Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


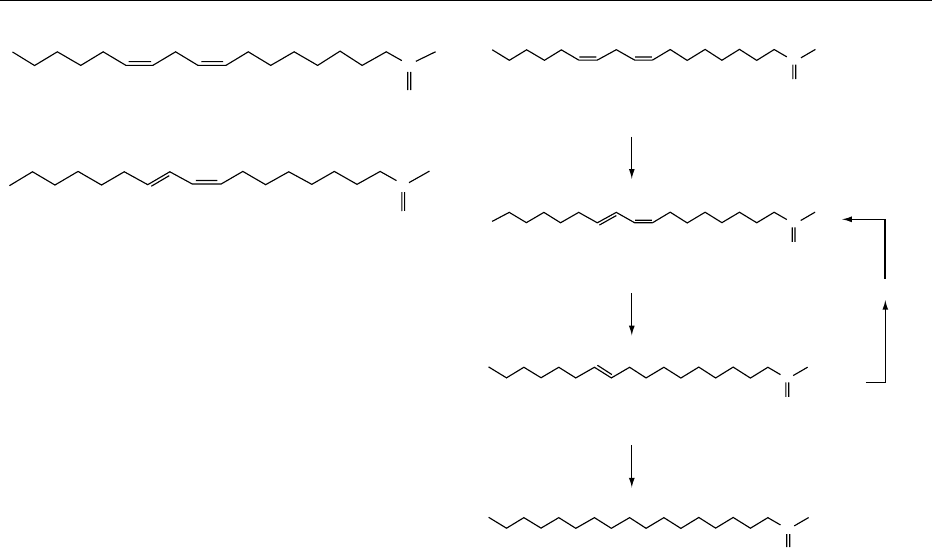
components. In recent years, the production of filled
milks has become increasingly important. In this case,
the milk fat is replaced by locally available and
cheaper vegetable fats.
Storage and Packing
0041 An adequate keeping quality of up to 12 months can
be achieved for both sweetened condensed milk and
evaporated milk, but this largely depends on the stor-
age conditions. At a storage temperature above 25
C,
the aging process and related physical, as well as
organoleptic, defects appear more rapidly. For recom-
bined products, the normal shelf-life stability, without
major deviations from the original aspect, is reduced
by about half of a fresh-milk product. (See Storage
Stability: Parameters Affecting Storage Stability.)
0042 The most commonly used packing is the tin plate
can, which offers absolute protection against light, is
basically crash proof, and has advantages for handling
and storage. Recycling is also an important factor. As
alternative packing, glass containers or paper/plastic
laminates can be used; however, depending on the
packing material, a considerable shelf-life reduction
should be taken into account. For paper/plastic lamin-
ates, aseptic filling techniques are used, especially for
evaporated milk. (See Canning: Principles.)
Nutritional Considerations
0043 Owing to the concentrated form, both sweetened
condensed milk and evaporated milk have an in-
creased compositional analysis compared with milk
(Table 1). For sweetened condensed milk, the figure
for carbohydrate includes the sucrose content neces-
sary for product conservation; the carbohydrate in
evaporated milk and unconcentrated milk is lactose
only. The rather high energy value of sweetened con-
densed milk is due mainly to the amount of carbohy-
drates present, whereas the protein and fat contents
compare more or less with evaporated milk. (See
Milk: Dietary Importance.)
Production and Usage
0044 World production amounts to over 4.5 million
tonnes. About one-third is produced as sweetened
condensed milk and two-thirds as evaporated milk.
As recently as 35–40 years ago, infant feeding was
still a major application for either sweetened or un-
sweetened concentrated milk. Owing to the nature of
the products, somewhat distinct usages are common
practice.
Sweetened Condensed Milk
0045Owing to the high sucrose content of > 40% and its
viscous consistency, this product is frequently used as
a jam-like bread spread.
0046An early, but no longer popular, use of sweetened
condensed milk was to dilute it with water and con-
sume it as a drink. Presently, coffee or tea whitening
and sweetening is the major use of sweetened con-
densed milk. In many countries, it is also used in
combination with cocoa or other milk modifiers in
the preparation of homemade drinks.
0047A growing application is in sweet dessert prepar-
ations like icecreams, cakes, cookies, etc. Specific to
the Latin American continent is its use for Dulce de
Leche, basically a caramelized sweetened condensed
milk obtained by boiling the can in water for 2–3h.
Different applications vary from country to country
and from one geographical region to another,
according to traditional consumption habits.
Evaporated Milk
0048A large area of application is coffee and tea whitening
and the preparation of milk-based beverages. The
other main field for general usage is in the culinary
sector to enhance the taste and texture of mashed
potatoes, pasta, quiches, soups, and a wide variety
of savory and sweet recipes. The main uses can also
vary specifically from one country to another.
Manufacturing Principles
0049The raw material used for both products is usually
cows’ milk, although in certain regions, it may be a
mixture of cows’ and buffalos’ milk. (See Buffalo:
Milk.)
Sweetened Condensed Milk
0050A flow diagram for the manufacture of sweetened
condensed milk is shown in Figure 1.
