As well as the risk to
public health, the production or quality of food may
be affected by residues, for example by inhibition of
fermentation processes during cheese production.
0003 This phenomenon of negative effects of lagging
drugs is known as the ‘residue problem.’ Controlling
this problem through residue determination in the
food chain from ‘stable to table’ is necessary to
guarantee food quality and safety and to secure
the consumer’s trust.
Test at Maximum Residue Limit
0004 In the case of registered substances, and thus sub-
stances allowed for veterinary use, international, na-
tional, and supranational legal bodies have agreed on
so-called ‘maximum residue limits’ (MRL) for each
edible product, and for each husbandry species. The
consumer may be exposed to a nonacceptable health
risk when this MRL is offended, i.e., the concentra-
tion of the indicator molecule is in excess of this
concentration. This makes statutory and nonstatu-
tory control of residues in food of major importance.
MRL values may differ from one (inter)national
authority to another, as consensus is not always
reached on quantitative public health risks.
0005Authorities have also introduced a definition
for residues of veterinary medicinal drugs, which,
according to EU council regulation 2377/90, is:
All pharmacologically active substances whether active
principles, excipients or degradation products, and their
metabolites which remain in food stuffs obtained from
animals to which the veterinary medicinal product in
question has been administered.
This description clearly does not include contamin-
ation arising from environmental substances, phyto-,
phyco-, or mycotoxins, or as a result of food hand-
ling, processing, and preparation. The description
highlights the environmental influences on the
original molecules administered to the animal. The
chemical appearance of a residue in muscle (ante
mortem) or in meat (post mortem) is not static. In
addition to biochemical processes in the animal, stor-
age, food processing, and food preparation, which
may include fermentation and heating, frequently
initiate reactions converting the original pharmacon
into derivatives. The residue analyst has to account
for these processes, since they may mask the presence
of residues.
0006Relatively soon after administration, furazolidone,
for example, is metabolized completely and occurs
as a so-called bound residue, which is a covalent
linkage of the residue molecule with predominantly
proteins. Bound residues are, by definition, not ex-
tractable. The remaining and nonreacting 3-ami-
no-2-oxazolidone fragment is extractable and is used
for furazolidone residue determination. The cepha-
losporin ceftiofur is converted into a reactive des-
furoyl ceftiofur species, which reacts with itself,
proteins, and other biomolecules. The residue is re-
leased chemically and stabilized prior to residue an-
alysis. Another example is that of the antibiotic
sulfadimidine, which may occur in the presence of
relatively high concentrations of glucose, as in saus-
ages, as an extractable sulfadiminyl glucose residue.
In the living animal, many veterinary drugs are sul-
fated or conjugated to glucuronic acid in order to
detoxify and excrete these substances. Incubation
with sulfatases and glucuronidases prior to residue
determination may be necessary to obtain reliable
results.
254 ANTIBIOTICS AND DRUGS/Residue Determination

0007 Authorities have to decide on the indicator residue
molecule for analysis. The MRL value of this indica-
tor molecule should correspond with the total
concentration of residue components, including
derivatives of ante- and post-mortem reactions.
Analysis Strategies
0008 Before going into a detailed description of residue
analysis techniques, it should be noted that two dif-
ferent situations occur, which dramatically influence
the desired degree of reliability of an analytical
method.
0009 In the situation of registration of pharmaceuticals,
methods are designed and validated in well-defined
experiments. A defined number of healthy animals
are treated, allowing statistical analysis and aver-
aging of errors. Target tissues may be sampled, and
these usually contain easily detectable amounts of
residues. In contrast, in an inspection or a monitoring
situation, a juridically useful judgment should be
made on the basis of a single sample for which the
history is generally unknown and the concentrations
are usually relatively low. In most cases, meat is
sampled, which is mostly not the ideal matrix to
trace the (illegal) use of pharmaceuticals. Further-
more, in contrast to the ‘registration methods’, time
pressure is relatively high in the field of ‘forensics’
especially in the case of inspection of carcasses in the
slaughter line. Swine are slaughtered at a rate of up to
12 carcasses per minute, whereas chickens are slaugh-
tered at a rate of up to 270 exemplars each minute.
The storage capacity becomes critical when a decision
on condemnation of a carcass has to be made. The
quality criteria for analytical methods in these
different situations may therefore vary considerably.
0010 The situation and analysis strategy therefore deter-
mine the characteristics of the assay of choice. The
ideal method, which is inexpensive, fast, specific,
selective, sensitive and allows a high throughput,
does not exist. Compromises have to be made. A
low-cost screening method with a high throughput
may be followed by a usually more expensive, more
selective, more sensitive, and specific confirmatory
method. In arbitrage situations, a reconfirmation
may be needed through so-called reference methods
involving expensive instrumental techniques requir-
ing highly skilled personnel.
Inventory Expected Residue Levels
0011 Of the enormous collection of veterinary drugs, anti-
biotics are used most frequently and in the largest
amounts compared with the other groups listed in
Table 1. In the EU, a considerable part of antibiotic
use is still for growth promotion. From the point of
view of residue analysis strategies, it is important to
consider the use of a drug before developing or intro-
ducing a new method in the residue laboratory. The
different levels at which veterinary drugs are applied
will result usually in proportional concentrations in
edible tissues. For that reason, legal antibiotic growth
promoters do not result in high levels of residues.
Tracing the use of illegal antibiotic growth promoters
in animal products may be more worthwhile, but will
require sensitive tests amenable for detecting rela-
tively low concentrations. Therapeutic use of antibi-
otics, however, may yield residues at concentrations
of milligrams per kilogram, when withdrawal times
are not obeyed, or as high as grams per kilogram at
the injection site. The withdrawal time is the pre-
scribed interval between administration and allow-
ance to bring products of a treated animal to the
market.
0012Besides antibiotics, the occurrence of substances
with hormonal activity is attracting much attention,
at least in the EU. Since steroid hormones are effective
at very low levels, tracing residues in meat needs
ultrasensitive assays for detecting concentrations as
low as the subnanogram per kilogram level. These
methods also may need to be able to differentiate
between endogenous and administered, exogenous
hormones, as in the case of nortestosterone of 17b-
estradiol, for example. In these situations, it is diffi-
cult to answer the questions ‘when is a residue a
residue, and when is a residue not a residue?’
