Bird R.B., Stewart W.E., Lightfoot E.N. Transport Phenomena
Подождите немного. Документ загружается.


s0.4
Concluding Comments
7
The conservation laws as applied to collisions of monatomic molecules can be ob-
tained from the results above as follows: Eqs. 0.3-1, 0.3-2, and 0.3-4 are directly applica-
ble; Eq. 0.3-6 is applicable if the internal energy contributions are omitted; and
Eq.
0.3-8
may be used if the internal angular momentum terms are discarded.
Much of this book will be concerned with setting up the conservation laws at the mi-
croscopic and macroscopic levels and applying them to problems of interest in engineer-
ing
and science. The above discussion should provide a good background for this
adventure. For a glimpse of the conservation laws for species mass, momentum, and en-
ergy at the microscopic and macroscopic levels, see Tables 19.2-1 and 23.5-1.
50.4
CONCLUDING COMMENTS
To use the macroscopic balances intelligently, it is necessary to use information about in-
terphase transport that comes from the equations of change. To use the equations of
change, we need the transport properties, which are described by various molecular the-
ories. Therefore, from a teaching point of view, it seems best to start at the molecular
level and work upward toward the larger systems.
All the discussions of theory are accompanied by examples to illustrate how the the-
ory is applied to problem solving, Then at the end of each chapter there are problems to
provide extra experience in using the ideas given in the chapter. The problems are
grouped into four classes:
Class A: Numerical problems, which are designed to highlight important equa-
tions in the text and to give a feeling for the orders of magnitude.
Class
B:
Analytical problems that require doing elementary derivations using
ideas mainly from the chapter.
Class
C:
More advanced analytical problems that may bring ideas from other chap-
ters or from other books.
Class
D:
Problems in which intermediate mathematical skills are required.
Many of the problems and illustrative examples are rather elementary in that they in-
volve oversimplified systems or very idealized models. It is, however, necessary to start
with these elementary problems in order to understand how the theory works and to de-
velop confidence in using it. In addition, some of these elementary examples can be very
useful in making order-of-magnitude estimates in complex problems.
Here are a few suggestions for studying the subject of transport phenomena:
Always read the text with pencil and paper in hand; work through the details of
the mathematical developments and supply any missing steps.
Whenever necessary, go back to the mathematics textbooks to brush up on calculus,
differential equations, vectors, etc. This is an excellent time to review the mathemat-
ics that was learned earlier (but possibly not as carefully as it should have been).
Make it a point to give
a
physical interpretation of key results; that is, get in the
habit of relating the physical ideas to the equations.
Always ask whether the results seem reasonable. If the results do not agree with
intuition, it is important to find out which is incorrect.
Make it a habit to check the dimensions of all results. This is one very good way of
locating errors in derivations.
We hope that the reader will share our enthusiasm for the subject of transport phe-
nomena. It will take some effort to learn the material, but the rewards will be worth the
time and energy required.

8
Chapter
0
The Subject of Transport Phenomena
QUESTIONS FOR DISCUSSION
What are the definitions of momentum, angular momentum, and kinetic energy for a single
particle? What are the dimensions of these quantities?
What are the dimensions of velocity, angular velocity, pressure, density, force, work, and
torque? What are some common units used for these quantities?
Verify that it is possible to go from
Eq.
0.3-3
to
Eq.
0.3-4.
Go through all the details needed to get Eq.
0.3-6
from
Eq.
0.3-5.
Suppose that the origin of coordinates
is
shifted to a new position. What effect would that
have on
Eq.
0.3-7?
Is
the equation changed?
Compare and contrast angular velocity and angular momentum.
What is meant by internal energy? Potential energy?
Is the law of conservation of mass always valid? What are the limitations?

Part
One
Momentum
Transport

This Page Intentionally Left Blank
Chapter
1
Viscosity and the Mechanisms
of Momentum Transport
51.1
Newton's law of viscosity (molecular momentum transport)
2
Generalization of Newton's law of viscosity
1.3
Pressure and temperature dependence of viscosity
~1.4'
Molecular theory of the viscosity of gases at low density
51.5'
Molecular theory of the viscosity of liquids
51.6'
Viscosity of suspensions and emulsions
1.7
Convective momentum transport
The first part of this book deals with the flow of viscous fluids. For fluids of low molecu-
lar weight, the physical property that characterizes the resistance to flow is the
viscosity.
