Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

Handbook of Clay Science
Edited by F. Bergaya, B.K.G. Theng and G. Lagaly
Developments in Clay Science, Vol. 1
r 2006 Published by Elsevier Ltd.
693
Chapter 11.3
CLAY LINERS AND WASTE DISPOSAL
K. CZURDA
Angewandte Geologie, Universita
¨
t Karlsruhe, D-76128 Karlsruhe, Germany
As populations grow and technologies advance, the type and quantity of waste
produced keeps on growing. As a result, waste disposal became a huge environmental
problem. This chapter is concerned with waste encapsulation in relation to environ-
mental clean-up and protection. The term ‘encapsulation’ refers to the sealing of
waste body by geological and engineered liner systems. In most cases, such systems
partly consist of clay liners with varied modifications. Thus, encapsulation systems
are as varied as the environments in which they are built, and the components of an
encapsulation system are as multiple and complex as the wastes themselves. For all
waste types, encapsulation is the only option if the waste has to be permanently
isolated from the accessible environment (Caldwell and Reith, 1993). The require-
ments for an encapsulation system are basically the same, whether the waste is mu-
nicipal refuse in a landfill, hospital debris in a low-level waste dump, or mixed wastes
of diverse industrial production/construction activities. This leads us to the need to
classify wastes because encapsulation systems consist of engineered liner components
according to the magnitude of the risks which are associated with the waste.
From the beginning, sophisticated engineered liners should meet two requirements:
(i) to guarantee practical imperviousness so as to prevent leachates from infiltrating
the environment and (ii) to possess retention or at least retardation properties pre-
venting contaminant migration by convection and diffusion (Drescher, 1997). In many
cases—but not absolutely—the surface barrier (layer) may be slightly permeable, al-
lowing further decomposition of sanitary wastes by precipitation moisture. Because of
the different functions of the surface and the base encapsulation barriers, different
state-of-the-art systems were developed (Fig. 11.3.1). In practice, clay liners are
designed so that most of the required properties are optimally expressed (Fig. 11.3.2).
11.3.1. WASTE CATEGORIES
Different waste compositions require different sealing units. For example, it is not
economical to use a multilayer system for inert construction wastes or to design an
DOI: 10.1016/S1572-4352(05)01022-6
identical system for sanitary landfills and toxic industrial wastes (Br adshaw et al.,
1992). For this reason, all regional waste repository regulations ha ve to categorise
wastes not according to the input of waste components but mainly in terms of
leaching the waste and quantifying the contaminant content (Czurda, 1992). The
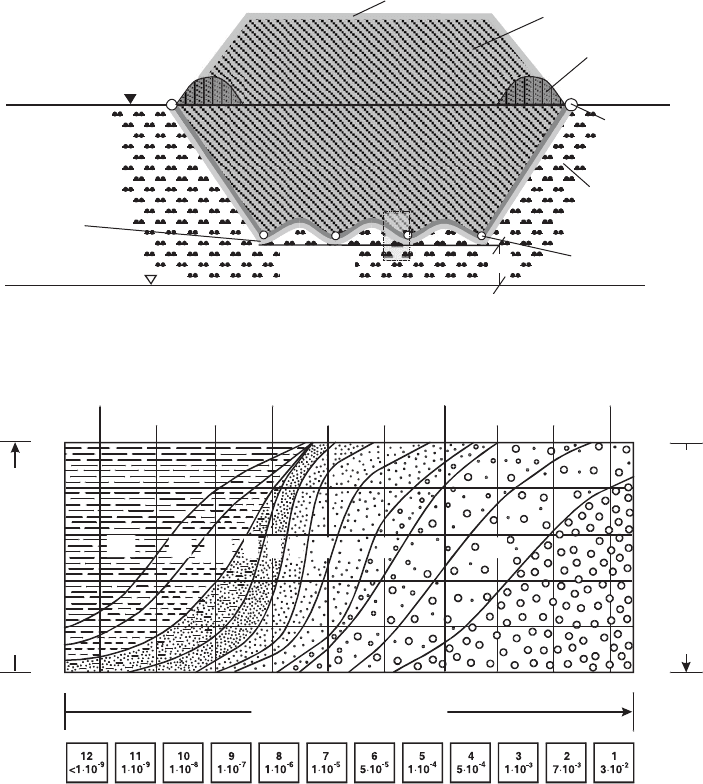
1m
Groundsurface
Technical
barrier
Geologic
barrier
Ring
drainage
Supporting
dam
Waste
Surface liner
Drainage
system
Groundwater table
Base liner
Fig. 11.3.1. Multibarrier system for waste encapsulation. Geologic in situ barriers and en-
gineered technical barriers (compacted mineral layers and geomembranes) are the main parts
of the system.
