Bird R.B., Stewart W.E., Lightfoot E.N. Transport Phenomena
Подождите немного. Документ загружается.

5C.6
The Error Function
857
cosh ix
=
cos x;
sinh
ix
=
i
sin x
(C.5-8,9)
J
cosh x dx
=
sinh x;
J
sinh
x
dx
=
cosh x (C.5-12,13)
It should be kept in mind that cosh x and cos x are both even functions of x, whereas
sinh
x
and sin
x
are odd functions of
x.
5C.6
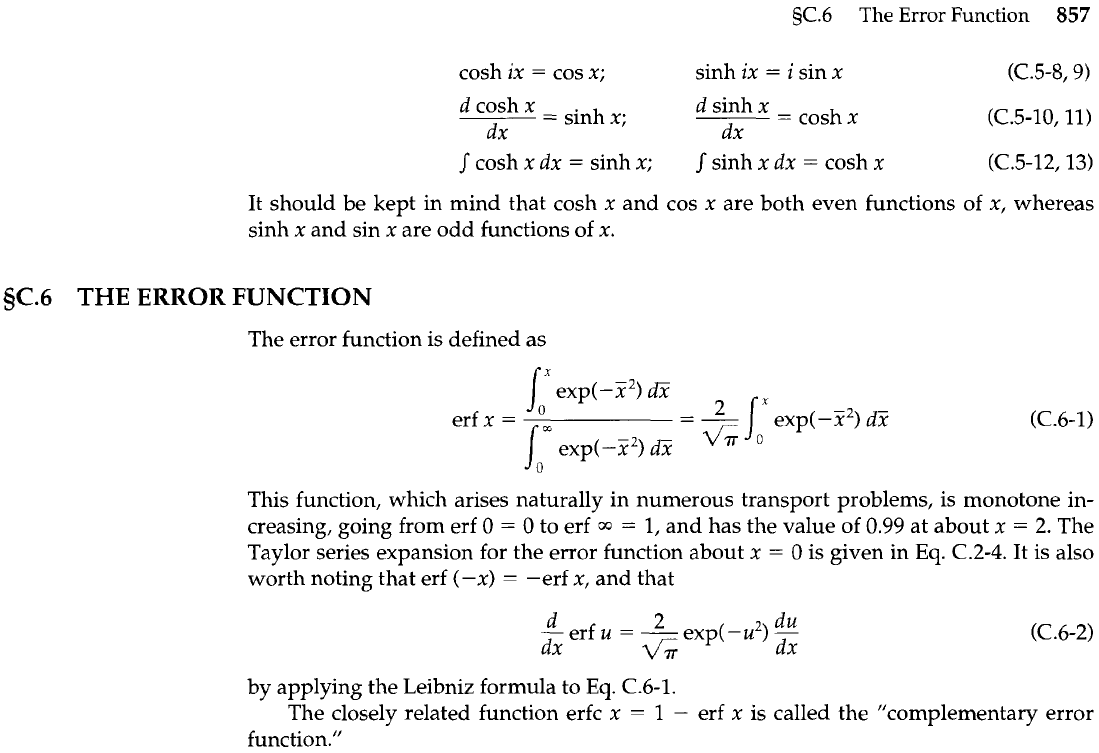
THE ERROR FUNCTION
The error function is defined as
This function, which arises naturally in numerous transport problems, is monotone in-
creasing, going from erf
0
=
0
to erf
=
1,
and has the value of 0.99 at about x
=
2.
The
Taylor series expansion for the error function about
x
=
0 is given in
Eq.
C.2-4.
It is also
worth noting that erf
(-x)
=
-erf x, and that
by applying the Leibniz formula to
Eq.
C.6-1.
The closely related function erfc x
=
1
-
erf
x
is called the "complementary error
function.''