tbl0001 Table 1 Typical comparative figures for standard concentrated products and milk (all values in grams per 100 g)
Product Protein Fat Carbohydrates Minerals (ash) Calcium Phosphorus
Sweetened condensed milk 7.8 8.0 55.2 1.8 0.28 0.23
Evaporated milk 6.5 7.5 9.8 1.4 0.24 0.19
Milk 3.2 3.5 4.6 0.7 0.12 0.09
1576 CONDENSED MILK
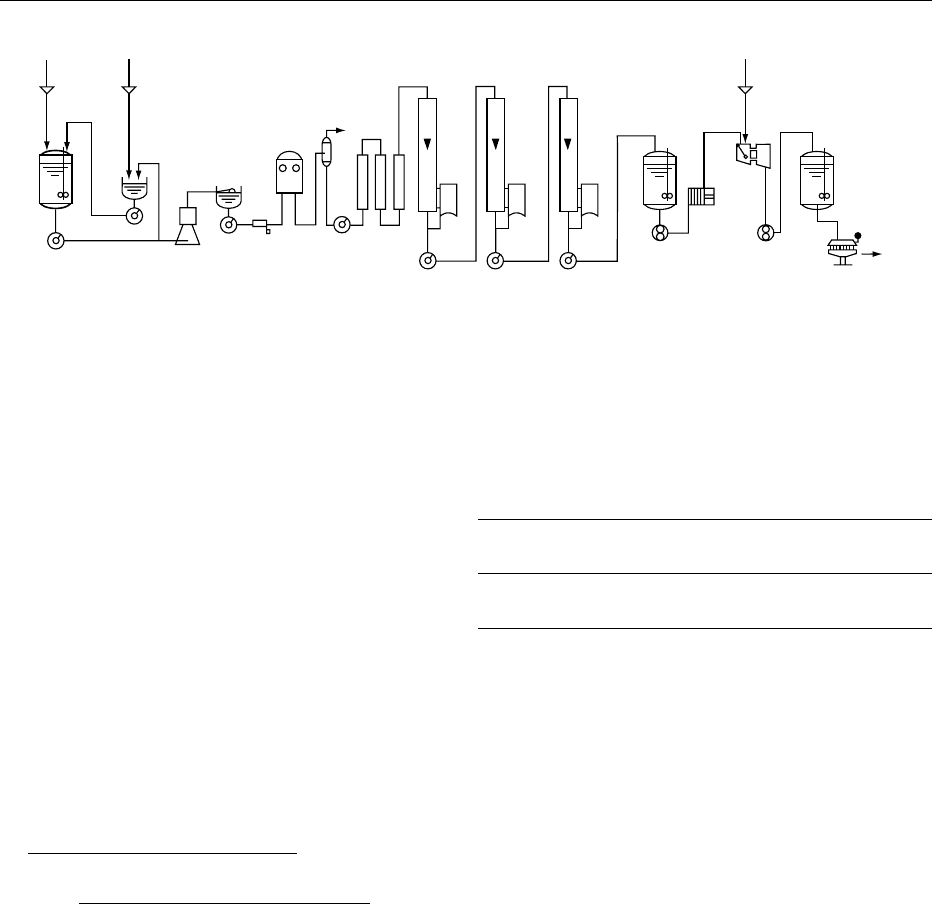
0051 Raw milk is collected and selected according to the
usual quality criteria. The milk is analyzed for fat and
solids-nonfat (SNF) content and the ratio between
both components is adjusted according to the fat/
SNF ratio of the final product to be manufactured
by the addition of either cream or skim milk.
0052 If the final product should have a composition of
8.0% fat and 21% SNF, the raw milk should be
adjusted to the same fat/SNF ratio (see Table 2).
0053 Sugar is dissolved in the cold milk, in principle by
recirculation through a dissolving vat. The amount of
sugar is determined by the quantity of milk prepared
for a batch standardization, its fat content, and the
compositional requirement of the sucrose content of
the final product, e.g.,
10 000 kg of milk 3:25% fat
100
sugar factor
sugar content of finished product
Fat
:
After standardization, the milk/sugar mixture under-
goes a heat treatment, which consists of a tempera-
ture/time combination usually above 100
C and
holding for seconds or minutes. This heat treatment
eliminates nearly all the bacteriological flora of the
milk, but the choice of temperature/time combination
greatly influences final product characteristics such
as the consistency and shelf-life stability. (See Heat
Treatment: Chemical and Microbiological Changes.)
0054 The viscosity of the product is an important
factor. It is rather difficult to indicate precise heat
treatment conditions, since they depend on the equip-
ment used and the characteristics of the raw material
milk which can produce different behavior through
regional, climatic, and seasonal influences. Constant
observation of viscosity and age thickening is neces-
sary to counteract any negative effect. In general,
lower heat treatments favor increased viscosities,
and higher, intensive heating tends to give lower vis-
cosities. The viscosity should be sufficient to avoid
phase separation during storage, and age thickening
should be moderate in order to keep the product
pourable during its storage life.
0055Concentration after heat treatment takes place
under vacuum and usually in a multiple-effect
evaporator.
0056Sweetened condensed milk is not a sterilized or
sterile product; its conservation is assured by the
bacteriostatic effect of the high sucrose concentra-
tion. The sugar-in-water ratio in the concentrated
product must be above 61%. However, osmophilic
organisms can develop in this medium, so it is import-
ant that the equipment after heat treatment and up to
filling is of a hygienic design and is operated under the
best hygienic conditions to avoid reinfection. Strict
controls for equipment cleaning and sterilization are
essential.
0057After evaporation, the concentration must be as
close as possible to the final product’s total solids
content; an adjustment by, for example, water should
be omitted because of the risk of contamination.
0058After evaporation, the product is cooled to
normally 20–25
C, and at the final concentration,
part of the lactose contained in the product is
Fresh milk Sugar
Lactose
Cooling/seeding
Storage/fillingHoldingEvaporationHeat treatment
123
Standardization
fig0001 Figure 1 Basic flow diagram for the manufacture of sweetened condensed milk (continuous process). Reproduced from Condensed
Milk, Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
tbl0002Table 2 Example adjustment of the fat and SNF content of raw
milk to achieve a final product composition of 8.0% fat and 21%
SNF
Finalproduct Ratio Standardized
milk
Ratio
Fat (%) 8.0
0.381
3.25
0.381
SNF (%) 21.0 8.52
CONDENSED MILK 1577
oversaturated. To avoid autocrystallization, fine-
milled and pasteurized dry lactose crystals are added
to the concentrate to initiate instant and controlled
crystallization. During storage, product stirring
should be maintained for several hours to finalize
the crystallization process.
0059 Filling, usually into metallic tin plate cans, is the
last delicate operation. Cans and lids must be steril-
ized, and this is often achieved by passing them
through a flaming installation. Air in the filling area
must be filtered and of an excellent bacteriological
quality; the air space in the can must be as low as
possible to restrict mold growth. It is not necessary to
perform an aseptic operation, but excellent hygienic
conditions must prevail.
