Blake A.J.(ed.) Crystal Structure Analysis
Подождите немного. Документ загружается.

This page intentionally left blank

8
Fourier syntheses
William Clegg
8.1 Introduction
The crystal structure we are trying to determine and its X-ray diffraction
pattern are related to each other by the mathematical process of Fourier
transformation; each is the Fourier transform of the other, as shown in
the introductory material. It is worth beginning here with a summary
of the fundamental relationships involved and some comments on the
notation and its meaning.
X-rays are scattered by the electrons in a crystal structure, so what
we are able to determine is the electron-density distribution, averaged
over time and hence over the vibrations of the atoms. Since the crystal
structure is periodic, we need determine only the contents of one unit
cell, and the presence of symmetry other than pure translation reduces
this even further, to the asymmetric unit of the structure, which is a
fraction of the unit cell in all cases except space group P1.
The electron density is a smoothly varying continuous function with a
single numerical value (in units of electrons per cubic Ångstrom, e Å
−3
)
at each point in the structure. For many of the calculations involved
in crystallography this is not a convenient function to work with, and
we describe the structure instead in terms of the positions and dis-
placements (vibrations) of discrete atoms, each with its own electron
density distribution about its centre. In most studies (except for high-
resolution charge-density experiments), atoms are taken to be spherical
in shape when stationary, ignoring valence effects such as bonding and
lone pairs of electrons, and their individual contributions to X-ray scat-
tering, known as atomic scattering factors, are calculated from electron
densities derived from quantum mechanics. These atomic scattering fac-
tors are known mathematical functions, varying with Bragg angle θ,
available in published tables (such as the International Tables for Crys-
tallography), and incorporated in standard crystallography computer
programs. The X-ray scattering effects of atoms are modified by atomic
displacements, which cause the at-rest electron density to be spread
out over a larger volume and usually unequally in different directions
(anisotropic), and this effect is described by a set of anisotropic dis-
placement parameters (adps) for each atom. The most commonly used
mathematical model uses six adps and can be represented graphically
as an ellipsoid. This model is a reasonable approximation to physical
reality in most cases.
103
104 Fourier syntheses
Thus, each symmetry-independent atom in the asymmetric unit of
a crystal structure is described by the following parameters: a known
atomic scattering factor (f), a set of displacement parameters (U values),
and three co-ordinates (x, y, z) specifying its position. (In some cases,
such as disordered structures, another parameter is used, giving the
site occupancy factor, because a site may be occupied by an atom in
some unit cells and not in others, at random, so on average we have to
specify a fraction of an atom here.) We give atomic positions relative to
one corner of one unit cell chosen as the origin, and measuredalong each
of the unit cell axes. Rather than using Å as units for the co-ordinates,
we give them as fractions of the unit cell axis lengths, and these fractions
do not have units. This means, for example, that the origin of the unit
cell has co-ordinates 0,0,0 and the point right in the centre of the unit cell
has co-ordinates
1
/
2,
1
/
2,
1
/
2. It is convenient to take most co-ordinates to lie
in the range 0–1, but molecules do not generally lie conveniently within
the confines of an arbitrarily defined unit cell, so some co-ordinates
may be negative or be greater than 1. A majority of co-ordinates outside
the range 0–1 simply means a poorly chosen unit cell origin, with the
molecule lying largely or entirely outside the ‘home’ unit cell. This is,
of course, not strictly incorrect, since all unit cells are exact copies of
each other by definition, and any integer can always be added to or
subtracted from all x, all y,orallz co-ordinates, but it is bad practice.