Anyone who has bought motor oil is aware of the fact that some oils are more "viscous"
than others and that viscosity is a function of the temperature.
We begin in 31.1 with the simple shear flow between parallel plates and discuss how
momentum is transferred through the fluid by viscous action. This is an elementary ex-
ample of
molecular momentum transport
and it serves to introduce "Newton's law of vis-
cosity" along with the definition of viscosity
p.
Next in 31.2 we show how Newton's law
can be generalized for arbitrary flow patterns. The effects of temperature and pressure
on the viscosities of gases and liquids are summarized in 51.3 by means of a dimension-
less plot. Then 51.4 tells how the viscosities of gases can be calculated from the kinetic
theory of gases, and in 51.5 a similar discussion is given for liquids. In
51.6
we make a
few comments about the viscosity of suspensions and emulsions.
Finally, we show in 31.7 that momentum can also be transferred by the bulk fluid
motion and that such
convective momentum transport
is proportional to the fluid density
p.
51.1
NEWTON'S LAW OF VISCOSITY (MOLECULAR
TRANSPORT OF MOMENTUM)
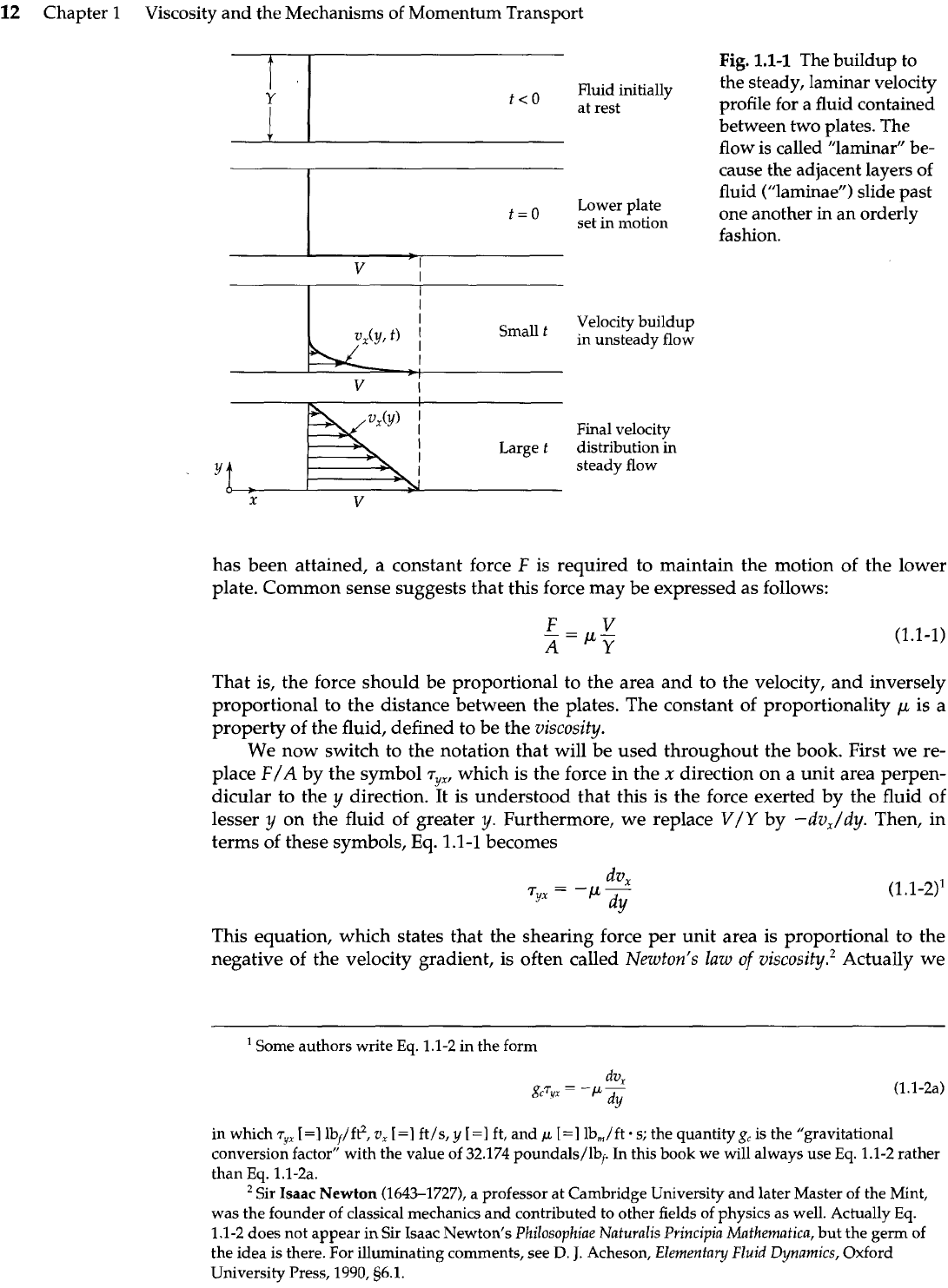
In Fig.