Evaporated Milk
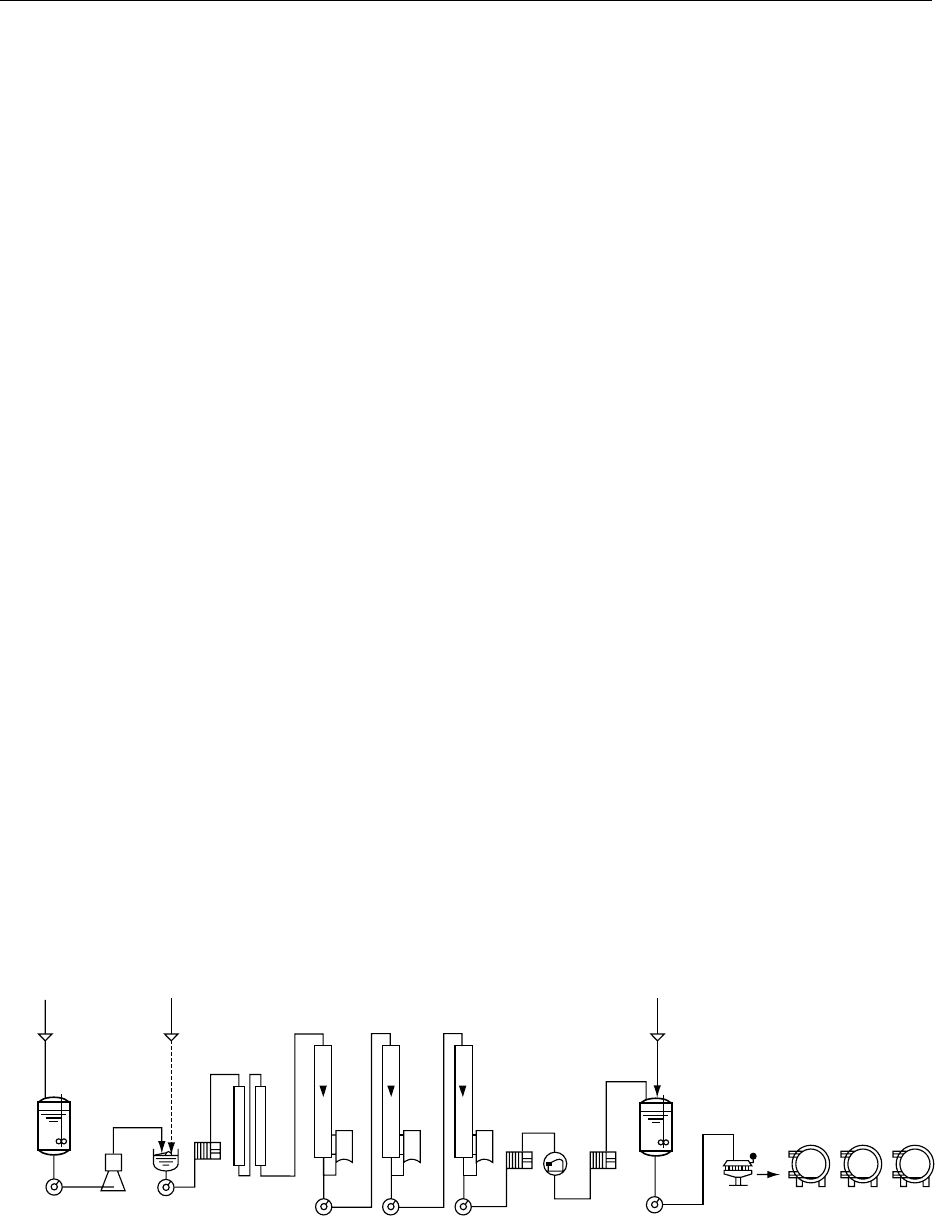
0060 A flow diagram for the manufacture of evaporated
milk is given in Figure 2.
0061 In principle, this product is poststerilized, which is
a conventional process, whereby after filling and
closing operations, the product is sterilized in the
final container (e.g., tin plate cans, glass bottles, etc.).
0062 For stability reasons, the product contains a small
percentage of stabilizing salts (either one or a com-
bination of the earlier-mentioned salts). Where legis-
lation allows, the hydrocolloid, carrageenan, may
be added. Stabilizing salts are indispensable as manu-
facturing and technological aids for adjusting or
regulating the heat stability of the product during
the poststerilization process. Carrageenan influences
phase stability (creaming and protein sedimentation)
during the product’s storage life.
0063 During the last 20 years, aseptically filled evapor-
ated milks, mainly in soft packs, have been developed
and commercialized. To realize aseptic filling, the
product has to be heat-treated in-line for final steril-
ization. Ultrahigh temperature (UHT)-type installa-
tions are used for this purpose. (See Heat Treatment:
Ultra-high Temperature (UHT) Treatments.)
0064The processing steps for milk standardization, heat
treatment, and concentration/evaporation are similar
or identical to those described for sweetened con-
densed milk, with the exception of the addition of
sugar.
0065After concentration, evaporated milk is homogen-
ized, cooled for intermediate storage, and sterilized
after filling.
0066Heat treatment/heat stability The ability of concen-
trated milk to withstand high-temperature steriliza-
tion is essential. This is commonly described as heat
stability and refers to the resistance of milk concen-
trate to coagulation during sterilization in containers.
0067Milk has a natural heat stability, which is influ-
enced mainly by compositional factors, like mineral
salt content, protein content, degree of acidity, etc.
The natural heat stability also varies according to
season and lactation period. In order to obtain milk
sterilizable at different levels of concentrations, this
natural heat stability must be improved and adapted.
In practice, this is achieved by temperature/time com-
bination of preliminary heat treatments on the milk
before concentration and the addition of certain
mineral salts. Precise indications are rather difficult,
owing to the various influencing factors. The optimal
heat stability has to be found by an empirical ap-
proach. In principle, more intensive heat treatments
increase the heat stability, but above optimum condi-
tions, a reverse effect is attained.
0068It must be understood also that processing oper-
ations, such as concentration and homogenization,
Stabilizing
salts
Filling Sterilization
Homogenization/cooling
StorageEvaporationHeat treatment
123
Standardization
Standardized
milk
Carrageenan
fig0002 Figure 2 Basic flow diagram for the manufacture of evaporated milk. Reproduced from Condensed Milk, Encyclopaedia of Food Sci-
ence, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
1578 CONDENSED MILK

have a destabilizing effect on the concentrate. The
various operations must be balanced so as to ensure
a product of optimum quality.
0069 Concentration After preheating, the milk is evapor-
ated under vacuum. It is of utmost importance that
the evaporator works under optimal hygienic condi-
tions, despite the fact that the product is sterilized
afterwards. In general, falling film evaporators are
commonly used today.
0070 Homogenization In order to obtain a satisfactory
homogenization effect, the homogenizer and, in par-
ticular, the homogenizing valves must be kept con-
stantly in the best mechanical conditions.
0071 The homogenization temperature should prefer-
ably be around 65
C.
0072 A homogenizing pressure of 200–250 bar is usu-
ally applied, and the best results are obtained by a
two-valve system in series. In principle, the second
valve is adjusted to 20–25% of the total pressure.
Excessive pressure has a destabilizing effect, which
is irreversible. Following homogenization, the
product is cooled for intermediate storage.
0073 Pilot sterilization It is advantageous to fill some
cans and to sterilize them under the given conditions
in order to verify the heat stability of the concentrate.
Corrective actions are further possible by addition
of stabilizing salt and adaptation of the sterilizing
conditions.
0074 Sterilization After filling into containers (cans), the
product is sterilized. The purpose of sterilization
is to obtain physical and bacteriological stability.
According to practical application, one speaks of
commercial sterility.
0075 The effect of sterility is expressed by the F value
as an integral function of the lethality of the micro-
organisms present, related to C. botulinum, at the
specific destruction temperature of 121
C. Usually,
an F value of 4 is considered satisfactory.