8.2 Forward and reverse Fourier transforms
The diffraction pattern (a set of discrete reflections, each a wave with
its own amplitude and relative phase) is the Fourier transform of the
crystal structure. The mathematical relationship for this is given by:
F(hkl) =
N
j=1
f
j
exp[2πi(hx
j
+ ky
j
+ lz
j
)]. (8.1)
Here, f
j
is the atomic scattering factor for the jth atom in the unit cell,
which has co-ordinates x
j
, y
j
, z
j
; f
j
incorporates the effects of atomic dis-
placements in this equation, in order to keep it simple. The integers h, k
and l are the indices for one particular reflection, occurring in a certain
direction, and this equation shows how the structure factor F for that
reflection is related to the crystal structure. What this equation means
in words is that each reflection in the diffraction pattern is a wave and
it is made up as a sum of waves scattered by the individual atoms, each
atom in accordance with its electron-density distribution (f
j
); in adding
up the waves scattered in this direction, their relative phases have to
be allowed for, and these depend on the positions of the atoms relative
to each other, as expressed in the exponential term. For mathematical
convenience and compactness, complex number notation is used (hence
the symbol i), allowing us to use just one symbol to represent both the
amplitude and phase together for a wave. F(hkl) is a complex number,

8.2 Forward and reverse Fourier transforms 105
as explained in Chapter 1 and Appendix A, with an amplitude and a
phase. Equation (8.1) applies once for each reflection (each direction in
which a discrete diffracted beam occurs) in order to obtain the complete
diffraction pattern, and each calculation involves the sum of N terms,
this being the number of atoms in the unit cell. The presence of sym-
metry in the structure allows the calculations to be simplified further,
because symmetry-equivalent reflections have the same amplitude and
related phases, but we shall keep with general equations here.
Equation (8.1) can be used to calculate the expected diffraction pattern
for any known structure, and it is used at various stages during a crystal-
structure determination, even when the ‘knownstructure’isincomplete.
We referto the resultof this as a set of calculated structure factors, F
c
(hkl)
or just F
c
.
Equation (8.1) also describes mathematically the physical process
observed when X-rays are diffracted by a crystal, which is the exper-
iment of collecting diffraction data. From the experiment, however, we
obtain only the amplitudes of the reflections (derived from the mea-
sured intensities) and not their phases. Thus, we have a set of observed
structure factors, but they are only |F
o
|. We do not have any observed
phases, so the observed diffraction pattern is, in this sense, incomplete.
One particular F is never measured in the diffraction experiment, but
is important for future use. This is the structure factor F(000), corre-
sponding to completely in-phase scattering by all atoms in the forward
direction with θ = 0, and it can not be physically separated from the
undiffracted beam. Setting all indices to zero in (8.1) and noting that
atomic displacement parameters have no effect at zero Bragg angle, we
find that F(000) has an amplitude equal to the total number of electrons
in one unit cell, and has a phase of zero.
That is half the story, which we may call the forward Fourier trans-
form. The other half is the reverse Fourier transform. The crystal
structure, expressed as electron density, is the Fourier transform of the
diffraction pattern. This relationship is expressed as:
ρ(xyz) =
1
V
hkl
F(hkl) exp[−2π i(hx + ky + lz)]. (8.2)
There is an obvious similarity to (8.1), with the terms for the diffraction
patternandfor the crystal structureexchanged between the leftandright
sides of the equation. The main differences otherwise are the inclusion
of the unit cell volume V in (8.2) (to make sure the units are correct,
since the crystal structure here is described by its electron density ρ
instead of by discrete atomic scattering factors that, like structure factor
amplitudes, have units of electrons rather than e Å
−3
), and the presence
of a minus sign in the exponential.
Equation (8.2) is the basis of all Fourier synthesis calculations in crys-
tallography. It shows how the electron density in the crystal structure
can, in principle, be obtained from the diffraction pattern. Like the
forward Fourier transform, it describes a physical process, but this time
106 Fourier syntheses
one that is unachievable in an experiment. It is the equivalent of the use
of lenses in an optical microscope to take light scattered by an object
being viewed, and recombine the scattered waves to produce a focused
image of the object; unfortunately X-rays can not be bent by lenses in
the same way as visible light, or we would be able to build an X-ray
supermicroscope and not have so much work to do! The equation says
that, in order to find the electron density at a particular point in the
structure, we have to take all the individual scattered X-ray waves (the
reflections F) and add them together, allowing for their different relative
phases. The phase differences will vary with the position at which we
are finding the electron density, because the waves will have different
path lengths in converging on that point, and this is the meaning of the
exponential term again; but the waves also have different phases from
their initial production in the diffraction process (given by the forward
Fourier transform), and these have to be included as well.