1.1-1
we show a pair of large parallel plates, each one with area
A,
separated by a
distance
Y.
In the space between them is a fluid-either a gas or a liquid. This system is
initially at rest, but at time
t
=
0
the lower plate is set in motion in the positive
x
direc-
tion at a constant velocity
V.
As time proceeds, the fluid gains momentum, and ulti-
mately the linear steady-state velocity profile shown in the figure is established. We
require that the flow be laminar ("laminar" flow is the orderly type of flow that one usu-
ally observes when syrup is poured, in contrast to "turbulent" flow, which is the irregu-
lar, chaotic flow one sees in a high-speed mixer). When the final state of steady motion
12
Chapter
1
Viscosity and the Mechanisms of Momentum Transport
Fluid initially
<
O
at rest
Lower plate
f
=O
set in motion
Velocity buildup
in unsteady flow
v
I
Fig.
1.1-1
The buildup to
the steady, laminar velocity
profile for a fluid contained
between two plates. The
flow is called "laminar" be-
cause the adjacent layers of
fluid ("laminae") slide past
one another in an orderly
fashion.
Final velocity
Large
t
distribution in
Yt
steady flow
has been attained,
a
constant force
F
is required to maintain the motion of the lower
plate. Common sense suggests that this force may be expressed as follows:
That is, the force should be proportional to the area and to the velocity, and inversely
proportional
to
the distance between the plates. The constant of proportionality
p
is a
property of the fluid, defined to be the
viscosity.
We now switch to the notation that will be used throughout the book. First we re-
place
F/A
by
the symbol
T,,,
which is the force in the
x
direction on a unit area perpen-
dicular to the
y
direction. It is understood that this is the force exerted by the fluid of
lesser
y
on the fluid of greater
y.
Furthermore, we replace
V/Y
by
-dvx/dy.
Then, in
terms of these symbols,
Eq.
1.1-1 becomes
This equation, which states that the shearing force per unit area is proportional to the
negative of the velocity gradient, is often called
Newton's
law
of
visco~ity.~
Actually we
Some authors write Eq.
1.1-2
in the form
in which
ryx
[=]
lbf/ft2,
v,
[=]
ft/s,
y
[=I
ft, and
p
[=]
lb,/ft. s; the quantityg, is the "gravitational
conversion factor" with the value of
32.174
poundals/lbf.
In
this book we will always use
Eq.
1.1-2
rather
than Eq.
1.1-2a.
Sir
Isaac
Newton
(1643-1727),
a professor at Cambridge University and later Master of the Mint,
was the founder of classical mechanics and contributed to other fields of physics as well. Actually Eq.
1.1-2
does not appear in Sir Isaac Newton's
Philosophiae Naturalis Principia
Mathematics,
but the germ of
the idea is there. For illuminating comments, see
D.
J.
Acheson,
Elementary
Fluid Dynamics,
Oxford
University Press,
1990,§6.1.

51.1
Newton's Law of Viscosity (Molecular Transport of Momentum)
13
should not refer to Eq. 1.1-2 as a "law," since Newton suggested it as an empiricism3-
the simplest proposal that could be made for relating the stress and the velocity gradi-
ent. However, it has been found that the resistance to flow of all gases and all liquids
with molecular weight of less than about 5000 is described by Eq. 1.1-2, and such fluids
are referred to as Newtonian fluids. Polymeric liquids, suspensions, pastes, slurries, and
other complex fluids are not described by
Eq.