0013Monitoring and inspection programs should be
aware of the use of ‘cocktails’ of illegal hormonal
compounds. The mixture will exert its biological
effect, but the individual components may not be
detected as a result of low concentrations per com-
ponent. The illegal use of b-agonists may co-occur, for
example, with corticoids and thyreostats. Residue
analysis strategies may therefore focus on the most
easily extractable and detectable residue in first
screening.
Sampling
0014In setting up a method, a few steps can be recognized
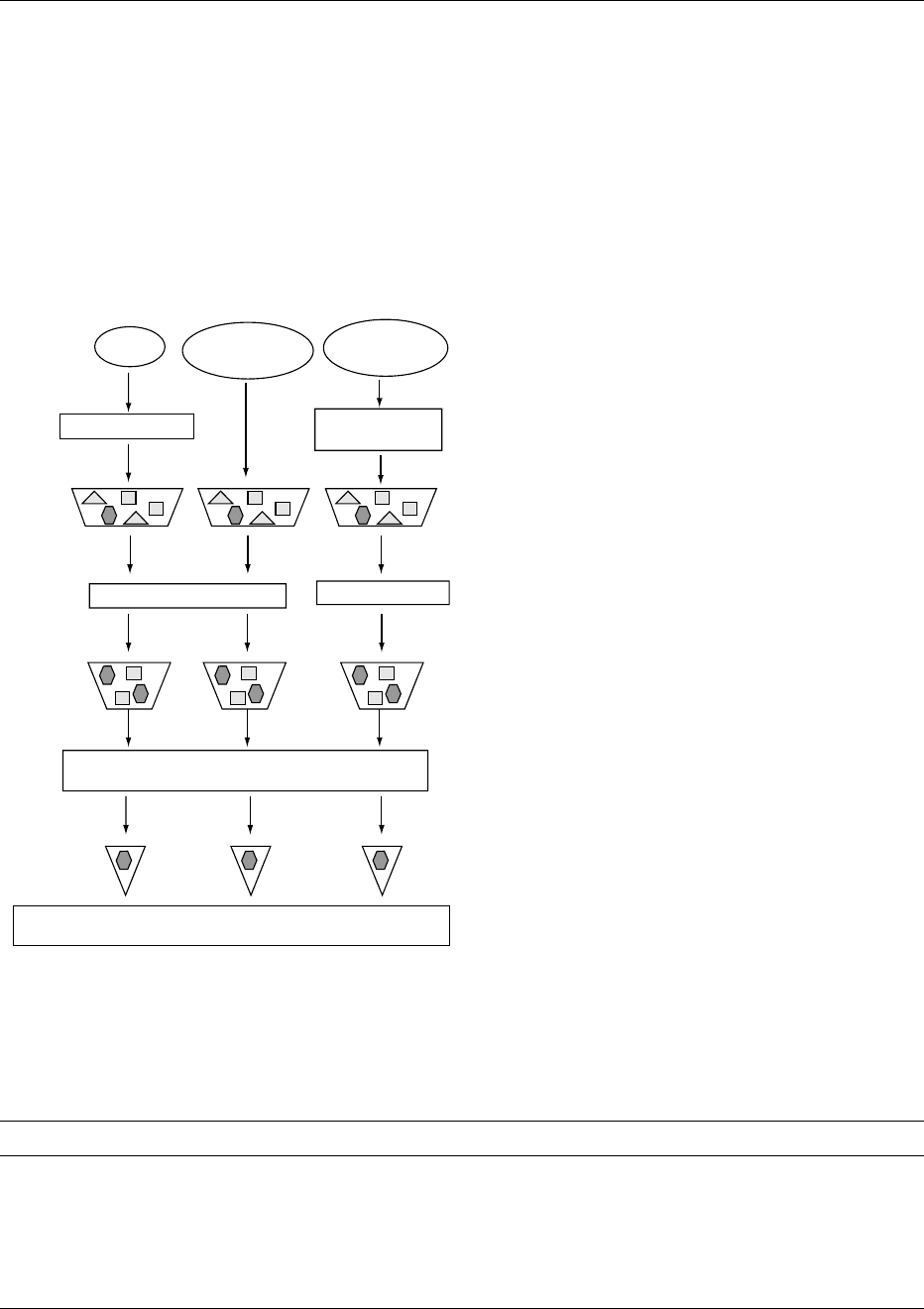
(Figure 1). Each step from sampling, extraction, and
concentration to (instrumental) analysis is essential
and should be carried out appropriately for successful
analysis. The statement: ‘collect the wrong sample, or
collect the right sample incorrectly, and you will trivi-
alize all that follows, rendering your data worthless’
emphasizes the importance of the first step, namely
sampling. The selection of the laboratory sample for
analysis, its transport, and storage should always
be carefully evaluated (cf. Table 2). The storage of a
ANTIBIOTICS AND DRUGS/Residue Determination 255

laboratory sample of homogenated or intact tissue
may influence the final determined residue content
dramatically. Furthermore, the test sample should
reflect the laboratory sample, which usually means
thorough homogenization of the latter. The labora-
tory sample should reflect the tissue, body fluid, or
total animal that is sampled. At the same time, the
result of an individual sample should not be over-
valued. Unfortunately, many mistakes have been
made at this level, sometimes with huge political
impact.
0015 When tracking the illegal use of prohibited sub-
stances, the choice for the most suitable matrix for
analysis is obvious, since the MRL in an edible prod-
uct does not have to be tested. In such cases, urine
(anabolics), feces (steroid hormones), bile (antibiot-
ics), retina (b-agonists), or hair (b-agonists), instead
of meat, may be most suitable.