Since this physical process can not actually be carried out, we have
to emulate it by calculation, using (8.2). Unfortunately, this is still not
possible in a direct way, because we do not have all the information
required. In (8.2), F(hkl) are complex numbers, with an amplitude and
a phase: although we have the structure factor amplitudes, we do not
know the intrinsic relative phases of the reflections. Much of the task of
solving a crystal structure is recovering the lost phase information, at
least as approximate values, so that the reverse Fourier transform can
be carried out.
Modified versions of (8.2) are used at various stages in a crystal-
structure determination, as our knowledge of the phases develops from
non-existenttoessentially complete, and these arereferredtoas different
kinds of Fourier syntheses or Fourier maps.
In order for (8.2) to give an accurate result for the electron density,
it is not only necessary to have phases and to have accurate values for
the reflection amplitudes (i.e. good data!); we should, in principle, also
include all possible reflections with indices between −∞ and +∞. This
is clearly unachievable, and the effect is to produce some distortions in
the electron density, which may be seen as small ripples surrounding
the atoms, most noticeable around atoms with high electron density.
It is, however, not usually a significant problem, since the form of
atomic scattering factors, together with atomic displacements, means
that diffraction intensities decrease at higher Bragg angles, and the
unmeasured high-index small amplitudes would not contribute much
to the Fourier summations anyway. Inclusion of F(000) is important
in order to obtain correct electron density values, since all other terms
effectively contribute no net electron density to the total in the unit cell,
because they are waves consisting of equal positive and negative parts.
A Fourier synthesis may be thought of as smearing out the correct total
number of electrons uniformly throughout the unit cell (this is the F(000)
term) and then redistributing this density by successive addition of
other waves, each of which will reduce the density in some regions and
increase it in others by the same amount; the final result has the electron
8.3 Some mathematical and computing considerations 107
density concentrated in discrete maxima corresponding to atoms, with
low or zero (but never negative) electron-density regions in between.
8.3 Some mathematical and computing
considerations
Since Fourier transform calculations, both forward and reverse, take up
a very high proportion of the amount of computing involved in crystal-
lography, they need to be carried out as efficiently as possible. The scale
of the task can be illustrated easily. For the forward Fourier transfor-
mation, consider a unit cell of dimensions 10×10×10 Å
3
containing 60
atoms. Typically, this will give about 7000 reflections up to a maximum
θ of 25
◦
with Mo-Kα radiation. Calculation of the diffraction pattern F
c
thus involves 7000 sums (ignoring symmetry), in each of which there
are 60 terms. This makes 420 000 calculations, each of which includes
exponentials, multiplications and additions. This is a relatively small
structure!
For the reverse Fourier transformation, consider the same crystal
structure. From (8.2) we obtain values of the electron density at discrete
points in the unit cell, not a continuous function. This means calculat-
ing values at selected points on a three-dimensional grid covering the
unit cell. In order to resolve adjacent atoms and make good use of the
available data, a grid spacing of about 0.3 Å is reasonable, giving about
37 000 grid points. So, (8.2) has to be used 37 000 times, each one being
a sum of 7000 terms, making a total of about 260 million calculations.
And this is for just one Fourier synthesis.
The presence of symmetry does reduce the size of the task, of course,
because symmetry-equivalent reflections have the same amplitude and
related (not generally equal) phases, so the forward Fourier trans-
formation only has to be carried out for the symmetry-unique data
set. Similarly, the electron density need be calculated only for the
asymmetric unit, and not for the complete unit cell.