1.1-2 and are referred to as non-Newtonian
fluids.
Polymeric liquids are discussed in Chapter
8.
Equation 1.1-2 may be interpreted in another fashion. In the neighborhood of the
moving solid surface at
y
=
0
the fluid acquires a certain amount of x-momentum. This
fluid, in turn, imparts momentum to the adjacent layer of liquid, causing it to remain in
motion in the
x
direction. Hence x-momentum is being transmitted through the fluid in
the positive
y
direction. Therefore
r,,
may also be interpreted as the flux
of
x-momentum
in the positive
y
direction, where the term "flux" means "flow per unit area." This interpre-
tation is consistent with the molecular picture of momentum transport and the kinetic
theories of gases and liquids. It also is in harmony with the analogous treatment given
later for heat and mass transport.
The idea in the preceding paragraph may be paraphrased by saying that momentum
goes "downhill" from a region of high velocity to a region of low velocity-just as a sled
goes downhill from a region of high elevation to a region of low elevation, or the way
heat flows from a region of high temperature to a region of low temperature. The veloc-
ity gradient can therefore be thought of as
a
"driving force" for momentum transport.
In what follows we shall sometimes refer to Newton's law in Eq. 1.1-2 in terms of
forces (which emphasizes the mechanical nature of the subject) and sometimes in terms
of momentum transport (which emphasizes the analogies with heat and mass transport).
This dual viewpoint should prove helpful in physical interpretations.
Often fluid dynamicists use the symbol
v
to represent the viscosity divided by the
density (mass per unit volume) of the fluid, thus:
This quantity is called the kinematic viscosity.
Next we make a few comments about the units of the quantities we have defined. If
we use the symbol
[=I
to mean "has units of," then in the SI system
r,,
[=I
N/m2
=
Pa,
v,
[=
J
m/s, and
y
[=I
m, so that
since the units on both sides of Eq. 1.1-2 must agree. We summarize the above and also
give the units for the c.g.s. system and the British system in Table 1.1-1. The conversion
tables in Appendix
F
will prove to be very useful for solving numerical problems involv-
ing diverse systems of units.
The viscosities of fluids vary over many orders of magnitude, with the viscosity of
air at 20°C being
1.8
x
Pa
.
s and that of glycerol being about
1
Pa
.
s, with some sili-
cone oils being even more viscous. In Tables 1.1-2,l. 1-3, and 1.1-4 experimental data4 are
A relation of the form of Eq. 1.1-2 does come out of the simple kinetic theory of gases (Eq. 1.4-7).
However, a rigorous theory for gases sketched in Appendix
D
makes it clear that Eq. 1.1-2 arises as the
first term in an expansion, and that additional (higher-order) terms are to
be
expected. Also, even an
elementary kinetic theory of liquids predicts non-Newtonian behavior (Eq. 1.5-6).
A
comprehensive presentation of experimental techniques for measuring transport properties can be
found in W. A. Wakeham,
A.
Nagashima, and
J.
V.
Sengers,
Measurement of the Transporf Properties offluids,
CRC Press, Boca Raton,
Fla.
(1991). Sources for experimental data are: Landolt-Bornstein,
Zahlenwerte und
Funktionen,
Vol. II,5, Springer (1968-1969);
International Critical Tables,
McGraw-Hill, New York (1926);
Y.
S. Touloukian,
P.
E. Liley, and S. C. Saxena,
Tkermopkysical Properties of Matter,
Plenum Press, New York
(1970); and also numerous handbooks of chemistry, physics, fluid dynamics, and heat transfer.