Appendix
D
The Kinetic Theory of Gases
5D.1
The Boltzmann equation
9D.2
The equations of change
5D.3
The molecular expressions for the fluxes
5D.4
The solution to the Boltzmann equation
5D.5
The fluxes in terms of the transport properties
5D.6
The transport properties in terms of the intermolecular forces
5D.7
Concluding comments
In Chapters
1,9,
and
17
we gave a brief account of the use of mean free path arguments
to get approximate expressions for the transport properties. Then we gave the rigorous
results from the Chapman-Enskog development for dilute monatomic gases. In this ap-
pendix we give a brief description of the Chapman-Enskog theory, just enough to show
what the theory involves and to show how it gives a sense of unity to the subject of
transport phenomena in gases. The reader who wishes to pursue the subject further can
consult the standard references.'
Dl
THE
BOLTZMANN
EQUATION^
The starting point for the kinetic theory of low-density, nonreacting mixtures of
monatomic gases is the
Boltzmann equation
for the velocity distribution function
f&
r,
t).
The quantity
f&,
r,
t)
dk,dr is the probable number of molecules of species
a,
which at
time
t
are located in the volume element dr at position
r,
and have velocities within the
range
dib.
about
i.,.
The Boltzmann equation, which describes how
fa
evolves with time, is
'
J.
H.
Ferziger and
H.
G.
Kaper,
Mathematical Theory of Transport Processes in Gases,
North-Holland,
Amsterdam (1972);
S.
Chapman and
T.
G.
Cowling,
The Mathematical Theory of Non-Uniform Gases,
3rd
edition, Cambridge University Press (1970);
J.
0.
Hirschfelder, C. F. Curtiss, and
R.
8.
Bird,
Molecular
Theory of Gases and Liquids,
2nd corrected printing, Wiley, New York (1964), Chapter 7;
E.
M.
Lifshitz and
L.
P.
Pitaevskii,
Physical Kinetics,
Pergamon, Oxford (1981), Chapter
1.
L.
Boltzmann,
Sitzungsberichte Keiserl. Akad. der Wissenschaften,
66
(2), 275-370 (1872); C.
Cercignani,
The Boltzmann Equation and Its Applications,
Springer-Verlag, New York (1988).
C.
F.
Curtiss,
J.
Chem. Phys.,
97,1416-1423,7679-7686 (1992), found it necessary to modify the Boltzmann equation to
account for the possibility of orbiting pairs of molecules; the modification, important only at very low
temperatures, was found to give much better agreement with the limited low-temperature experimental
data.

5D.3
Molecular Expressions for the Fluxes
859
in which d/dr is identical to the V-operator, and
d/d&
is a similar operator involving ve-
locities rather than positions. The quantity
g,
is the external force per unit mass acting on
a molecule of species a, and
J,
is a very complicated five-fold integral term accounting
for the change in f, because of molecular collisions. The
J,
term involves the intermolecu-
lar potential energy function (e.g., the Lennard-Jones potential) and the details of the col-
lision trajectories. The Boltzmann equation may be thought of as a continuity equation in
the six-dimensional position-velocity space, and
J,
serves as a source term. The veloc-
ity distribution function is "normalized" to the number density of species
a;
that is,
Sf,(&, r, t)dra
=
nab t).
5D.2
THE EQUATIONS OF CHANGE
When the Boltzmann equation is multiplied by some molecular property rl/,(r,) and then
integrated over all molecular velocities, the general equation of change is obtained:
An integration by parts is performed to get this result, and use is made of the fact that
fa
is zero at infinite velocities. If
$,
is a quantity that is conserved during a collision (see
§0.3), then the term containing
J,
can be shown to be zero.'
Now let
be successively the conserved quantities for monatomic molecules: the
mass
ma,
the momentum
ma&,
and the energy
$m,(i,
.
r,). When these are substituted for
+,
into Eq. D.2-1, and when a sum over all species
a
is performed for the second and
third of these, we get the equations of change for mass of
a,
momentum, and energy as
follows:
In the last of these equations the internal energy per unit volume is defined to be
Thus we see that the equations of continuity, motion, and energy are direct conse-
quences of the conservation laws for mass, momentum, and energy discussed in Chapter
0. Equations D.2-2 to 4 should be compared with Eqs. 19.1-7,3.2-9, and Eq.
(B)
and foot-
note (b) of Table 19.2-4, which were derived by continuum arguments.