In addition, there are various well-known mathematical procedures
for simplifying the calculations involved, because of the properties of
sines and cosines of sums of terms, as shown in Appendix A. The
details of these do not need to concern us here; although Fourier
calculations were carried out by hand in the early pioneering days
of crystallography before the widespread availability of fast comput-
ers (and were often restricted to one- and two-dimensional synthe-
ses rather than full three-dimensional studies, to provide projections
of electron density, from which full structures were subsequently
deduced), these calculations are now performed at very high speed in
‘black boxes’.
It should be noted that the phases of reflections can take any value
between 0 and 360
◦
(0 and 2π radians) for non-centrosymmetric struc-
tures. By contrast, phases are restricted to a choice of two values,
0 and 180
◦
(0 and π radians) when a structure is centrosymmetric. This
108 Fourier syntheses
considerably simplifies the mathematics, since the complex exponen-
tial terms collapse to real cosines, with disappearance of the imaginary
sine components. In pictorial physical terms, this means that each of the
waves being added together in (8.2) can only be completely in phase (0,
crest-to-crest) or completely out of phase (180
◦
, crest-to-trough), and
the problem of finding the unknown phases reduces to the smaller
(but still considerable) task of finding the unknown signs, positive or
negative, for the reflection amplitudes |F| in order to add the waves
together.
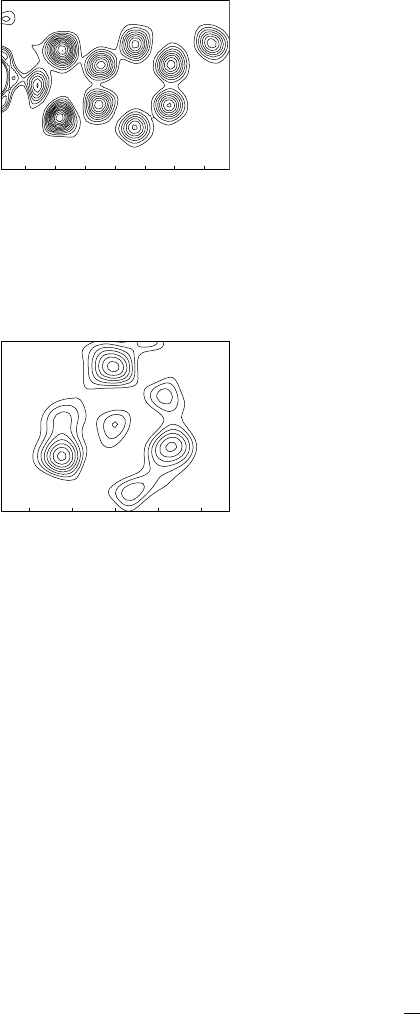
Fig. 8.1 Contoured section through a
Fourier synthesis in a plane containing B,
C, O and H atoms. The edge of a Pt atom
bonded to B is seen at the left. H atoms are
not visible; the ten clear peaks correspond
to atoms.
Fig. 8.2 Contoured section through a
Fourier synthesis in the plane containing
three methyl carbon atoms of a two-fold
disordered tert-butyl group. The major
component atoms are clearly seen as the
largest peaks, but the minor components
do not all give separate maxima. The small
peak at the centre is the outer edge of the
central carbon atom of the group, which
lies below this plane, where the electron
density is higher and reaches its maximum
for this atom.
A Fourier synthesis is a three-dimensional function, usually obtained
as a set of values on a three-dimensional grid. In chemical crystallog-
raphy, it is rare for such a result to be presented in full. Normally, the
positions of maxima (also called peaks) in the synthesis are found by
interpolation between the grid points (effectively a form of curve fitting
in three dimensions) as part of the computing procedure, and these posi-
tions, together with the corresponding values of the electron density, are
listed and made available as potential atom sites for visual inspection
or, more likely, interpretation through a molecular graphics program.
In most cases, this works satisfactorily, but it causes problems when
atom sites are not clearly resolved from each other, giving no discrete
maximum in the synthesis. This is the norm in protein crystallogra-
phy, where data often do not extend to atomic resolution, and different
techniques are used. With atomic-resolution data, the most common
occurrence of this problem is in cases of disorder, when the alternative
sites may be too close together to give separate maxima. Inspection of
the full Fourier synthesis in the region of the disorder may be necessary.