Extraction and Clean-up
0016 Milk and diluted honey, but also urine, serum used to
trace contamination of the animal, may be analyzed
in some cases without extensive sample processing
(Figure 1). Solid matrices, including fat, hair, meat,
kidney, retina, and skin, however necessitate extrac-
tion of residues prior to analysis. Specific solvents are
mixed with the homogenized matrix to facilitate solu-
bilization of the residues. As an exception, the collec-
tion of meat drip through heating or compressing, or
of pre-urine from the kidney cortex may be sufficient
for microbiological growth-inhibition assays (see
below).
0017Matrices are complex, and it should be noted that
the residue analyst looks for a single particle in a heap
of a million to billion other potentially interfering
particles. These particles are also for a part solubil-
ized and extracted from the matrix. Clean-up of
samples is therefore necessary in most cases to over-
come any interference of the analysis method.
0018A good knowledge of the chemical nature of the
analyte in terms of stability, pK
a
value(s), polarity,
and functional groups will support the design of a
selective, effective, and appropriate clean-up proced-
ure. Before the availability of sorbent materials in
the mid-1980s, sample clean-up was carried out by
tbl0001 Table 1 Classes and examples of veterinary drugs
a
Activity/class Subclass Examples
b
Antibiotics, including
growth-promoters
and chemotherapeutics
Nitrofurans Furazolidine, nitrofurazone, furaltadone
(Fluoro)quinolones Enrofloxacin, oxolinic acid, flumequine
Tetracyclines Oxytetracycline, doxycycline, chlortetracycline
Penicillins and cephalosporins Amoxiciline, cloxacillin, penicillin G, ampicillin, ceftiofur
Aminoglycosides Gentamicin, lincomycin, streptomycin
Sulfonamides Sulfadimidine, sulphaquinoxaline
Macrolides Erythromycin A, spiramycin, tylosine
Peptides Virginiamycin, bacitracin
Miscellaneous Chloramphenicol, carbadox, salinomycin
Anti-ectoparasitica Phosphoesters, carbamates,
pyrethroids a.o.
Dichlorvos, carbaryl, deltamethrine, lufenurone, cyromazin, lindane
Anthelmintics Imidazothiazoles Levamisole, dexamisol
Benzimidazoles Mebendazole, fenbendazole, lobendazole
Tetrahydropyrimides Pyrantel
Avermectins Ivermectin, abamectin, moxidectine
Salicylanilides Closantel, rafoxanide, niclosamide
Coccidiostats
c
amprolium, dimitridazole, monensin,
Antimycotica nystatine, griseofulvine, enilconazole
Hormones Glucocorticosteroids Dexamethasone, cortisone (hydrocortisone)
Androgenic and estrogenic
sex steroids
17b-Estradiol, methyltestosteron, trenbolone
Progastegens Megestrol, medroxyprogesteronacetate
Resorcylic acid lactones Zeranol, taleranol
Stilbenes Diethylstilboestrol, hexoestrol, dienoestrol
b-agonists Clenbuterol, salbutamol, bromobuterol
Thyreostats Tapazole, thiouracil
Proteins Somatropins
Tranquilizers Azaperone, carazolol, xylazine
a
This table is a summary and not a complete list of compounds and classes of compounds used in veterinary medicine.
b
Some of these compounds are registered veterinary drugs. The EU-accepted MRL values in food from bees, cattle, deer, horses, pigs, poultry,
salmonidae,sheep,etc.arepublishedontheinternetathttp://eudrams1.is.eudra.org
c
Many antibiotics are used as coccidiostats as well.
256 ANTIBIOTICS AND DRUGS/Residue Determination
liquid/liquid partition and/or atmospheric pressure
chromatography. Sample concentration was obtained
through rotary evaporation. A major improvement
in this laborious work was obtained by the launch
of commercially available, and thus standardized,
solid-phase extraction (SPE) cartridges. The cart-
ridges enabled selectivity and concentration in a
single step with minimal sample and solvent con-
sumption. The choice of stationary phases and sup-
portive material in SPE is comparable with that used
in high-performance liquid chromatography (HPLC),
including normal- and reversed-phase, partition and
ion-exchange chromatography. Combinations of
phases within a single cartridge are available as
well. For example, so-called ‘restricted access mater-
ials’, such as alkyl-diol-silica particles, combine the
ability of gel filtration and solid-phase extraction
within one material. These special SPE materials
allow the extraction of aqueous extracts or liquid
samples like milk or urine without prior deproteina-
tion. With the advent of chelating agents and the
availability of antibodies and receptors, it is now
possible to develop cartridges facilitating affinity
extraction with a high selectivity.
0019More recently, disks consisting of membranes of
polymers with high, reversed and ion-exchange
phase capacity have been introduced. These disks
are used as filters, allow fast-flow processing, purify
and concentrate analytes like SPE cartridges with
small to large sample volumes. Interfaces are avail-
able, enabling the coupling of such disks to HPLC
columns.
0020Another sorbent extraction approach is matrix
solid-phase dispersion, which allows homogenization
and extraction by mixing of sorbent material in a
single run omitting liquid extraction of the homogen-
ate as a separate step. Columns prepared with the
mixture are eluted in a similar way to SPE cartridges.
Alternatives for these extraction procedures are
supercritical fluid extraction and accelerated solvent
extraction. The approaches are slowly gaining popu-
larity but are still used in niche applications.