5D.3
THE MOLECULAR EXPRESSIONS FOR THE FLUXES
At the same time the equations of change are obtained, the molecular expressions for the
fluxes are generated as integrals over the distribution function:
at
equilibrium
j,<r, t)
=
m,S(r,
-
v)fadr, -0 (D.3-I)
at
equilibrium
dr,
t)
=
m$(&
-
v)(
ib.
-
v)f,dL
-
a
at
equilibrium
q(r,
t)
=
$m,J.(ib,
-
v)'(+,
-
v)f,dr,
-
0
a

860
Appendix
D
The Kinetic Theory of Gases
In these expressions the fluxes involve integrals over the products of mass, momentum,
and energy with the "diffusion velocity"
(i;
-
v) of species
a.
Note the similarity be-
tween the structure of these molecular fluxes (or "diffusive fluxes") and that of the con-
vective fluxes of mass p,v, momentum pw, and kinetic energy ipv2v appearing in the
equations of change, where v is the local instantaneous mass average velocity of the gas
mixture. Thus the molecular fluxes represent the diffusive movement of mass, momen-
tum, and energy above and beyond that described by the convective fluxes. Note also
that the molecular theory automatically generates the molecular work term
-
(V
[m
.
v])
in the energy equation.
5D.4
THE SOLUTION TO THE BOLTZMANN EQUATION
If the gas mixture were at rest, the velocity distribution function would be given by the
Maxwell-Boltzmann distribution function (known from equilibrium statistical mechan-
ics). Then we would find, as shown in 5D.3, that
j,
=
0,
that
.rr
=
pi3
=
n~T6, and that
q
=
0.
The derivation of p
=
KT
is given in Problem 1C.3.
On the other hand, when there are concentration, velocity, and temperature gradi-
ents, the distribution function is given as the Maxwell-Boltzmann distribution multi-
plied by a "correction factor":
where
4,
<<
1. In this expression n,, v, and T are functions of position r and time t.
Since the deviations from equilibrium result from the temperature, velocity, and concen-
tration gradients,
$,(r,,
r, t) can be represented, near equilibrium, as a linear function of
the various gradients,
in which the vector
A,,
the tensor B,, and the vectors Cap, all functions of
r,,
r, and
t,
are
given as the solutions of integrodifferential equations.' The quantities d, are "general-
ized diffusional driving forces" that include concentration gradients, the pressure gradi-
ent, and external force differences, defined as
in which x,,
w,,
and p, are the mole fraction, mass fraction, and partial pressure, respec-
tively. Equation D.4-3, valid only for a mixture of monatomic gases at low density, is
generalized for other fluids in the discussion of the thermodynamics of irreversible
processes in 524.1.
5D.5
THE FLUXES
IN
TERMS OF THE TRANSPORT PROPERTIES
When Eqs. D.4-1 to 3 are substituted into Eqs. D.3-1 to 3, we get the expressions for the
fluxes in terms of d,, Vv, and VT:
5D.7
Concluding Comments
861
In these equations the transport properties appear: the viscosity p, the thermal conduc-
tivity
k,
the multicomponent thermal diffusion coefficients
D:,
and the multicomponent
Fick diffusivities
Dap
(the
Bop
are the Maxwell-Stefan diffusivities, closely related to the
Dm&.
Thus the kinetic theory predicts the "cross effects": the transport of mass resulting
from a temperature gradient (thermal diffusion) and the transport of energy resulting
from a concentration gradient (the diffusion-thermo effect).
The pressure term in Eq. D.5-2 comes from the first term in the expansion in Eq. D.4-
1 (that is, the Maxwell-Boltzmann distribution), and the viscosity term comes from the
second term (that is, the
+,
term containing the gradients). The kinetic theory of
monatomic gases at low density predicts that the dilatational viscosity will be zero.