This can involve taking planar sections through the three-dimensional
synthesis. Sections parallel to the unit cell faces are straightforward,
as these will correspond to the grid points on which the synthesis has
been performed, but sections in arbitrary orientations can also be calcu-
lated, either explicitly at appropriate points or by interpolation between
the points of the standard grid. The sections can be contoured with lines
joiningpointsofequalelectron density, like the contours showing moun-
tains on geographical maps, and this helps to show regions of electron
density that can correspond to atom sites, even if disorder is a problem.
Examples are shown in Fig. 8.1 and Fig. 8.2.
8.4 Uses of different kinds of
Fourier syntheses
All Fourier syntheses are essentially variations on (8.2). This may be
written in a slightly different but equivalent way to help show what the
variations are.
ρ(xyz) =
1
V
hkl
|
F(hkl)
|
exp[iφ(hkl)]exp[−2π i(hx + ky + lz)]. (8.3)
8.4 Uses of different kinds of Fourier syntheses 109
Here, the structure factor F has been separated into its amplitude |F|
and its phase φ, both of which are needed in order to carry out the
calculation.
Different kinds of Fourier syntheses use different coefficients instead
of the amplitudes |F|, and they may also in some cases apply weights
to the individual terms in the sum, so that not all reflections contribute
strictlyin proportionto these coefficients.These areall attemptstoobtain
asmuch useful information as possible at differentstages of the structure
determination, even if the phases are not well known.
8.4.1 Patterson syntheses
These are discussed in detail in the next chapter. The coefficients are
|F
o
|
2
instead of |F
o
|, and all phases are set equal to zero. In this case
all necessary information is known and the synthesis can be readily
performed. The result, of course, is not the electron density distribution
for the structure, but it is related to it in what is often a useful way, as
is explained later. There are some slight variations even within this use,
and these are covered in the Patterson synthesis chapter (Chapter 9).
8.4.2 E-maps
These are an important part of direct methods for solving crystal struc-
tures, and are discussed more fully in Chapter 10. The coefficients are
|E
o
|, the so-called normalized observed structure factor amplitudes,
which represent the diffraction pattern expected for point atoms (with
their electron density concentrated into a single point instead of spread
out over a finite volume) of equal size, at rest. E-values are calculated,
with a number of assumptions and approximations, from the observed
amplitudes |F
o
|, and only the largest values are used, weaker reflections
being ignored because they contribute less to the Fourier synthesis any-
way. Phases for this selected subset of the full data are estimated by
a range of techniques under the general heading of ‘direct methods’,
and usually a number of different phase sets are produced and used to
calculated E-maps. These maps tend to contain sharper (stronger and
narrower) maxima than normal Fourier syntheses (F-maps), and this
can help to show up possible atoms, but they also tend to contain more
noise (peaks, usually of smaller size, that do not correspond to genuine
atoms).
8.4.3 Full electron-density maps,
using (8.2) or (8.3) as they stand
These actually tend not to be used very often in chemical crystallog-
raphy, except for demonstration purposes, because the other types of
syntheses have particular advantages at different stages. However, let
us consider how we can carry out such a synthesis without having any
110 Fourier syntheses
experimental phases. Such a procedure can be used when some of the
atoms have been located (perhaps from direct methods or a Patterson
synthesis) and others still remain to be found. Once we have some
atoms, we can use them as a model structure, which we know is not
complete, but it contains all the information we currently have. From
the model structure we can use (8.1) to calculate what its diffraction
pattern would be. This will not be identical to the observed diffraction
pattern, but it should show some resemblance to it, the more nearly so
as we include more atoms in the correct positions. There are various
measures of agreement between the sets of observed and calculated
amplitudes, |F
o
| and |F
c
|, but the important thing is that the calcu-
lated diffraction pattern includes phases, φ
c
as well as amplitudes |F
c
|.