0076 Today, modern instruments are available for
measuring the time/temperature profile within any
type of sterilizer.
0077 Usually, rotary and continuous sterilizers are used.
Major Product Defects
Bacteriological Problems
0078 For evaporated milk, the sterilization processes
usually applied are proven and sufficiently safe to
guarantee product safety and commercial sterility. If
problems arise, they are mainly traced back to an
insufficient can integrity. Faulty soldering, welding
of can bodies, or closure seams are the weakest
points. However, the presence of excessive numbers
of thermophilic spores can cause severe spoilage.
Appropriate line and finished product controls must
be put in place. (See Spoilage: Bacterial Spoilage.)
0079Sweetened condensed milk is less vulnerable to
spoilage because of its high sugar content, but it is
not protected against osmophilic organisms. Plant
and manufacturing hygiene are the keys to success.
Physical Instability
0080Instability may be of various individual defects, some
of which may be interrelated. Separation problems
can, and mainly are, a result of inadequate product
viscosities. Certain texture problems may be related
to nonoptimal heat treatments, but for sweetened
condensed milk, it may also be a result of coarse
lactose crystallization.
0081Age thickening is a constant problem for sweetened
condensed milk. The increase of viscosity over the
storage time is always present and, from a manufac-
turing point of view, can be corrected only by an
optimal heat treatment of the milk prior to concen-
tration. Considerable practical experience is required
to keep this phenomenon under control.
0082Age gelation of evaporated milk is a similar phe-
nomenon to age thickening for sweetened condensed
milk but often appears only and suddenly after long
storage times. The product can remain with normal
physical properties up to 10 months or more and can
then thicken within a few weeks. This gelation is
strictly a storage defect and should not be confused
with thickening or coagulation during the steriliza-
tion process or with coagulation resulting from
microbiological activity. The main factors influencing
age gelation are, as above, insufficient and nonopti-
mal preheating conditions of the milk before evapor-
ation, marginal sterilization conditions, and an
insufficient stabilizing salt level.
0083Inferior raw milk quality and final product storage
conditions can also play an important role within the
complex context of age gelation or thickening for
either evaporated or sweetened condensed milk.
Products Manufactured by Recombining
0084As already mentioned, traditional dairy products like
sweetened and unsweetened condensed milk are
made by recombination using skim milk powder
and anhydrous milk fat for the dairy components.
The following is a brief description of these processes.
(See Recombined and Filled Milks.)
0085When recombining sweetened condensed milk (see
Figure 3), according to a process using only flash
CONDENSED MILK 1579
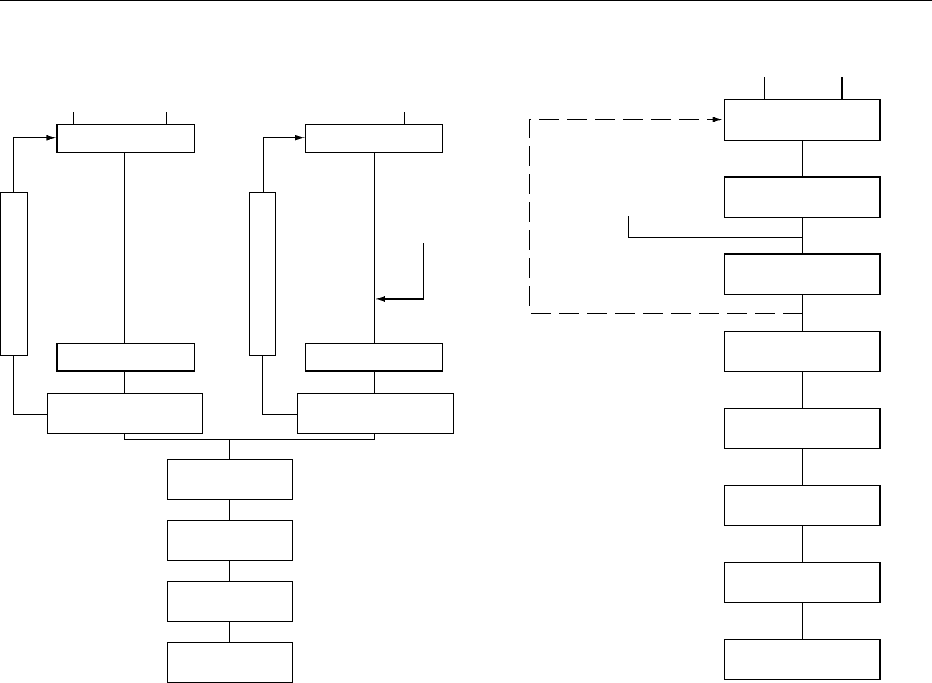
cooling evaporation, the skim milk solids have to be
dissolved at a concentration of 40%, where some
formation of lumps is practically unavoidable. How-
ever, these lumps are easily dispersible by mechanical
force, as obtained through a colloid mill. A recircula-
tion system has a beneficial effect on skim milk
powder hydration.
0086 In principle, dissolving sugar in a low-temperature
medium does not pose a major problem but is some-
what time-consuming. Therefore, the circulated
solution is heated after powder dosage in order to
make the sugar dissolve rapidly.
0087 A slightly simplified process for recombining can
be used for evaporated milk (see Figure 4).
0088 Dissolving skim milk powder at a low concentration
generally poses less problems. Skim milk powder is
certainly the most critical raw material and, depending
on its characteristics, directly affects the physical
properties of the final recombined product. This influ-
ence depends to a large extent on the heat treatment
given to the liquid skim milk before drying. Seasonal
and regional influences may also play a role.
0089Since milk proteins are sensitive to heat, the extent
of their denaturation reflects the heat treatment ap-
plied and is used for classifying skim milk powders.
The latter have been classified by the American Dairy
Products Institute into three groups according to the
level of undenatured whey protein nitrogen present in
the powder after manufacture. In general terms, this
is expressed as the whey protein nitrogen index (WPN
index) (see Table 3). Whereas the high-heat-type
powders are, in principle, the most appropriate for
sterilized products, the low-heat range powders are
usually more suitable for nonsterilized recombined
products such as sweetened condensed milk.
0090Anhydrous milk fat is the principal source of fat
used in recombined dairy products. It contributes
significantly to the taste and milky character of the
product. This is a clear indication for the quality
requirements for this raw material. In certain cases,
Mixing vessel Mixing vessel
Butteroil
Sugar
Colloid mill Colloid mill
Pasteurization
Flash cooling
evaporation
Lactose seeding
Storage and
filling
Without heating
Heating to 658 C
Intermediate storage
(308 C)
Water
1ST PHASE 2ND PHASE
Skim milk
powder
Intermediate storage
(508 C)
fig0003 Figure 3 Flow chart for the recombination of sweetened con-
densed milk. Reproduced from Condensed Milk, Encyclopaedia of
Food Science, Food Technology and Nutrition, Macrae R, Robinson
RK and Sadler MJ (eds), 1993, Academic Press.