0021After extraction, the analyte-containing solution
often has to be concentrated by removing excess solv-
ent through evaporation or sublimation by applying a
vaccum or a flow of an inert gas (nitrogen) sometimes
in combination with heat. Loss of a part of the
amount of the analyte can occur if unsuitable glass-
or plastic ware is used. Many analytes, like penicillins
and avermectins, tend to become adsorbed by free
silanol groups of glass surfaces. Deactivation of
free silanol groups through silanization of glassware
Eggs
Laboratory
sample
Milk, urine,
serum
Homogenization
Tissue, feces,
feed
Grinding and
homogenization
Test
sample
Centrifugation or filtration
Liquid extraction
Extraction, clean-up and concentration: SPE
(evaporation of solvent)
Test portion
Analysis of analyte: high-performance liquid
chromatography, gas chromatography, mass-spectrometry
fig0001 Figure 1 Typical scheme for sample processing involving SPE
and determination of residues of veterinary drugs in different
matrices.
tbl0002 Table 2 Stability of veterinary drug residues in different matrices upon cold storage and heating
Pharmaceutical Storage at 18
CStorageatþ4
C Heated (Percentage activityrecovered)
Penicillin Stable in muscle for 14 days Stable in muscle for 14 days 10% in pork at 80
C for 10 min
Degraded in kidney Degraded in kidney 0% in sterilized sausages at 125
C for 65 min
Furazolidone Unstable Unstable
Oxytetracycline Stable for 6 months Stable for 6 months No recoverable activity in pork at 100
C for 10 min
Sulfadimidine
a
Degraded in liver Degraded in liver 100% in well-done steak at 80
C for 30 min
Chloramphenicol Degraded in liver Degraded in liver and kidney 37% in pork at 80
C for 10 min
Spectinomycin Degraded in liver and kidney Degraded in liver and kidney
a
Sulfadimidine reacts with nitrite and glucose in sausages.
ANTIBIOTICS AND DRUGS/Residue Determination 257

using chlorsilane often enhances the recovery in such
situations.
Analysis of Analyte
0022 Overviewing all available methods for separation and
detection of residues in the nonprocessed test sample
or in the obtained extract, an enormous gamma of
(immuno)chemical and physicochemical approaches
can be disentangled (Table 3). Thin-layer chromatog-
raphy (TLC) was applied frequently in the early days
of residue analyses and was closely followed in time
by gas chromatography (GC). Analytes on TLC plates
were visualized by generating chromophores or fluor-
escents by applying (through spraying or dipping)
derivatizing molecules. Thermal conductivity, flame
ionization, and, in certain applications, electron-
capture and nitrogen phosphorus (NPD), detectors
were popular in GC analysis. In current residue GC
methods, the universality, selectivity and specificity
of the mass spectrometer (MS) in combination with
electron-impact ionization (EI) is by far preferred.
0023 HPLC, in combination with a range of different
detectors, became popular in the 1980s and 1990s.
The stationary phases used predominantly are
reversed-phase materials, especially phenyl-hexyl,
octyl- or dodecyl-modified silica gels, but also, parti-
tion and ion-exchange chromatography is found in
residue-determination protocols. Highly end-capped
silica gels or polymeric stationary phases may be
necessary to prevent tailing of amphoteric residues,
such as tetracyclines. Polymeric phases also allow
elution under alkaline conditions without solving
the supporting silica material.
0024 In addition to adequate extraction, clean-up and
chromatography, the choice of detection system is
an important parameter to achieve selectivity and
specificity. Analytes, which are not detected by light
absorption, refractive index, or fluorescence at the
level they are expected to occur in animal-derived
matrices, may require chemical modifications
rendering them as fluorescents, chromophores, or
UV-light absorbing compounds. For example, specti-
nomycin is converted postcolumn into a fluorescent
derivative following oxidation and reaction of two
o-phthaldialdehyde molecules with the two primary
amines into conjugated iso-indoles. In such cases,
chemical modification may also introduce a tremen-
dous improvement in the selectivity of the method.
0025Alternative detection systems compatible with LC
that have been used in residue analyses comprise
evaporative laser-scattering and electrochemical de-
tection, including (pulsed) amperometric detection.
0026In electrochromatography, electroosmotic flow is
used for mobile phase delivery. Packed and open
tubular columns are used in these systems, giving
very small band widths, since extremely small par-
ticles can be used as the stationary phase. The devel-
opment of heat is, however, a major drawback of this
separation technique. Instead of chromatography,
capillary electrophoresis with a high resolving power
may be considered as well. However, the maximum
sample size, which is very small, limits its use in
residue analysis. To obtain namely dissolved extracts,
relatively large solvent volumes are required, which
reduce residue concentrations.
0027The identification of analytes in TLC, GC, and LC
separation systems is carried out by comparison of
the migration or elution positions with those of stand-
ard substances under identical chromatographic and
detection conditions. However, comigration or coelu-
tion is usually not considered as a proof of identity of
the analyte, especially if juridical consequences are
involved. For this reason, combination of chromatog-
raphy with spectrometric (MS) or spectroscopic de-
tection techniques (NMR, IR) may provide data with
tbl0003 Table 3 Examples of residue analysis approaches
Analyte Matrix type Analyticalmethod Sample pretreatment
Penicillins
(group-specific
method)
Meat GC-NPD or GC-EI-MS Acetonitrile/buffer extraction, multiple liquid–
liquid partition ion-exchange-SPE, elution
with sodium chloride, liquid/liquid partition,
derivatization
Penicillins Milk HPLC, photochemical postcolumn
derivatization, UV detection at 300 nm
or electrochemical detection
Centrifugation and ultrafiltration, on-line SPE
(restricted-access material)
Avermectins Liver HPLC, fluorescence detection
(l
ex
: 365 nm l
ex
470 nm)
Acetone/water extraction, liquid–liquid partition,
SPE, concentration, precolumn derivatization
Quinolones Poultry meat HPLC-APCl-MS/MS Phosphate-buffer extraction, SPE
Tetracyclines Kidney HPLC-ESI-MS/MS Mcllvain-buffer extraction, on-line SPE
Sulfamethazine,
Sulfdaizine
Meat, milk Biosensor (surface plasmon resonance) Homogenization with buffer, centrifugation
258 ANTIBIOTICS AND DRUGS/Residue Determination
sufficient evidence. Of the spectroscopic approaches,
nuclear magnetic resonance (NMR) and Fourier-
transform infrared (FTIR) spectroscopy are most suit-
able, although certainly not in routine settings. The
so-called hyphenated techniques, like in TLC-MS,
GC-MS, LC-MS, LC-NMR, and GC-FTIR, have
proved very powerful techniques for separation and
molecular identification of the residue.