131011
0
20
40
60
80
100
percent coarser by weight
8 4 212 9 7 6
0
20
40
60
80
100
percent finer by weight
0.001 0.002 0.006 0.02 0.06 0.2 0.6 2 6 20 10060
grain diameter [mm]
range of hydraulic permeability (k) [m·s
-1
]
clay
silt
middle
sand
middle
gravel
middle
stones
fine coarse fine finecoarse coarse
5
Fig. 11.3.2. Practical imperviousness is the main function of liner systems. Hydraulic perme-
abilities are expressed as k coefficients in ms
1
. The range of kp10
7
ms
1
is considered as one
of the most important barrier features of mineral sealing units in the national regulations.
Grain size distribution areas 10, 11, and 12 refer to this.
Chapter 11.3: Clay Liners and Waste Disposal694

European Union, most European countries, the USA, Canada, Japan, etc. follow
this system, ending up with similar waste categories. Taking radioactive waste into
account leads to the following classification scheme:
inert construction and industrial waste,
domestic waste,
toxic industrial waste,
incineration ashes and slags, and
radioactive waste.
Nuclear waste repositories have to follow special national regulations and are not
further treated in this chapter.
As an example for waste assignments, some thres hold values accordi ng to
German regulations (Gassner, 1991;TASiedlungsabfall, 1993; Deponieverordnung,
2002) are shown in Table 11.3.1. They are close to European Union values
(EU-Richtlinie, 1999).
11.3.2. MINERAL BARRIERS
Clay rocks, clay mineral admixtures, and zeolite admixtures are the most impor-
tant and widely used natural materials for constructing mineral barriers within en-
gineered sealing layers. These materials are also used as constituents of in situ
geological barriers, i.e., waste deposit location. The use of alternative materials such
as amorphous silica, fly ashes, fly ash zeolites, clay remnant s from coal flotation, etc.
is not discussed.
Table 11.3.1. Assignment criteria for waste categories according to the German regulations
TA-A and TA-Si. Selected examples. Category I is inert waste, category II is domestic waste,
and category III is toxic industrial waste. The leachable parts are listed as examples for very
common toxic waste constituents
I II III
Conductivity p6000 mS/cm p50000 mS/cm p100000 mS/cm
Uniaxial strength X50 kN/m
2
X50 kN/m
2
X50 kN/m
2
TOC
p20 mg/L p100 mg/L p200 mg/L
Phenols p0.2 mg/L p50 mg/L p100 mg/L
Mercury p0.005 mg/L p0.020 mg/L p0.100 mg/L
Cadmium p 0.050 mg/L p0.100 mg/L p0.500 mg/L
Lead p0.200 mg/L p1000 mg/L p2000 mg/L
Sulphate p500 mg/L p1400 mg/L p5000 mg/L
Soluble part p3% p6% p10%
Total Organic Carbon
11.3.2. Mineral Barriers 695
A. Clay Rocks and Clay Minerals
In using clays and zeolites and other fine-grain material for sealing purposes, two
main issues are relevant: (i) leachate retention due to low-hydraulic conductivities
and (ii) toxic constituent retention or retardation due to adsorption, precipitation,
redox processes, and other mechanisms. Soil barriers, containing enough clay min-
erals with adequate properties to provide low permeability, are used extensively to
prevent rapid advective migration of various leachates from waste disposal sites
(Hiltmann and Stribrny, 1998). The clayey barriers vary from thin geosynthetic clay
liners (GCL) of 1–3 cm thickness, to compacted clay liners (CCL) up to 300 cm in
thickness, to natural undisturbed clayey barriers up to 30 m or more in thickness.