Water
Mixing vessel
total solids 25−27%
Heating
45−558 C
Colloid mill
Butteroil
Deaeration
Pasteurization
Homogenization
Cooling
Storage before
filling/sterilization
Skim milk
powder
fig0004Figure 4 A flow chart for the recombination of evaporated milk.
Reproduced from Condensed Milk, Encyclopaedia of Food Science,
Food Technology and Nutrition, Macrae R, Robinson RK and Sadler
MJ (eds), 1993, Academic Press.
1580 CONDENSED MILK
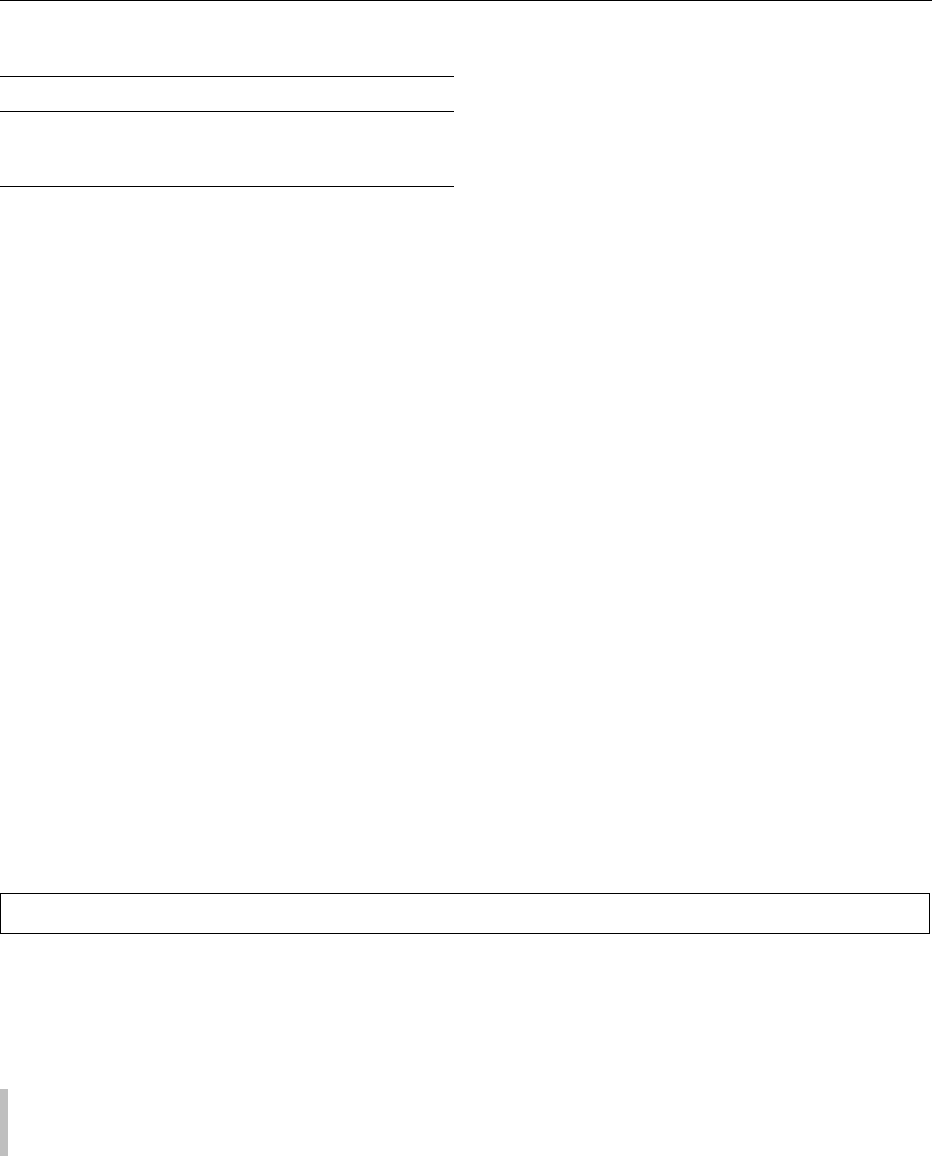
vegetable oils are used as butterfat substitutes, pri-
marily for economic reasons. Such products are inter-
nationally recognized as ‘filled milks.’ The vegetable
oils used in filled milks should be double-refined and
deodorized and have a low peroxide value.
0091 Water is a basic ingredient of all recombined dairy
products. In general, a good drinking water quality is
sufficient and acceptable. However, as water taste,
odor, and possibly also color may influence the final
product, special monitoring of water quality is
important.
0092 Buttermilk powder can be and is used in recom-
bined products. From the technological point of view,
buttermilk powder is an emulsifying aid for the fat,
since it contains a relatively large amount of the
phospholipids that are lost in the separation process
of skim milk and anhydrous milk fat. For most appli-
cations, only sweet buttermilk is suitable; buttermilk
powder obtained from acidified cream for butter pro-
duction is not suitable. (See Emulsifiers: Uses in Pro-
cessed Foods.)
0093 As the natural vitamin content of fresh milk is
usually reduced slightly during processing of the raw
materials for recombining, vitamins A, D, and B are
usually added. For filled milks, further addition of
vitamin E is carried out and is recommended for
nutritional purposes.
0094Various processing aids, mainly phosphates but
also other emulsifiers or stabilizers, are added to
achieve specific product characteristics and consist-
encies.
0095All additives must be in accordance with legal pre-
scriptions or comply with the Food and Agriculture
Organization/World Health Organization standard
(Code of Principles). (See Legislation: Additives.)
See also: Buffalo: Milk; Canning: Principles; Heat
Treatment: Ultra-high Temperature (UHT) Treatments;
Chemical and Microbiological Changes; Legislation:
Additives; Milk: Dietary Importance; Recombined and
Filled Milks; Spoilage: Bacterial Spoilage; Storage
Stability: Parameters Affecting Storage Stability
Further Reading
Codex Alimentarium (1984) Rome: Food and Agriculture
Organization of the United Nations/World Health
Organization.
Hunziker OF (1935) Condensed Milk and Milk, 5th edn.
La Grange, IL: Hunziker.
Pizzoferrato L, Manzi P, Vivanti V et al. (1998) Mailland
reaction in milk-based foods: nutritional consequences.