Although these are not the same as the true phases we would really like
to know, they are currently the nearest thing we have to them. A Fourier
synthesis using coefficients |F
c
| with the phases φ
c
would just repro-
duce the same model structure and get us nowhere, but combining the
true observed amplitudes |F
o
|with the ‘current-best-estimate’ phases φ
c
gives us a new electron-density map. If the calculated phases are not too
far from the correct phases (as is usually the case if the model structure
has atoms in approximately correct places and these are a significant
proportion of the electron density of the structure), then this usually
shows the atoms of the model structure again, together with new fea-
tures not in the model structure but demanded by the diffraction data,
i.e. more genuine atoms. Because of all the approximations involved in
this process, there may also be peaks in the electron-density map that
do not correspond to real atoms, and the results need to be interpreted
in the light of chemical structural sense and what is expected. Addi-
tion of these new genuine atoms gives a better model structure, and
the whole process can be repeated, giving better calculated phases and
yet another new, and clearer, Fourier synthesis. This is done repeatedly
until all the atoms have been found and the model structure essentially
reproduces itself.
8.4.4 Difference syntheses
These are widely used in preference to full electron-density syntheses
for expanding partial structures. The coefficients are |F
o
|−|F
c
| and the
phases are obtained from a model structure as described above. The
result is effectively an electron-density map from which the features
already in the model structure are removed, so that new features stand
out more clearly, and it usually makes it easier to find new atoms. This
is rather like saying that, if the tallest peaks in a range of mountains
were somehow taken away, the foothills would appear to be much more
impressive! Peaks lying at the positions of atoms in the model structure,
or negative difference electron density there, indicate that the model
has either too little or too much electron density in those places, and can
indicatea wronglyassigned atom type, e.g.Ninstead of O orNinstead of
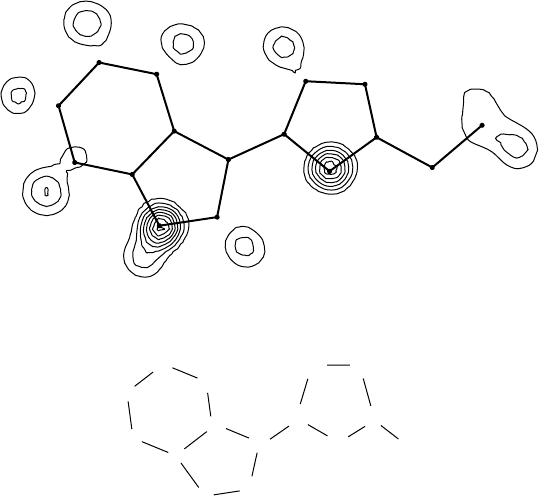
8.4 Uses of different kinds of Fourier syntheses 111
C
C
C
C
CC
Et
CN
N
C
C
C
C
C
Fig. 8.3 A section through a difference synthesis showing the effect of wrongly assigned
atom types and missing hydrogen atoms; the assumed model structure is shown, together
with the positions of its atoms and bonds in the map.
C for these respective effects. An example is shown in Fig. 8.3. There are
potentially some considerable problems with difference syntheses when
the proportion of known atoms is quite small, because the calculated
phases can have large errors.Also, weak reflections with relatively large
uncertainties in their intensities can cause disproportionate errors, and
it may be best not to use the weakest reflections; alternatively they can
be given reduced weights, as discussed below. It is important to ensure
that the observed and calculated data are on the same scale. Another
reason why difference syntheses can be better than full F
o
syntheses is
that series termination errors (small ripple effects due to the lack of data
beyond the measured θ
max
) cancel out through use of the differences
instead of full amplitudes.
8.4.5 2F
o
− F
c
syntheses
The use of coefficients 2|F
o
|−|F
c
| with phases calculated from a model
structure combines theadvantages of standard F
o
and difference synthe-
ses. The resulting map shows both the known and the as-yet unknown
features of the structures, with the new atoms emphasized, and it is
less subject to some of the errors of the simple difference synthesis.
It is more widely used in protein crystallography than by chemical
crystallographers.