0028 The evolution, and sometimes revolution, of mass
selectors, ionization techniques, and their interfacing,
has made the mass spectrometer standard equipment
in a residue analysis laboratory. Mass selectors com-
prise sector, quadropole, ion-trap, time-of-flight
instruments, and combinations thereof. In particular,
mild-ionization techniques, such as atmospheric pres-
sure chemical ionization (APCI) and electrospray
ionization (ESI), facilitate MS analysis of small to
relatively large and hydrophobic to hydrophilic mol-
ecules. APCI and ESI are therefore very suitable for
residue determination. Using mass spectrometer-
induced molecular fragmentation, it is possible to
elucidate the chemical structure of the residue. A
major pitfall is matrix-caused interference, which
may reduce the response of the analyte to nil.
0037 Besides physicochemical methods, the use of
microbiological growth-inhibition assays to test
meat and milk for the presence of antibiotics is popu-
lar over a long period of time. In terms of the number
of analyses performed each year, this type of residue
determination is most important. In addition to the
low cost, this may be a result of an early implementa-
tion of such tests in national legislation. Examples of
such methods are the EU Four Plate Test, the New
Dutch Kidney Test, and the Delvotest SP, which use
very antibiotic-sensitive bacterial reporter strains,
such as Bacillus subtilis and Bacillus stearothermo-
philus var. calidolactis. These bacteria are inoculated
under optimal conditions with and without sample.
After culturing, results are read from visible inhib-
ition zones or from the color change of the bacterial
suspension in agar gels.
0029 The availability of relatively large amounts of
immunoglobulins in the 1980s, of which the pro-
duction could be assured, enabled the development
of immunoassays, like radio-immunoassays, enzyme-
immunoassays, enzyme-linked immunosorbent
assays, and later strip- and dip stick-based immuno-
assays. Specific enzymes and receptors have been used
in a similar way in such analysis approaches, which are
referred to as receptor assays, such as the commercially
available CHARM Test II and Penzyme III. The major
advantage of immunoglobulins and receptors is their
high specificity, which reduces the sample clean-up
to a minimum. However, their high specificity is a
disadvantage in multiresidue methods.
0030In immuno- and receptor assays, the binding of
small-molecular-weight compounds to their corres-
ponding receptors or immunoglobulins is not directly
detectable. These assays are therefore mostly com-
petitive assays in which the analyte competes with a
radio-isotope- or enzyme-labeled reporter compound
for a limited number of binding sites. In contrast to
conventional immuno- and receptor assays, advanced
biosensor techniques do not require this labeling in
most cases. From the point of view of signal transduc-
tion, biosensors fall into four categories, namely
mass, optical, electrical, and thermal. Of the few
applications so far, the ‘surface plasmon resonance’
optical biosensor has shown benefits in screening
residues, including ciprofloxacin, clenbuterol, enro-
floxacin, gentamycin, streptamycin, sulfadiazine, and
sulfamethazine.
0031The ability of tetracyclines to fluoresce and to form
complexes with alkali metals has revealed its presence
in many cases (Figure 2). The article entitled ‘The dog
with the luminous bone’ in the Daily Telegraph sev-
eral years ago is certainly an example of a tetracycline
contamination of bone tissue.
Quality Assurance
0032The validation of a developed method, but also that
of an existing method newly introduced in a lab, is of
absolute importance to assure reliable outcomes. In
fact, international trading requiring certification of
foodstuffs dictates that the performance of analytical
methods be supported by validation data. The topics
that have to be assessed are limit of detection
and quantification, specificity, selectivity, accuracy,
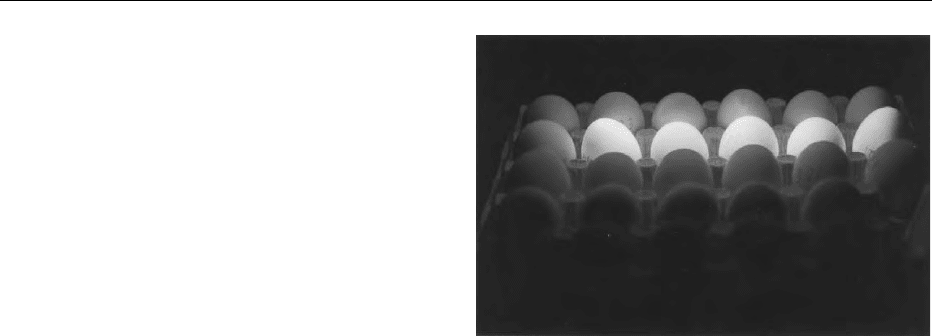
Figure 2 Eggs examined under UV light. The white/yellow
fluorescence of the eggs in the middle is a result of tetracycline
residues complexed with calcium in the eggshell. Positive eggs
were collected from tetracycline treated laying hens. Courtesy of
Dr. Michael Petz and Dr. Georg Zurhelle, University of Wuppertal,
Germany.
ANTIBIOTICS AND DRUGS/Residue Determination 259
precision, reproducibility, repeatability, etc. The ro-
bustness of the method should also attract attention.
Critical points that may influence the measurement
like minor changes in pH, temperature, SPE flow rate,
the use of different SPE batches or of different pro-
ducers, etc. therefore have to be evaluated as well.
0033 In analysis strategies, the predictive value of the
method should be considered. The false-negative
rate of validated screening methods should be less
than 5% (b-error) at the level of interest, whereas
up to 20% false positive outcomes are not appreci-
ated but are generally accepted. In contrast, false-
positive results are not accepted in confirmatory
methods, which usually carry a higher risk with sys-
temic errors and interference. Criteria for the positive
identification and quantification of residues are
increasingly gaining attention. The suitability of con-
firmatory and/or reference methods for registered
veterinary drugs, as proposed, is given in Table 4.In
the case of banned substances, methods have to be
based on molecular spectrometry, and the number of
so-called ‘identification points’ (Table 5) should be at
least four for positive identification.
0034Besides validation of methods, quality-assurance
systems, such as good laboratory practice (GLP) are
also essential for residue laboratories. Such systems
not only ensure clarity on the performance of the
method, but also increase the traceability of the
results. Furthermore, GLP or similar systems assure
appropriate laboratory management and properly
trained analysts.