14
Chapter
1
Viscosity and the Mechanisms of Momentum Transport
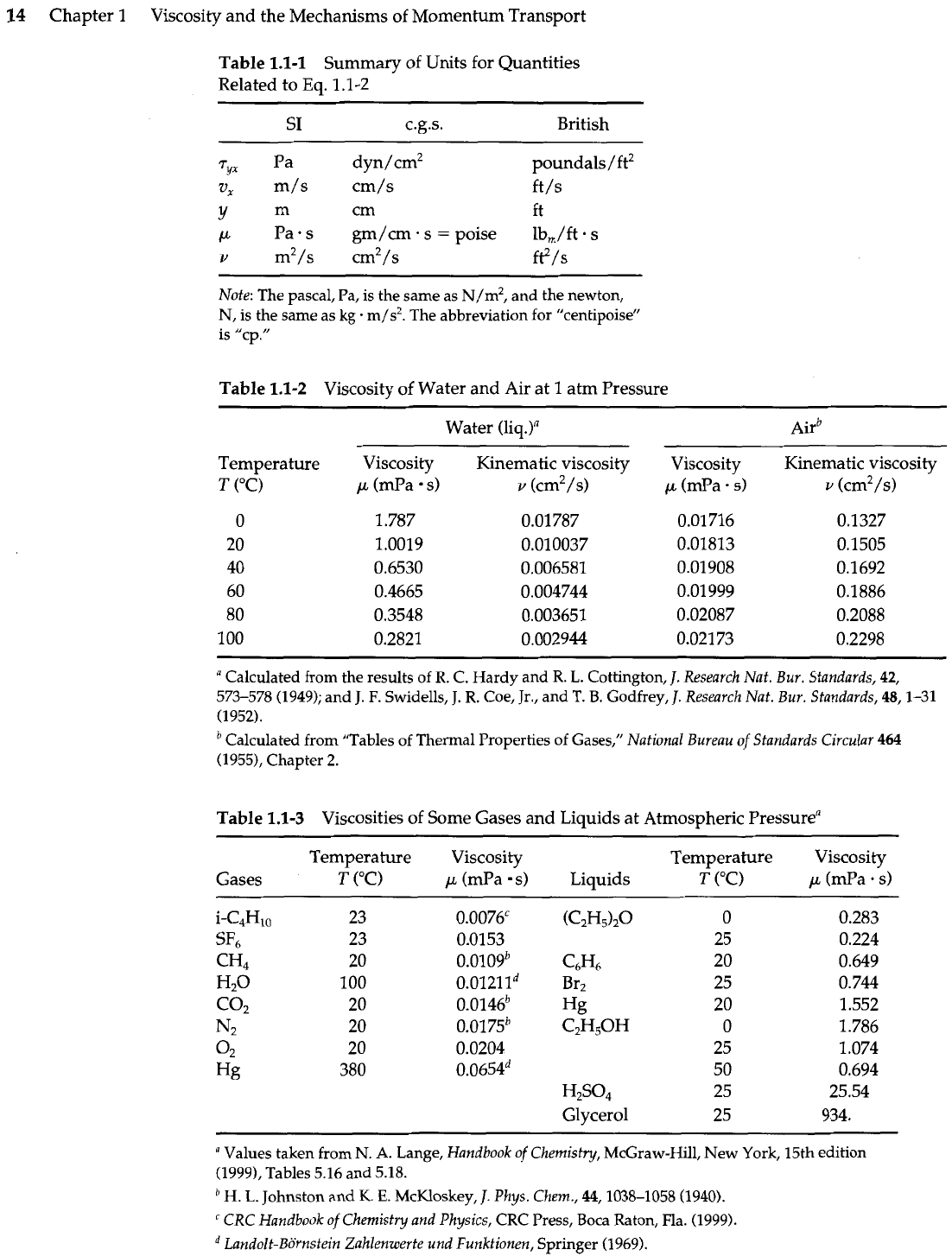
Table
1.1-1
Summary of Units for Quantities
Related to Eq.
1 .l-2
--
SI c.g.s. British
Note:
The pascal, Pa, is the same as N/m2, and the newton,
N,
is the same as kg
-
m/s2. The abbreviation for "centipoise"
is "cp."
Table
1.1-2
Viscosity of Water and Air at
1
atm Pressure
Water (liq.)"
Temperature
T
("C)
Viscosity
p
(mPa s)
1.787
1.0019
0.6530
0.4665
0.3548
0.2821
Kinematic viscosity
v
(cm2/s)
0.01 787
0.010037
0.006581
0.004744
0.003651
0.002944
Viscosity
p
(mPa. s)
Kinematic viscosity
v
(cm2/s)
Talculated from the results of
R.
C. Hardy and
R.
L.
Cottington,
J.
Research Nut. Bur. Standards,
42,
573-578 (1949); and
J.
F.