5D.6
THE TRANSPORT PROPERTIES
IN
TERMS
OF
THE INTERMOLECULAR FORCES
The transport properties in Eqs. D.5-1 to
3
are given by the kinetic theory as complicated
multiple integrals involving the intermolecular forces that describe binary collisions in
the gas mixture. Once an expression has been chosen for the intermolecular force law
(such as the Lennard-Jones (6-12) potential of Eq. 1.4-lo), these integrals can be evalu-
ated numerically. For a pure gas, the three transport
properties-self-diffusivity,
viscos-
ity, and thermal conductivity-are then given by:
The dimensionless "collision integrals"
a,
=
flk
.=
1.1% contain all the information
about the intermolecular forces and the binary collision dynamics. They are given in
Table E.2 as functions of
KT/&.
If we set the collision integrals equal to unity, we then get
the transport properties for a gas composed of rigid spheres.
Thus the transport properties, needed in the equations of change, have been obtained
from kinetic theory in terms of the two parameters
u
an<
E
of the in_termolecular potential
energy function. From these expressions we get Pr
=
C,p/k
=
$(c,/C,)
=
$(;)
=
and
Sc
=
p/p9
=
z(fl,/o,)
=
$,
these values being quite
good for pure monatomic gases.
sD.7
CONCLUDING COMMENTS
The above discussion emphasizes the close connections among mass, momentum, and
energy transport, and it is seen how all three transport phenomena can be explained
in
terms of a molecular theory for low-density, monatomic gases. It is also important to see
that the continuum equations of continuity, motion, and energy can all be derived from
one starting point-the Boltzmann equation-and that the molecular expressions for the
fluxes and transport properties are generated in the process. In addition, the discussion
of the dependence of the fluxes on the driving forces is very closely related to the irre-
versible thermodynamics approach in Chapter
24.
This appendix has dealt only with low-density, monatomic gases. Similar discus-
C.
F.
Curtiss,
J.
Chem. Phys.,
24,225-241 (1956);
C. Muckenfuss and C.
F.
Curtiss,
J.
Chem. Phys.,
29,
1257-1277 (1958);
L.
A.
Viehland and
C.
F.
Curtiss,
1.
Chem. Phys.,
60,492-520 (1974);
D.
Russell and
C.
F.
Curtiss,
1.
Chem. Phys.,
60,514-520 (1974).
J.
H.
Irving and
J.
G.
Kirkwood,
J.
Chem. Phys.,
18,817-829 (1950);
R.
J.
Bearman and
J.
G.
Kirkwood,
J.
Chem. Phys.,
28,136-145 (1958).
C. F.
Curtiss and
R.
B. Bird,
Adv. Polymer Sci.,
125, 1-101 (1996);
Proc.
Nat.
Acad. Sci.,
93,7440-7445
(1996);
J.
Chem. Phys.,
106,9899-9921 (1997), 107,5254-5267 (1997), 111,10362-10370 (1999).
862
Appendix
D
The Kinetic Theory of Gases
sions are available for polyatomic gases? monatomic liquids? and polymeric liquids5 In
kinetic theories for monatomic liquids, the expressions for the momentum and heat
fluxes contain terms similar to those in Eqs. D.3-2 and 3, but also contributions associ-
ated with forces between molecules; for polymers, one has the latter contribution, but
also additional forces within the polymer chain. In all of these theories one can derive
the equations of change from an equation for a distribution function and then get formal
expressions for the transport properties.