Outlook in Residue Analysis
0035‘Multianalyte’, i.e., testing various residues in a single
run with a single instrument, is a magical word in the
field of residue analysis. It requires generic extraction
methods and highly resolving and robust analytical
techniques. The fulfillment of this ideal still seems to
be far away. Computerization and miniaturization
and the introduction of SPE cartridges already have
revolutionized residue analysis in the past. Advances
in computerization and miniaturization will certainly
increase sensitivity and selectivity further. This and
further technical improvements in instrumental tech-
niques will make multianalyte analyses possible to an
increasing degree. Automation and analysis within
minutes and even seconds, but also the type of matri-
ces (body fluids and excreta), will transform future
residue analysis labs into clinical chemistry labs.
0036Recent scandals have shown that residue determin-
ation at the slaughter-line stage is not effectual in
protecting public health. The need for high analysis
frequencies to guarantee consumers’ trust is obvious.
However, it is impossible to test every product for all
possible contaminations. Analysis should be per-
formed in the food chain, starting in the stable and
at the feed mill. Laboratory samples should be col-
lected not only from animals, but also from the feed
and drinking water in order to trace cross- or other
accidental contamination. Furthermore, inspection
systems will not focus on the individual animal any-
more, but on the complete herd or flock. Analysis of a
number easily collectable samples will need to reflect
the complete herd and predict the level of contamin-
ation of the animals, so that its products can be
processed and placed on the market accordingly. At
the same time, future veterinary control systems are
being developed that will monitor residue levels to be
in compliance with withdrawal times and MRL
values. The residue lab may therefore be (at least in
part) situated in the slaughterhouse or located in a
vehicle visiting farms, e.g., for certification purposes.
In particular, biosensor systems are attracting consid-
erable attention for such targets. (See Antibiotics and
Drugs: Uses in Food Production.)
tbl0004 Table 4 Suitability of methods for confirmatory analysis of
nonbanned veterinary medicinal products
Technique Criterion
LC or GC in combination with MS Suitable (at least three
identification points)
a
LC-diode array detector Suitable
Two-dimensional
TLC-spectrophotometry
Suitable
GC-electron capture detector Suitable, only if combined
with other methods
LC-immunogram Suitable, in combination
with other techniques
LC-UV/VIS (single wavelength) Suitable, in combination
with other techniques
a
See Table 5 for clarification of identification points. Adapted from the
pending revised EU commission Decision 93/256/EC.
tbl0005 Table 5 Awarding mass-spectrometric approaches for
confirmatory analysis of banned veterinary products with so-
called identification points
a
MS technique Identification points
awarded per ion
Low-resolution (LR) MS 1.0
LR-MS
n
precursor ion
b
1.0
LR-MS
n
transition products 1.5
High resolution (HR) MS 2.0
HR-MS
n
precursor ion 2.0
HR-MS
n
transition products 2.5
a
At least four identification points are necessary for confirmation of
banned veterinary drugs. Adapted from the pending revised EU
Commission Decision 93/256/EC.
b
MS
n
spectra are generated with tandem MS machines (MS
2
) or with
ion-trap mass selectors.
260 ANTIBIOTICS AND DRUGS/Residue Determination

See also: Antibiotics and Drugs: Uses in Food
Production; Chromatography: Thin-layer
Chromatography; High-performance Liquid
Chromatography; Gas Chromatography; Combined
Chromatography and Mass Spectrometry;
Contamination of Food; Quality Assurance and
Quality Control; Spectroscopy: Overview; Infrared and
Raman; Near-infrared; Fluorescence; Atomic Emission
and Absorption; Nuclear Magnetic Resonance
Further Reading
Commission of the European Communities, Directorate
General SANCO (2002) Commission Decision of Imple-
menting Council Directive 96/23/EC concerning the per-
formance of analytical methods and the interpretation of
results. Draft document SANCO/1085/2000 Rev. 7.
Funk W, Dammann V and Donnevert G (1995) Quality
Assurance in Analytical Chemistry. Weinheim: Wiley-
VCH.
Haagsma N and Ruiter A (eds) (1996) Residues of Veterin-
ary Drugs in Food. Proceedings of the EuroResidue III
Conference, Veldhoven, May 3–6. Utrecht: Utrecht Uni-
versity.
Horwitz W (ed.) (2000) Official Methods of Analysis of
AOAC INTERNATIONAL, 17th edn. Arlington, VA:
AOAC International.
Nollet LML (ed.) (1996) Residues and other food com-
ponent analysis. In: Handbook of Food Analysis (vol. 2)
New York: Marcel Dekker.
Oka H, Nakazawa H, Harada K and MacNeil JD (eds)
(1995) Chemical Analysis of Antibiotics Used in Agri-
culture, p. 452. Arlington, VA: AOAC International.
O’Keeffe M (ed.) (2000) Residue Analysis in Food – Prin-
ciples and Applications. Singapore: Harwood Academic
Publishers.
Stephany RW (1989) Molecular spectroscopy in forensic
residue analysis – a general overview of variability, ap-
plicability and cost-effectiveness. Journal of Chromatog-
raphy 489: 3–9.
Van Ginkel LA and Ruiter A (eds) (2000) Residues of
Veterinary Drugs in Food. Proceedings of the Euro-
Residue IV Conference, Veldhoven, May 8–10. Biltho-
ven: National Institute of Public Health and the
Environment.
ANTIOXIDANTS
Contents
Natural Antioxidants
Synthetic Antioxidants
Synthetic Antioxidants, Characterization and Analysis
Role of Antioxidant Nutrients in Defense Systems
Natural Antioxidants
M H Gordon, The University of Reading, Reading, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Natural antioxidants have been of interest for many
years because of their ability to retard the develop-
ment of off-flavors in foods. However, an upsurge in
interest in these components has occurred in recent
years because of their importance for the prevention
of diseases mediated by free radical reactions in vivo.
The onset of a variety of major health problems,
including cancer, atherosclerosis, rheumatoid arth-
ritis, inflammatory bowel disease, immune system
decline, brain dysfunction, cataracts, and malaria
may be delayed by natural antioxidants.