Swidells, J. R. Coe, Jr., and T.
B.
Godfrey,
J.
Research Naf. Bur. Standards,
48,l-31
(1952).
Calculated from "Tables
of
Thermal Properties of Gases,"
National Bureau of Standards Circular
464
(1955), Chapter
2.
Table
1.1-3
Viscosities of Some Gases and Liquids at Atmospheric Pressurea
-
-
--
Temperature Viscosity Temperature Viscosity
Gases
T
CC)
p
(mPa s) Liquids
T
rC)
p
(mPa. s)
(C&&,O
O
25
C6H6
20
Br2
25
Hg
20
C2H50H
0
25
50
H2S0,
25
Glycerol
25
Talues taken from N.
A.
Lange,
Handbook of Chemistry,
McGraw-Hill, New York, 15th edition
(1999), Tables 5.16 and 5.18.
H.
L.
Johnston
and
K.
E.
McKloskey,
J.
Phys.
Chern.,
44,1038-1058 (1940).
CRC Handbook of Chemistry and Physics,
CRC Press, Boca Raton,
Fla.
(1999).
Landolt-Bornstein Zahlenwerfe und Funktionen,
Springer (1969).
$1.1 Newton's Law of Viscosity (Molecular Transport of Momentum)
15
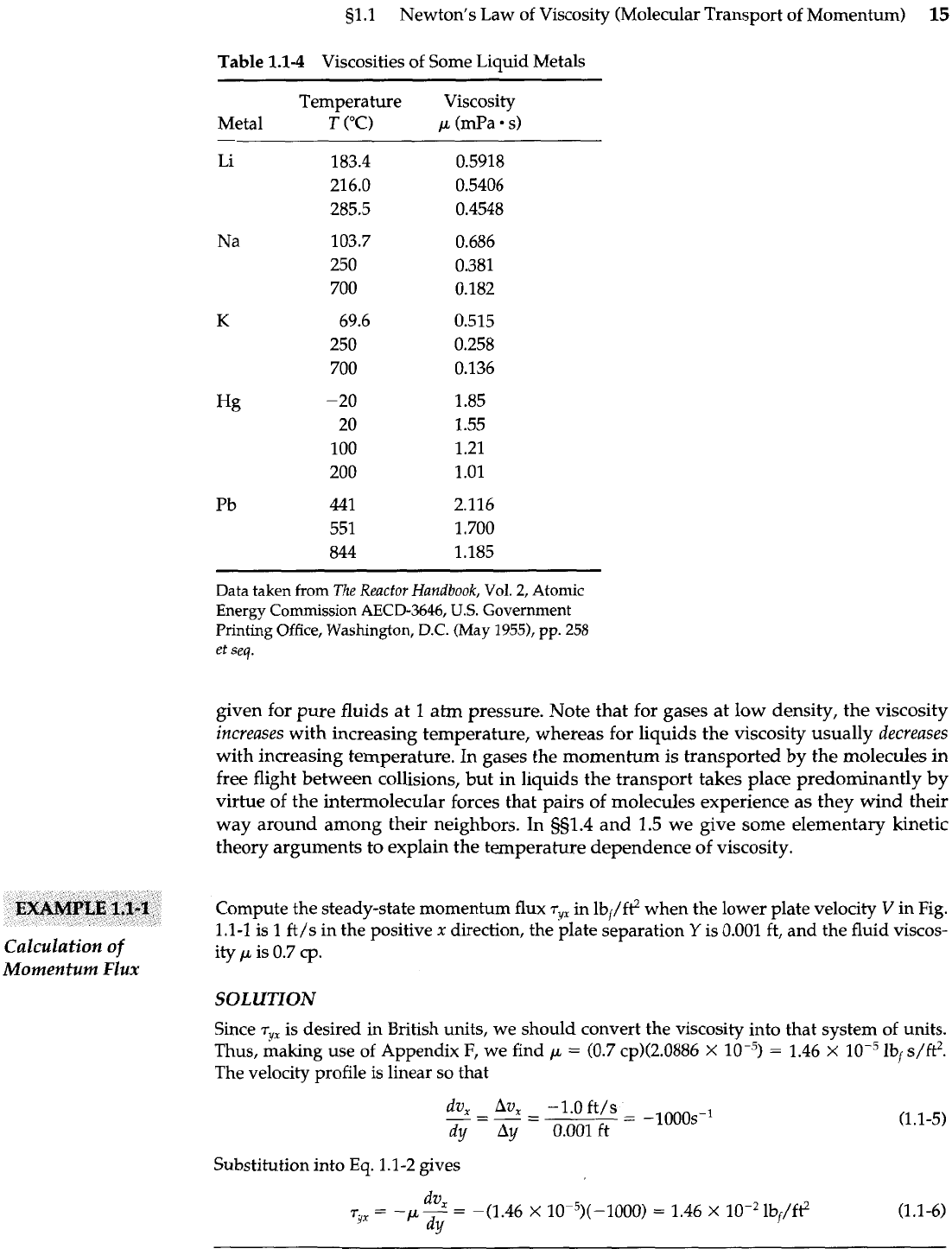
Table
1.1-4
Viscosities of Some Liquid Metals
Temperature Viscosity
Metal
T
("(3
p
(mPa s)
--
-
--
Data taken from
The Reactor Handbook,
Vol.