The hydraulic conductivity of undisturbed clayey deposits depends on the miner-
alogy, environment of deposition, and stress history of the deposits. The same holds
true for GCL and CCL.
Clays attract water, other polar liqui ds, and cations. A dried-out clay will expand
as it adsorbs water between its layers and particles when placed in an aqueous
solution. If toxic ions are present in the solution, they can adsorb on the charged clay
surface mineral by ion exchange. Thus, clays can accept or release ions depending on
the concentration of the ions in solution relative to that on the surface. These ions,
e.g., from the leachate, are not finally fixed but can participate in further exchange
processes depending on the chemical environment.
The nature of the cation s initially present at the clay mineral surface (derived, for
example, from the marine environment) is of decisive influence on adsorption po-
tentials. According to the diameter of hydrated cations and their valency they are
differently adsorbed by the clay surface mineral and are therefore exchangeable in
different quantities. For example, Na
+
-bentonites are especially suitable for base
liner construction. Because of their high-swelling potential and adsorpt ion capacity,
they fulfil the requirements for a high degree of imperviousness, and a high con-
taminant retention potential. Table 11.3.2 shows the cation exchange capacity (C EC)
and specific surface areas of some clay minerals and other materials. The theoretical
specific surface areas of smectites and vermiculites of 750–800 m
2
/g are only effective
when the contaminants can fully penetrate the interlayer space. This may be the case
for ion exchange with pure inorganic ions (see Chapter 12.10) and suitable organic
cations (see Chapter 7.3) but doe s not often apply to the adsorption of neutral (non-
ionic) compounds.
B. Zeolites
Zeolites show a high potential as contaminant adsorbents due to their highexchange
capacity and selectivity for certain cations, such as NH
4
+
,Pb
2+
,Cd
2+
,Sr
2+
, and
other metal ions, especially after ‘activation’ by sodium chloride. The selec tivity of
certain zeolites for specific chemicals is controlled by their pore size and charge
Chapter 11.3: Clay Liners and Waste Disposal696

properties. By analogy with clay minerals, the substitution of Al
3+
for Si
4+
in the
structure leads to a net negative charge and a high CEC for most natural zeoli tes.
Natural zeolites occur in sediments, lava vesicules, deuteric-altered plutonic rocks,
and hydrothermal systems associated with alkaline volcanic rocks. Since natural
zeolites generally derive from volcanic glass, they are of widespread occurrence.
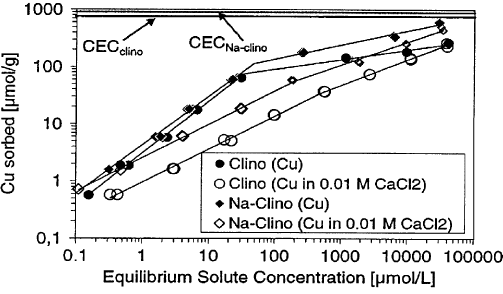
Fig. 11.3.3 shows the isotherms for the adsorption of Cu
2+
from deionised water
and a 0.01 M CaCl
2
solution by clinoptilolite (a very common natural zeolite) and
Na
+
–clinoptilolite (Huttenloch et al., 2001). The isotherms are curvi-linear, and can
be described by the Freundlich equation: c
s
¼ K
f
C
w
n
where c
s
denotes the amount of
the solute adsorbed per unit mass of adsorbent, c
w
is the equ ilibrium solute con-
centration, K
f
and n are empirical parameters specific of the adsorbate-adsorbent
interaction.
11.3.3. WASTE DEPOSIT MULTIBARRIER SYSTEMS
Waste deposits can in principle be constructed as underground storages and at the
surface as slope storage, slope dump, or depression storage. Common domestic waste,
incineration residues, inert construction wastes, etc. may be stored at the surface.
Underground storage as a special deposition mode is not treated in this chapter.
A. Base Liners
Base liner systems must be able to prevent leakage of contaminants from the waste,
and their infiltration into the subsoil. These systems have to prove a high potential for
retaining toxic materials by adsorption, precipitation, and/or redox processes (Rowe
et al., 1995). Adsorption on mineral surfaces mainly occurs by ion exchange. In many
cases toxicants can be retarded during their migration through the sealing layers.