Appendix
Tables
for
Prediction
Transport Properties
El
Intermolecular force parameters and critical properties
5E.2
Functions for prediction of transport properties of gases at low densities
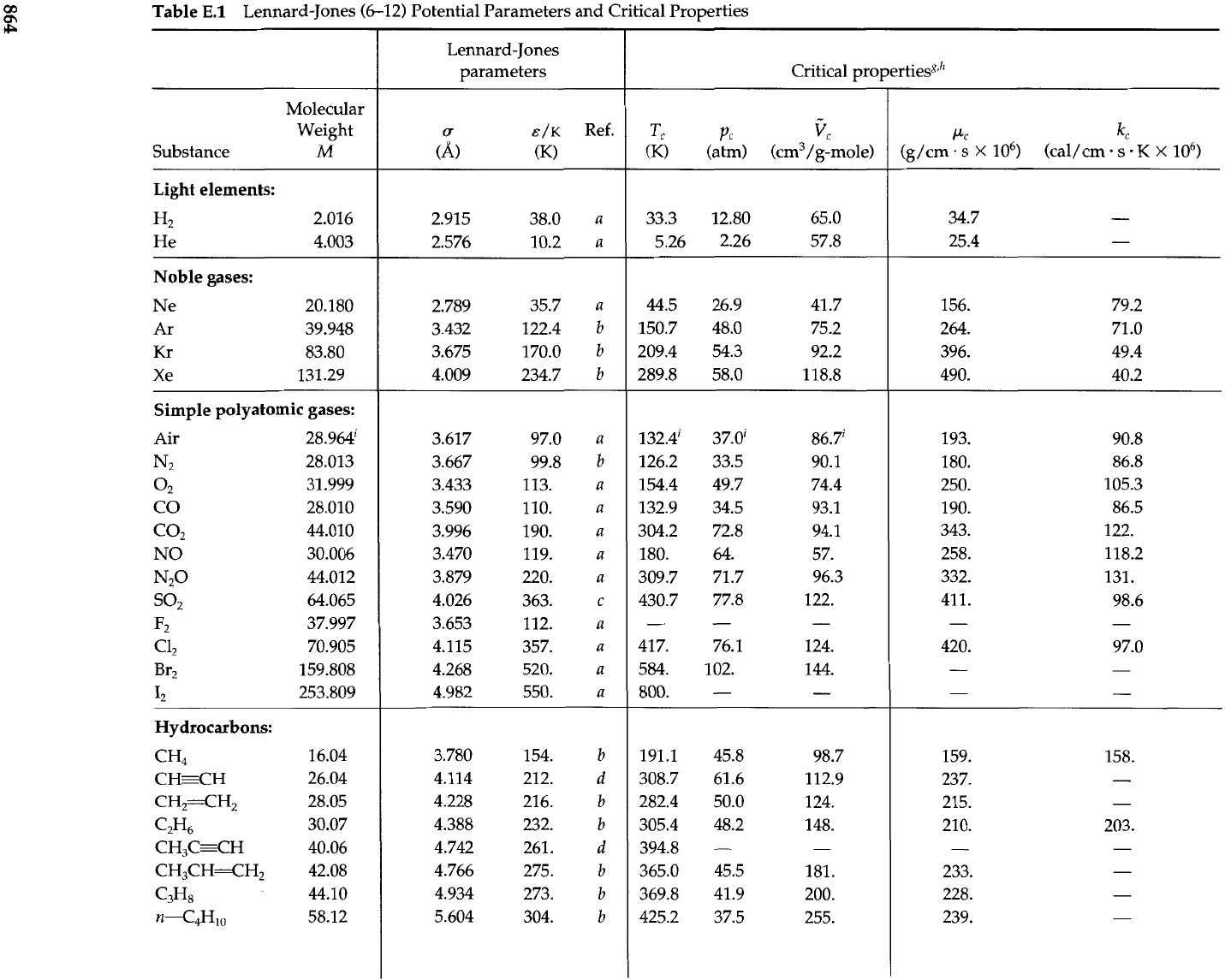
Table
E.l
Lennard-Jones (6-12) Potential Parameters and Critical Properties
Molecular
Weight
Substance
M
Light elements:
H2 2.016
He 4.003
Noble gases:
Ne 20.180
Ar 39.948
Kr
83.80
Xe 131.29
Simple polyatomic gases:
Air 28.9~~
N2
28.013
02
31.999
CO 28.010
co2
44.010
NO 30.006
No
44.012
so2
64.065
F2 37.997
c12
70.905
Br2 159.808
12 253.809
Hydrocarbons:
CH4
CHgH
CH2=CH2
C2H6
CH,C=CH
CH3CH=CH2
C3H8
n--C4H10
Lennard-Jones
parameters
IT
E/K
Ref.