2,
Atomic
Energy Commission
AECD-3646,
U.S.
Government
Printing
Office,
Washington, D.C. (May
1955),
pp.
258
et seq.
given for pure fluids at
1
atm pressure. Note that for gases at low density, the viscosity
increases
with increasing temperature, whereas for liquids the viscosity usually
decreases
with increasing temperature. In gases the momentum is transported by the molecules in
free flight between collisions, but in liquids the transport takes place predominantly by
virtue of the intermoIecular forces that pairs of molecules experience as they wind their
way around among their neighbors. In
g51.4
and
1.5
we give some elementary kinetic
theory arguments to explain the temperature dependence of viscosity.
Compute the steady-state momentum flux
T,,
in lbf/ft? when the lower plate velocity
V
in Fig.
1.1-1 is I ft/s in the positive
x
direction, the plate separation
Y
is 0.001 ft, and the fluid viscos-
Calculation of
ity
p
is 0.7 cp.
Momentum Flux
SOLUTION
Since
T~,
is desired in British units, we should convert the viscosity into that system of units.
Thus, making use of Appendix
F,
we find
p
=
(0.7 cp)(2.0886
X
=
1.46
x
Ibf
s/ft2.
The velocity profile is linear so that
dv,
-
Av,
-
-1.0 ft/s
=
-10oos-~
dy
Ay
0.001 ft
Substitution into
Eq.
1.1-2 gives
16
Chapter 1 Viscosity and the Mechanisms of Momentum Transport
1.2
GENERALIZATION OF NEWTON'S LAW
OF
VISCOSITY
In the previous section the viscosity was defined by
Eq.
1.1-2, in terms of a simple
steady-state shearing flow in which
v,
is a function of
y
alone, and
v,
and
v,
are zero.
Usually we are interested in more complicated flows in which the three velocity compo-
nents may depend on all three coordinates and possibly on time. Therefore we must
have an expression more general than Eq. 1.1-2, but it must simplify to Eq. 1.1-2 for
steady-state shearing flow.
This generalization is not simple;
in
fact, it took mathematicians about a century and a
half to do this. It is not appropriate for us to give all the details of this development here,
since they can be found in many fluid dynamics books.' Instead we explain briefly the main
ideas that led to the discovery of the required generalization of Newton's law of viscosity.
To do this we consider a very general flow pattern, in which the fluid velocity may
be
in various directions at various places and may depend on the time
t.
The velocity
components are then given by
In such
a
situation, there will be nine stress components
ril
(where
i
and
j
may take on
the designations
x,
y, and
z),
instead of the component
T~
that appears in
Eq.
1.1-2.
We
therefore must begin by defining these stress components.
In
Fig.