Journal of Food Protection 61: 235–239.
Ramonaityte DT (2001) Copper, zinc, tin and lead in
canned evaporated milk, produced in Lithuania: the
initial content and its change at storage. Food Additives
and Contaminants. 18(1): 31–37.
Webb BH, Johnson AH and Alford JA (1974) Fundamen-
tals of Dairy Chemistry. Westport, CT: AVI.
Confectionery See Sweets and Candies: Sugar Confectionery
CONJUGATED LINOLEIC ACID
C E Fernie, Scottish Crop Research Institute,
Invergowrie, Dundee, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
What is Conjugated Linoleic Acid (CLA)?
0001 Conjugated Linoleic Acid (CLA) is the collective name
given to a group of 18:2 free fatty acid positional and
geometric isomers, containing two conjugated double
bonds (Figure 1). Most fatty acids commonly occur-
ring in nature contain methylene-interrupted double
bonds, e.g., cis-9, cis-12 18:2 (linoleic acid). However,
conjugated dienes have been known for more than 50
years. They are seen as the products of both autoox-
idation and of partial hydrogenation of polyunsatur-
ated fatty acids, as well as being minor components of
dairy and ruminant lipids. There is increasing interest
in CLA, as in animal trials, it has shown potentially
beneficial effects on a variety of medical conditions
such as cancer and type 2 diabetes, as well as affecting
body composition parameters such as bone compos-
ition and lean body mass. As a result, this is an area of
tbl0003 Table 3 WPN index (values are in milligrams of nitrogen per
gram of powder)
Classification WPNindex
High heat 1.5
Medium heat 1.51–5.99
Low heat 6.0
CONJUGATED LINOLEIC ACID 1581
continuing growth in research in a wide variety of
scientific fields. This review will attempt to give an
overview of the current state of knowledge in this
developing area of interest.
Occurrence in Natural Products
0002 The most common source of natural CLA in the
human diet is from the meat and milk products of
ruminant animals. CLA isomers are present in these
materials at levels of approximately 0.5% of total
fat on average, although seasonal variations are
observed. The predominant isomer is the cis-9,
trans-11 acid, at levels of 80–90% of the total CLA,
with minor amounts of a wide number of other
isomers present, ranging from the 6,8 through to the
13,15 positional isomers, in almost all of the different
geometric configurations possible (i.e., cis, cis; cis,
trans/trans, cis and trans, trans), particularly in milk
fat and cheese. Biohydrogenation of linoleic acid is
thought to be the main mechanism of formation of
natural CLA. The process involves the action of the
ruminant bacterium Butyrivibrio fibrisolvens on diet-
ary linoleic acid (via linoleic acid isomerase) to give
CLA as an intermediate in the formation of trans-11
18:1 (vaccenic acid) and subsequently 18:0 (stearic
acid). As levels of CLA produced by this mechanism
do not seem to be high enough to account for the
amounts of CLA in ruminant tissues, a second mech-
anism has been proposed, involving the action of the
D-9 desaturase enzyme on vaccenic acid within adi-
pose and mammary tissues to give the cis-9, trans-11
CLA isomer (Figure 2).
0003 The variety of conjugated isomers in ruminant milk
and tissue is thought to be due to a combination of
double bond migration, and the action of specific cis,
trans isomerases in the rumen.
0004 Altering the feeding regime of the animals can vary
the levels of CLA in ruminant fats. Pasture-fed
animals have higher levels of CLA than those given
commercial feed. However, feed enriched with unsat-
urated fatty acids can also increase the CLA levels in
milk and meat. There can be wide variations in CLA
production between animals of different breeds, and
at different stages of lactation. This may relate to the
D-9 desaturase level of the individual animals.
0005CLA has been reported in fish, and its occurrence in
farmed fish is probably due to the CLA content of
vegetable oil in fish food. However, there is concern
that CLA can be misidentified in fish tissues, as it
elutes on some gas chromatographic columns in the
same region as the naturally occurring 18:4 isomers.
This may have been the case in early reports of low
levels of CLA in trout, shrimp, and mussels, as cur-
rently no mechanisms are known to account for CLA
formation in marine foods.
0006CLA is also present in small amounts, as a result of
the hydrogenation process, in margarines, cooking
oils and other partially hydrogenated fats.
Commercial Production
0007Mixtures of CLA isomers are available commercially,
for use in scientific studies and as reference standards,
and also as dietary supplements. These products,
which are usually in the free fatty acid form, are
typically produced by the alkaline isomerisation of a
OH
C
O
12
3
46
57
8
910
11
12
13
14
15
16
17
18
Linoleic acid
18
17
1614
1312
11
109
8
7
6
5
4
3
21
C
O
OH
Cis-9, trans-11 con
j
u
g
ated linoleic acid
15
fig0001 Figure 1 Linoleic acid is a methylene-interrupted fatty acid, i.e.,
it has a CH
2
group between the two double bonds, at position 11.
CLA is a conjugated fatty acid as there are no groups between the
double bonds in the carbon chain.
18
17151311 9 7 5 3
O
C
OH
16141210 8 6 4 21
18
171513 11
171513
171513
11
11
9753
9753
O
C
OH
OH
OH
16141210
181614
181614
1210
1210
86421
O
C
18642
97 5 3
O
C
18642
18:0
(stearic acid)
Trans-11 18:1
(vaccenic acid)
Cis-9, trans-11 18:2
(Conjugated linoleic acid)
Cis-9, cis-12 18:2
(linoleic acid)
∆-9 desaturase
fig0002Figure 2 Biohydrogenation of linoleic acid to produce CLA,
both as an intermediate towards stearic acid and by desaturation
of vaccenic acid.
1582 CONJUGATED LINOLEIC ACID

linoleate-rich vegetable oil, such as sunflower or saf-
flower oil. The CLA produced from this process in
aqueous solution generally contains four main CLA
isomers: trans-8, cis-10; cis-9, trans-11; trans-10,
cis-12, and cis-11, trans-13. Under harsh reaction
conditions, these isomers can be present in approxi-
mately equal proportions. It is possible, however, to
manipulate the reaction temperature and time to give
a mixture that is greater than 90% of the cis-9, trans-
11 and trans-10, cis-12 isomers, in approximately
equal proportions. This is more desirable, particu-
larly for supplement usage. By using propylene glycol
instead of water, with an appropriate basic catalyst
(e.g., potassium hydroxide), the amounts of the minor
isomers can be reduced to around 1%, but small
amounts of isomerization will always occur.