(A,
(K)
Critical properties8,h
Tc
PC
k
(K) (atm) (cm3/g-mole)
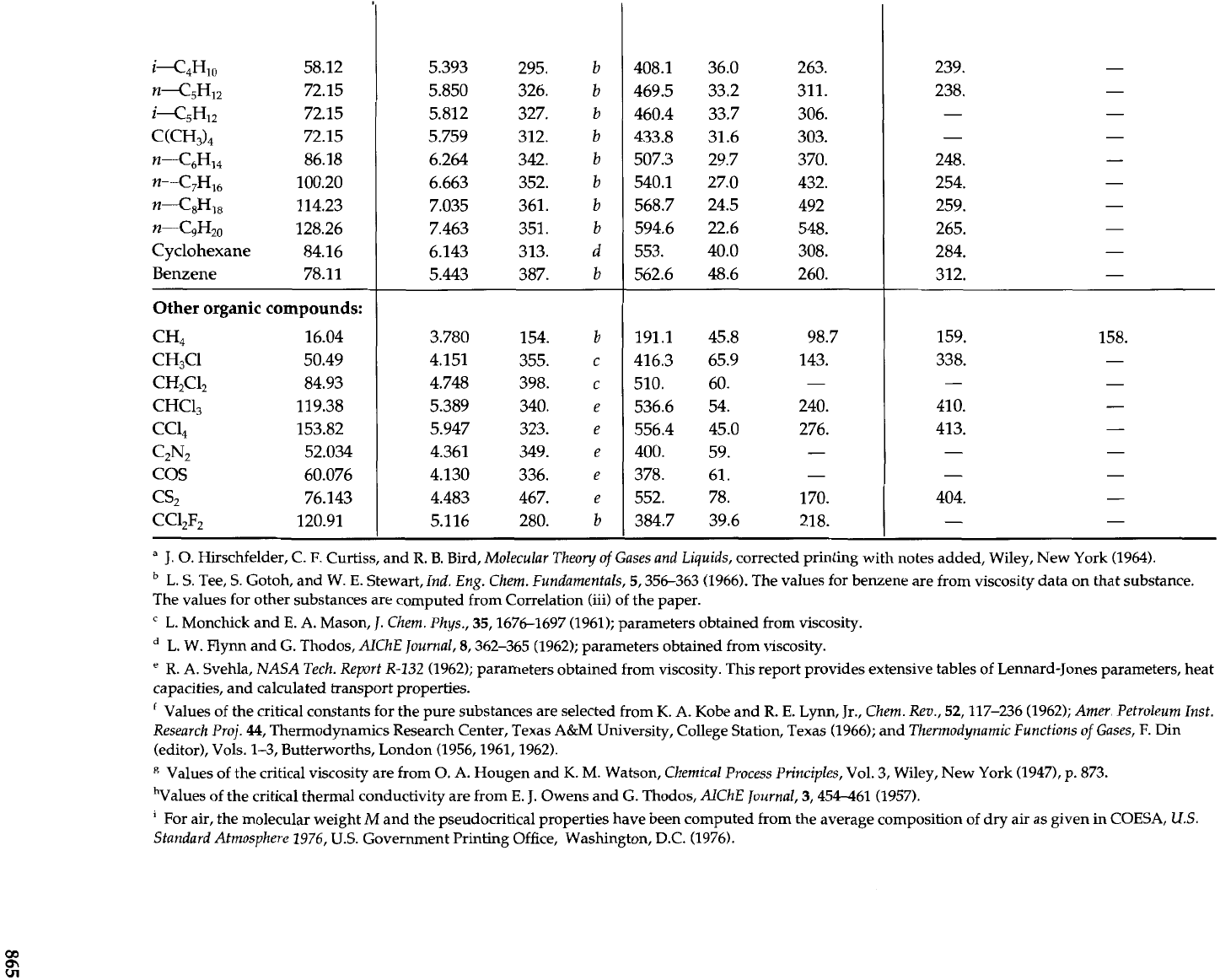
Other
organic compounds:
cH4 16.04
CH3C1 50.49
CH2C12 84.93
CHC1, 119.38
CCl, 153.82
C2N2 52.034
COS 60.076
cs2
76.143
CC12F2 120.91
a
J.