0002 Natural antioxidants are primarily plant phenolic
compounds which may occur in all parts of the
plant. Nonphenolics including carotenoids and
phospholipids may also show antioxidant activity
under some conditions. Plant phenolics are multi-
functional. They can act as radical scavengers, metal
chelators, singlet oxygen quenchers, or reducing
agents. Though they are considered to be safe at
normal levels of consumption they have not been
toxicologically tested in many cases and therefore
the possibility of mutagenic or carcinogenic activity
cannot be discounted for some natural phenolics at
unnaturally high levels of consumption. Some anti-
oxidants add color, aftertaste, or off-flavor to a prod-
uct and this may restrict their use in some types of
foods.
0003Natural antioxidants can be found in a wide range
of food raw materials. Tocopherols and related com-
pounds occur at levels of about 50–1000 mg kg
1
in
vegetable oils. Animal fats contain very low levels
(e.g., butter oil 20–50 mg kg
1
), but cereal germ oils
are very rich sources (1500–5000 mg kg
1
).
ANTIOXIDANTS/Natural Antioxidants 261
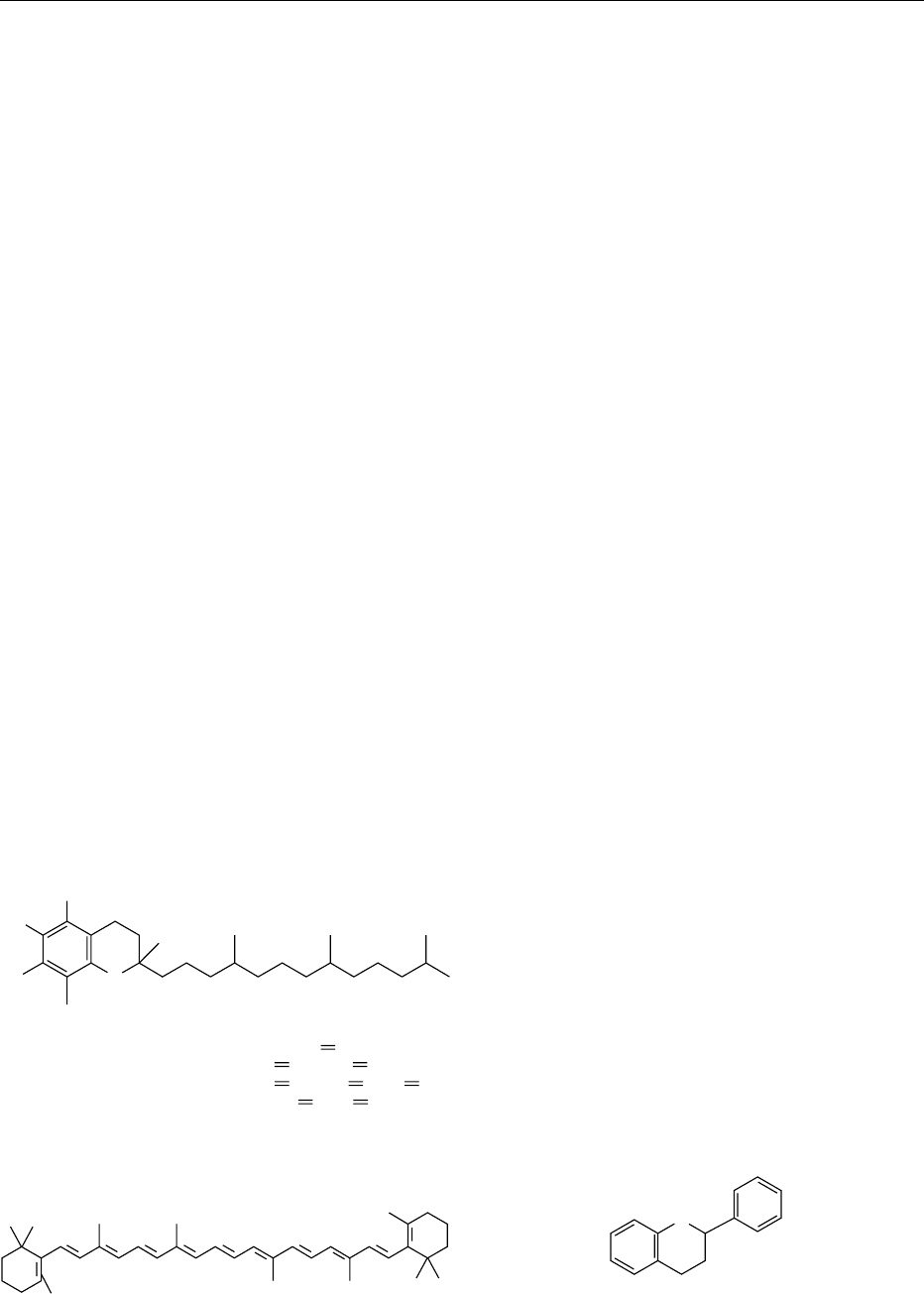
0004 Tocopherols are added to some foods as anti-
oxidants. They occur with various degrees of
methylation of the dihydrochromanol ring as a, b, g
and d-tocopherol. They have the chemical structure
shown in Figure 1.
0004 The activity of the tocopherols depends on their
concentration, the food system, and the presence of
heavy metals since they can act as prooxidants with
iron or copper. Tocopherols are strongly effective as
antioxidants in emulsions, but they are less effective
in oils. Their antioxidant activity under common test
conditions in oils decreases from d-tocopherol>
g-tocopherol > b-tocopherol > a-tocopherol. They are
most effective in the presence of a synergist like as-
corbic or citric acid. In biological systems, however,
a-tocopherol or vitamin E is the most active anti-
oxidant with a strong antioxidant effect in biological
membranes. Tocopherols are commercially produced
and they are used as food additives. (See Tocopherols:
Properties and Determination.)
0009 About 50 carotenoids can be found in the human
diet as minor components of fruit and vegetables.