0008 The processes described above all give mixtures of
CLA isomers. It is also possible to obtain CLA
enriched in one particular isomer. Enzymes derived
from fungi such as Candida rugosa and Geotrichum
candidum show a preference for the cis-9, trans-11
isomer, and, by preferentially esterifying this CLA
isomer, give an unesterified fraction that is rich in
the trans-10, cis-12 isomer. This has been demon-
strated on a laboratory scale, but has yet to be opti-
mized for commercial production. Alternatively, a
series of selective crystallisations can be used to frac-
tionate the cis-9, trans-11 and trans-10, cis-12
isomers, to give material of high isomeric purity. In
the future, the production of CLA is likely to expand
to include production of CLA-containing triacylgly-
cerols, as this is the form of lipid most generally
present in foodstuffs.
Analysis of CLA
0009 The primary concern in analyzing CLA is the com-
plete separation and quantitative identification of
the individual isomers in the sample. The form
of the sample will also determine the approach to
analysis. Samples are generally either in the free fatty
acid form, or present as esterified CLA as a compon-
ent in a biological matrix, from which the CLA needs
to be extracted first. A number of analytical tech-
niques have proven useful.
13
C NMR allows identifi-
cation and quantification of all the positional and
geometrical isomers in a single analysis, but has the
drawback of requiring a comparatively large amount
of sample, so it is not helpful for analyzing biological
extracts. Gas chromatography (GC), gas chroma-
tography–mass spectrometry (GC-MS) and high-
performance liquid chromatography (HPLC) can all
give large amounts of information regarding sample
composition, but for these methods, the sample must
be extracted and also derivatized prior to analysis.
A combination of the above techniques is often re-
quired to give a comprehensive analysis.
0010As CLA is prone to isomerization, care must be
taken in the extraction method chosen to obtain
CLA from tissue samples. A mild alkaline hydrolysis
can be used, for instance, to give the CLA as free fatty
acid, which can then be derivatized for analysis using
one of the methods described below.
0011Prior to GC analysis, fatty acids must be converted
to derivatives (usually methyl esters). Although there
are standard methods used to derivatize methylene-
interrupted fatty acids, it has become apparent over
the last few years that some of these methods, in
particular those using an acidic catalyst, are unsuit-
able for use with conjugated free fatty acids. Under
vigorous conditions, they lead to isomerization of
the sample and artefact formation. Though base-
catalyzed methods do not normally esterify methyl-
ene-interrupted free fatty acids, esterification of free
CLA can be achieved, but some of the methods used
have also shown artefact formation on reaction with
CLA. Phase transfer methods using methyl iodide in a
basic buffer solution have been utilized on a small
scale with consistent results. Despite the drawbacks,
acidic methods can give good quantitative results
providing that the reaction is carried out under mild
conditions with fresh reagent. Esterified CLA can be
converted to the methyl esters by the standard
method of transesterification with sodium methoxide
in methanol solution. The most useful GC columns
for resolving complex mixtures of CLA isomers are
longer, more polar columns such as the 100-m Cp-Sil
88 or the 120-m BPX-70 capillary columns. Neither
of these columns will completely resolve a complex
mixture of CLA isomers, although they will give good
results on most commercial samples. For this reason,
complex mixtures of biological CLA should also be
analyzed by either HPLC, or GC-MS, or both.
0012HPLC has proved especially useful in the analysis
of conjugated dienes, such as CLA. A UV detector is
usually employed as conjugated dienes show a strong
absorbance in the region of 230–235 nm. Silver-ion
HPLC, employing more than one column, can be used
to separate all the positional and geometric isomers
in a commercial CLA mixture, as methyl esters.
Reversed-phase HPLC has been used successfully to
concentrate the CLA methyl esters of biological
samples for further analysis.
0013For GC-MS, a different type of derivative, usually
containing nitrogen, is required to give good-quality
spectra of CLA. Both dimethyloxazoline (DMOX)
derivatives and 4-methyl-1,2,4-triazoline-3,5-dione
(MTAD) adducts are commonly used and can be
used to locate the conjugated double bond positions
in both CLA and its metabolites.
CONJUGATED LINOLEIC ACID 1583

Biological Activity
0014 As mentioned above, CLA has exhibited biological
effects in a number of major medical conditions.
However, many of the CLA mixtures used for early
trials were poorly characterized. As a result, there is
little conclusive information as to which CLA isomers
are the most active and under which circumstances. It
is only recently that individual pure cis-9, trans-11
and trans-10, cis-12 isomers have become available
for clinical trials. Consequently, the discussions
below assume that studies were carried out with com-
mercial isomer mixtures of free fatty acids, although
where a particular isomer has demonstrated a repro-
ducible effect this is indicated.
Anticarcinogenicity
0015 The interest in CLA as a biologically active material,
rather than as a minor lipid component or an oxida-
tion product, began in the early 1980s, when re-
searchers at the University of Wisconsin reported the
discovery of an extract from grilled minced beef,
which was found to inhibit mutagenesis. Since then,
many studies have been performed, both in vivo using
a number of different animals, and in vitro with a
variety of animal and human cell lines. Although other
lipids, such as fish oil, have been shown to have anti-
carcinogenic effects, CLA appears to be potent at
much lower concentrations (e.g., from 0.1% w/w in
rat tumors). A number of studies have concluded that
the cis-9, trans-11 isomer is the active CLA isomer
against cancer, but this remains to be proven.
0016 In vivo, dietary supplementation with CLA de-
creased the number and size of mammary tumors in
rats and mice, independent of the amount and types
of other fat in the diet. However, no effect was seen in
trials using tumor cells from hormone nonresponsive
cell lines such as the WAZ-2T (-SA) and MDA-MB-
231 cell lines. Further studies are required in this
field, as this may be relevant to elucidating the mech-
anism by which CLA acts on tumors. In tests on skin
carcinogenesis in mice, topically applied CLA was
found to be effective in reducing the size and number
of skin tumors, although dietary supplementation
with CLA only affected the number and not the size
of the tumors. Fewer data have been published on
the effect of CLA on prostate cancer and on fore-
stomach and colon cancers, with conflicting data
being obtained in different mouse and rat models.
0017 Much of the in vitro work on carcinogenesis has
been done on the estrogen-responsive MCF-7 human
breast cancer cell line. In the majority of these
studies, CLA has been shown to reduce cell growth
in a dose-dependent manner. Again, however, estro-
gen-unresponsive cell lines failed to show tumor
inhibition on treatment with CLA. Limited numbers
of studies on a variety of other human cancer cell lines
have also shown tumor inhibition in colorectal, liver,
prostate, and lung cells.
0018A number of studies have been conducted to try
and elucidate the mechanism by which CLA acts on
cancer cells. Initial suggestions that CLA acts as an
antioxidant have recently been contradicted by a
number of trials. CLA can block the formation of
carcinogen DNA adducts in rats in certain organs.