0. Hirschfelder, C.
F.
Curtiss, and
R.
B. Bird,
Molecular Theoy of Gases and Liquids,
corrected printing with notes added, Wiley, New York (1964).
L.
S. Tee,
S.
Gotoh, and
W.
E.
Stewart,
Ind. Eng. Chem. Fundamentals,
5,356-363 (1966). The values for benzene are from viscosity data on that substance.
The values for other substances are computed from Correlation (iii) of the paper.
"
L.
Monchick and
E.
A.
Mason,
J.
Chem. Phys.,
35,1676-1697 (1961); parameters obtained from viscosity.
L. W. Flynn and
G.
Thodos,
AIChE
Journal,
8,362-365 (1962); parameters obtained from viscosity.
'
R.
A.
Svehla,
NASA Tech. Report
R-132
(1962); parameters obtained from viscosity. This report provides extensive tables of Lennard-Jones parameters, heat
capacities, and calculated transport properties.
Values of the critical constants for the pure substances are selected from
K.
A.
Kobe and
R.
E.
Lynn, Jr.,
Chem. Rev.,
52,117-236 (1962);
Amer. Petroleum Inst.
Research Proj.
44,
Thermodynamics Research Center, Texas A&M University, College Station, Texas (1966); and
Thermodynamic Functions
of
Gases,
F. Din
(editor), Vols. 1-3, Butterworths, London (1956,1961,1962).
R
Values of the critical viscosity are from
0.
A.
Hougen and
K.
M.
Watson,
Chemical Process Principles,
Vol.
3,
Wiley, New York (1947),
p.
873.
hValues of the critical thermal conductivity are from
E.
J.
Owens and
G.
Thodos,
AlChE Journal,
3,454-461 (1957).
For air, the molecular weight
M