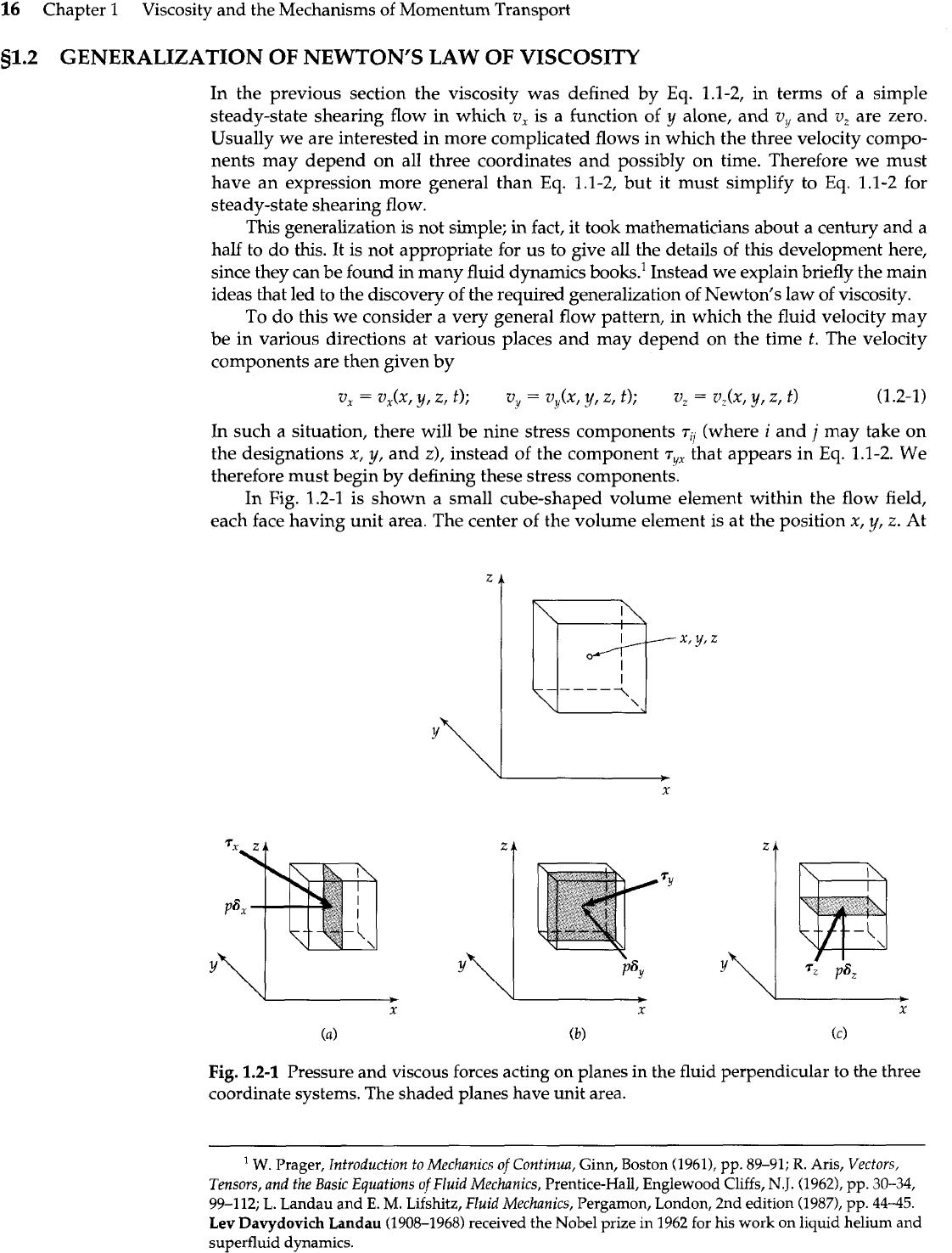
1.2-1 is shown a small cube-shaped volume element within the flow field,
each face having unit area. The center of the volume element is at the position x,
y,
z.
At
Fig.
1.2-1
Pressure and viscous forces acting on planes in the fluid perpendicular to the three
coordinate systems. The shaded planes have unit area.
W.
Prager,
Introduction to Mechanics of Continua,
Ginn, Boston (1961),
pp.
89-91;
R.
Aris,
Vectors,
Tensors, and the Basic Equations of Fluid Mechanics,
Prentice-Hall, Englewood Cliffs,
N.J.
(19621,
pp.
30-34,
99-112; L. Landau and
E.
M.
Lifshitz,
Fluid Mechanics,
Pergamon, London, 2nd edition (1987),
pp.
44-45.
Lev Davydovich Landau
(1908-1968) received the Nobel prize in 1962 for his work on liquid helium and
superfluid dynamics.