Another potential mode of action would be by
affecting the production of eicosanoids implicated in
tumorigenesis. CLA is incorporated into both neutral
lipids and phospholipids, at varying levels, depending
on the tissue type, and can be metabolized to give
18:3, 20:3, and 20:4 fatty acids containing conju-
gated double bonds. Dietary supplementation with
CLA has also been shown to reduce the synthesis of
the eicosanoid prostaglandin E
2
in mouse epidermis.
However, the direct method of action has still to be
determined.
0019There appears to be a direct link between levels of
CLA in breast tissue during maturation and the
limiting of lateral branching of the terminal end bud
cells of the developing breast, leading to a decreased
risk of breast cancer in later life. Again, the mechan-
ism for this effect has yet to be shown. Interestingly, a
number of studies examining human dietary CLA
intake and breast cancer risk world-wide have failed
to show any consistent positive or negative effect of
dietary CLA on cancer development. This may be due
to limitations of the accuracy of the dietary surveys
conducted.
Body Composition
0020In animal studies, CLA reduced body fat and
increased lean body mass in growing animals, for
a number of species, including mice, rats, and pigs.
Other physiological factors connected with these
effects in different studies were a reduction in lipopro-
tein lipase activity, an increase in the enzyme carnitine
palmitoyl transferase (associated with b-oxidation of
lipids), and reduction in adipocyte size (but not cell
number). Reduced body fat and increased body pro-
tein and water have been directly associated with the
trans-10, cis-12 isomer. Potential mechanisms for
these effects include alteration of membrane structure,
effects on eicosanoid and cytokine production, and
changes in peroxisome proliferator-activated receptor
(PPAR) activity.
0021Feeding of CLA also leads to a loss of monounsatu-
rated fatty acids, which is an effect seen in treatment to
reduce obesity. It has recently been demonstrated that
this effect is due to repression of the expression of the
stearoyl-coenzyme A desaturase gene in fat cells by the
1584 CONJUGATED LINOLEIC ACID

trans-10, cis-12 isomer, leading to smaller lipid drop-
lets containing lower levels of monounsaturated fatty
acids. This is not seen with the cis-9, trans-11 isomer.
0022 A few studies of the influence of CLA on human
body composition have been conducted. The amounts
of CLA fed were variable (between 1.7 and 7 g per
day), and a number of the subjects studied were either
athletes or body builders. In general, although there
was little effect on body weight, in most of the sub-
jects, body fat was either reduced or stayed constant,
and lean body mass was either constant or increased.
However, these variations were in most cases not sig-
nificant. The existing data are not consistent enough
to show a dose-dependent response, and the effects of
exercise should be taken into consideration. Also,
studies in humans were conducted on adults, whereas
animal data were collected from growing subjects. It
has been speculated that feeding CLA affects plasma
leptin levels and thus acts as a control on food intake.
However, recent data in humans showed that, al-
though supplementation decreased leptin levels in
the short term, after approximately 7 weeks levels
returned to baseline values, and no alterations in
either appetite or body composition were observed.
Further studies are required with individual isomers,
in particular the trans-10, cis-12 isomer to assess fully
the effects and the potential to treat obesity.
Immunity
0023 In mice, dietary CLA is incorporated into liver lipids
by displacing linoleic and arachidonic acids, thus
modifying the n-6 fatty acid composition of the phos-
pholipids. Subsequent experiments in rats have shown
that this phospholipid modification affects eicosanoid
production. For instance, feeding 1% CLA for 2 weeks
reduced serum prostaglandin E
2
levels by approxi-
mately 50%. CLA can also lower the levels of some
leukotrienes (e.g., LTB
4
) in rats. This may then affect
cytokine production, but further studies are required
in this area. It has effects on immunoglobulin produc-
tion, specifically increasing levels of IgA and IgG,
which are both associated with antiallergic effects.
0024 Few data are currently available from human stud-
ies, but a recent trial on women showed no changes in
immune function during the course of the study. This
may be due to a dose effect, as the levels of CLA per
weight in animal models tend to be much greater than
the levels fed in the human studies.
Atherosclerosis
0025 Experiments on atherosclerosis have been conducted
on both rabbits and hamsters. Feeding CLA at 0.1–
0.5% of the diet in rabbits for 22 weeks showed a
reduction in plasma triacylglycerol, total cholesterol,
and LDL-cholesterol levels. There was also an effect
on plaque formation, with a reduction in the CLA fed
animals. In hamsters, CLA feeding at similar levels
was found to reduce total cholesterol and fatty streak
area, although linoleic acid feeding showed similar
effects. An isomer effect was seen in the hamster
model, with only the trans-10, cis-12 isomer decreas-
ing triacylglycerol and cholesterol. Contradictory
data have been obtained in C57BL/6 mice, in which
an increase in aortic fatty streaks was reported.
Bone Metabolism
0026Lipids play an important part in bone metabolism as
they are known to influence both bone modeling and
remodeling. Both CLA and n-3 fatty acids modulate
the production of prostaglandin E
2
in chicks and rats,
leading to increased bone formation and decreased
resorption. CLA is thought to act on prostaglandin
E
2
production by influencing the cyclooxygenase
enzymes, which produce prostaglandin E
2
from ara-
chidonic acid. This may occur through the production
of competitive 20:4 conjugated CLA metabolites. As
also seen in other studies, CLA reduces the level of
LTB
4
, which is a strong bone resorption factor. CLA
may influence cartilage functions in growing animals.
As yet, few experiments have been conducted with
CLA, and further data are required to elucidate its
mechanism of action.
Diabetes
0027In type 2 diabetes, treatment aims to reduce insulin
resistance and improve glucose uptake. In in vitro
tests, chemicals such as thiazolidinediones show
these effects by directly affecting PPARg expression.
CLA, in particular the cis-9, trans-11 isomer, has also
been shown to bind to, and activate, PPARg, as have
other unsaturated fatty acids. In Zuker diabetic fatty
(fa/fa; ZDF) rats, a diet containing 1.5% CLA pro-
duced normal glycemic responses to tolerance tests,
as well as reduced hyperinsulinemia.
Enhanced CLA Levels and Food
Production
0028In animals, there are two different reasons to try to
increase the levels of CLA in tissue and/or milk.
Firstly, CLA has effects on body composition, leading
to, for instance, increased lean meat and lowered
levels of fat in pigs and cows. This is becoming more
desirable as public concern increases due to suggested
links between increased dietary levels of fat and con-
ditions such as heart disease and obesity. The second
reason is because of the potentially beneficial effects
of CLA itself in a number of diseases. Although
CONJUGATED LINOLEIC ACID 1585