Carotenoids, including b-carotene (Figure 2), demon-
strate antioxidant properties under certain condi-
tions. Due to their multiple conjugated double-bond
system, the carotenoids act as singlet oxygen
quenchers during photosynthesis. They are also able
to scavenge free radicals and act as antioxidants at
low partial pressure of oxygen.
0010 There is considerable interest in the nutritional
properties of carotenoids. Besides the provitamin A
property of some of the carotenoids, the antioxidant
properties may also be of nutritional significance.
Epidemiological studies have found that consumption
of fruit and vegetables, which contain carotenoids, is
associated with a reduced risk for several chronic
diseases, including cardiovascular and photosensitiv-
ity diseases, cataracts, and some cancers. However,
the results from large controlled trials involving
b-carotene supplementation do not always support
these beneficial associations, providing in some
cases evidence for adverse effects, including an
increase in the incidence of lung cancer and overall
mortality in smokers.
0011It has been suggested that other carotenoids,
including a-carotene, lycopene, lutein, and zea-
xanthin, may show a higher potency than b-carotene
in the suppression of the development of various
types of cancer, including lung, breast, and stomach
cancer. Dietary consumption of tomato products con-
taining lycopene is associated with reduced risk of
cancer and coronary heart disease. Although the anti-
oxidant properties of lycopene may be responsible for
its beneficial properties, other mechanisms, including
effects on intercellular gap junction communication,
or on the hormonal or immune system, may be partly
responsible. (See Carotenoids: Occurrence, Proper-
ties, and Determination.)
0012Flavonoidsareanimportantclassofnaturalantioxi-
dants that occur in a wide variety of fruit, vegetables,
leaves, and flowers. They are found mainly as glyco-
sides and methylated derivatives. The most important
subgroups are the colorless catechins, the red-purple-
colored anthocyanidins, the yellow flavonols and fla-
vones, as well as the colorless proanthocyanidins.
0013The flavonoids share a common C
6
-C
3
-C
6
skel-
eton. The flavonoid aglycone normally consists of
an aromatic ring (A) condensed with a six-membered
oxygen-containing ring (C) which carries a phenyl
substituent in the 2-position (B). The six-membered
ring condensed with the benzene ring is either a
g-pyrone ring (flavonols and flavones) or its dihydro
derivatives (flavanols and flavanones).
Flavonoid Structure(Figure3)
0014The position of the attachment of the B-ring to
the central C-ring divides the flavonoid class into
5,7,8-Trimethyltocol (α-tocopherol)
7,8-Dimethyltocol (β-tocopherol)
5,8-Dimethyltocol (γ-tocopherol)
8-Methyltocol (δ-tocopherol)
R
1
,R
2
,R
3
CH
3
R
1
H, R
2
,R
3
CH
3
R
1
CH
3
, R
2
H, R
3
CH
3
R
1
,R
2
H, R
3
CH
3
HO
R
1
5
6
7
8
2
O
4' 8'
R
3
R
2
fig0001 Figure 1 Tocopherol structure.
fig0002 Figure 2 Structure of b-carotene.
O
3'
4'
5'
6'2
3
45
6
7
8
2'
A
B
fig0003Figure 3 Flavonoid structure.
262 ANTIOXIDANTS/Natural Antioxidants
flavonoids (2-position) and isoflavonoids (3-pos-
ition). Flavonols including quercetin and myricetin
are important natural antioxidants. Quercetin occurs
as the glycoside quercetrin in onions, apple skins, and
black tea. Anthocyanidins differ from the other fla-
vonoids in having a charged oxygen in the C-ring.
The C-ring is open in the chalcones. Flavonoids are
often hydroxylated in position C-3,5,7,3
0
,4
0
or 5
0
.
Flavonol and Flavone Structure(Figure4)
0019 Attention was focused on the nutritional significance
of dietary flavonols and flavones by the Zutphen
Elderly Study in The Netherlands. The study started
in 1960 with 878 men aged 40–59 years and con-
tinued until 1985 with 555 of the original subjects
and 250 replacements aged 65–84 years. Nutritional
intake was studied by cross-checked dietary history
for 5 years. The mean baseline of flavonoid intake
was 25.9 mg day
1
(as aglycones). The major flavo-
noids in the food were quercetin, myricetin, kaemp-
ferol, apigenin, and luteolin. Their sources of intake
were mainly tea (61%), onions (13%), and apples
(10%).
002 0 After adjustment for age, body mass index,
smoking, serum total and high-density lipoprotein
cholesterol, blood pressure, physical activity, coffee
consumption, and intake of other antioxidants and
fiber, the flavonoid intake was significantly inversely
associated with mortality from coronary heart disease
and showed an inverse relationship with the incidence
of myocardial infarction.
002 1 In Mediterranean countries, wine is an important
source of flavonoids (10–20 mg l
1
combined flavo-
noids). This may partly contribute to the low risk of
coronary heart disease of wine drinkers who consume
a high level of saturated fats in France, the so-called
French paradox.
002 2 Tea catechins are very strong antioxidants. For
the tea catechins, stronger activity than BHA or
a-tocopherol in lard autooxidation systems was
shown with activity increasing in the order:
( )-epicatechin < ()-epicatechin gallate< ()-epi-
gallocatechin < ()-epigallocatechin gallate (Figures
5–8).
R
1
R
2
OH
X
B
A
O
OOH
HO
quercetin R
1
OH, R
2
H
myricetin R
1
,R
2
OH
quercitrin R
1
OH, R
2
H
luteolin R
1
OH, R
2
H
Flavonol aglycone: X OH
Flavonol: X glucose
Flavone aglycone: X H
fig0004 Figure 4 Flavonol and flavone structure.
HO
OH
O
OH
OH
OH
fig0005Figure 5 Epicatechin.
HO
OH
O
O
O
C
OH
OH
OH
OH
OH
OH
fig0008Figure 8 Epigallocatechin gallate.
HO
OH
O
OH
OH
OH
OH
fig0007Figure 7 Epigallocatechin.
HO
OH
O
O
O
C
OH
OH
OH
OH
OH
fig0006Figure 6 Epicatechin gallate.
ANTIOXIDANTS/Natural Antioxidants 263