and the pseudocritical properties have been computed from the average composition of dry air as given in COESA,
U.S.
Standard Atmosphere
1976,
U.S.
Government Printing Office, Washington, D.C. (1976).
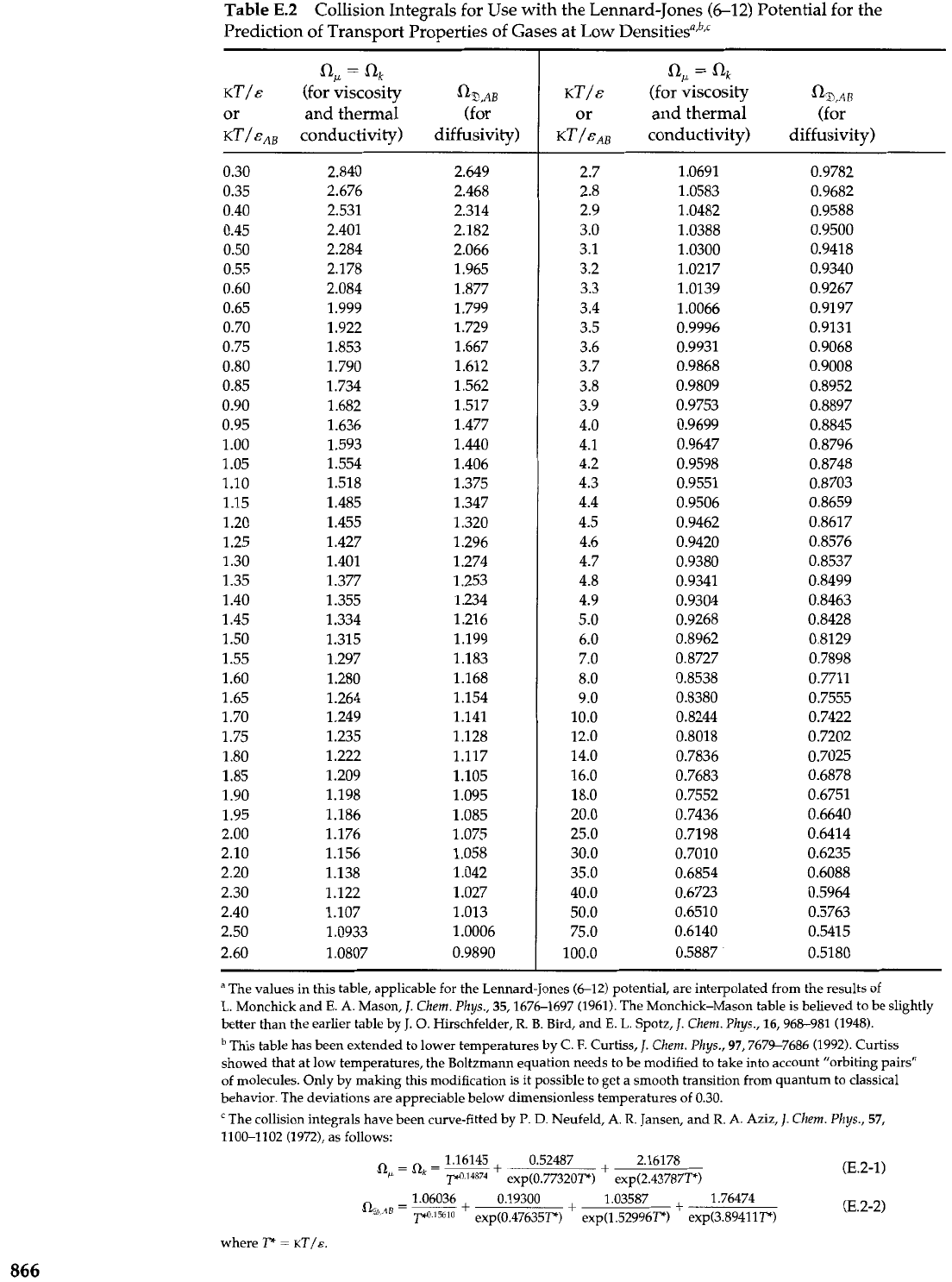
Table
E.2
Collision Integrals for Use with the Lennard-Jones
(6-12)
Potential for the
Prediction of Transport Properties of Gases at Low ~ensities",~,'
a,
=
ak
KT/&
(for viscosity
%AB
or and thermal (for
KT/&~,
conductivity) diffusivity)
a,
=
I(Zk
KT/&
(for viscosity
%,A,
or and thermal (for
KT/E~~
conductivity)
diffusivity)
"
The values in this table, applicable for the Lennard-Jones (6-12) potential, are interpolated from the results of
L.
Monchick and
E.
A. Mason,
J.
Chem. Phys.,
35,1676-1697 (1961). The Monchick-Mason table is believed to be slightly
better than the earlier table by
J.
0.
Hirschfelder,
R. B.
Bird, and
E.
L.
Spotz,
J.
Chem.
Phys.,
16,968-981 (1948).
This table has been extended to lower temperatures by C.
F.
Curtiss,
J.
Chem. Phys.,
97,7679-7686 (1992). Curtiss
showed that at low temperatures, the Boltzmann equation needs to be modified to take into account "orbiting pairs"
of molecules. Only by making this modification is it possible to get a smooth transition from quantum to classical
behavior. The deviations are appreciable below dimensionless temperatures of 0.30.
'The collision integrals have been curve-fitted by
P.
D.
Neufeld, A.
R.
Jansen, and
R.
A. Aziz,
J.
Chem. Phys.,
57,
1100-1102 (1972), as follows:
where
F
=
KT/&.