Table 11.3.2. Cation exchange capacity (CEC) and specific surface area of some clay minerals
and other materials
Adsorbent CEC (cmol(+)/kg)
Specific surface area (m
2
/g)
Allophane 50–100 500–700
Kaolinite 3–15 10–20
Illite 10–40 50–100
Montmorillonite 70–120 10–800
y
Vermiculite 130–210 1–800
y
Fe– +Al–(hydr)oxides (pH 8.0) 3–25 25–40
Humic material 150–250 about 800
¼ meq/100 g.
y
depending on the participation of internal surfaces; lowest value represents the external specific surface
area.
11.3.3. Waste Deposit Multibarrier Systems 697
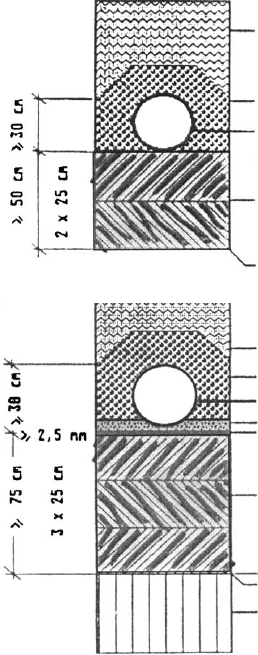
Figs. 11.3.4a, b shows an example of a base liner constructed according to Ger-
man regulations for inert and domestic wastes. The essential components are com-
pacted clay layers, and in case of domestic wastes, a geomem brane in addition to the
mineral layers and of course the geological barrier (Fig. 11.3.5). The basal system
contains a leachate collecting layer, connected to a purification plant. There are
different leakage detection systems on the market.
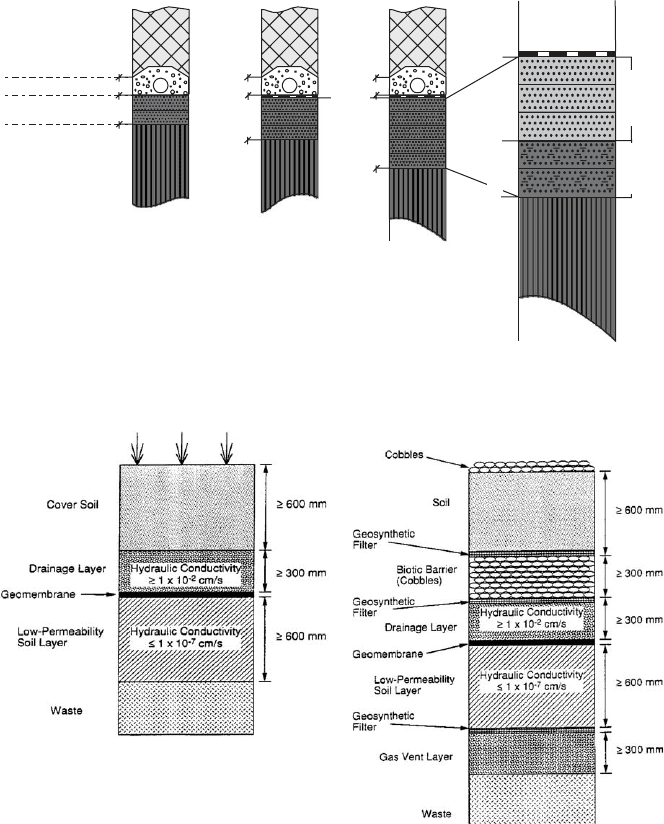
B. Surface Liners
The exclusive function of the surface liner is to prevent precipitation water from
infiltrating into the waste. In case of household wastes, the capping system has to
have a gas drainage system. Capping layers for all types of waste are therefore
constructed with a drainage layer (usually 16–32 mm gravel) in case of leaks in the
system.
As in the case of basal systems, CCL and geomem branes are the prevailing sealing
elements (Fig. 11.3.6a, b). But there is an important difference in the clay mineral
composition of the CCLs. Whereas the base CCL-clay should contain 2:1 layer clay
minerals (e.g ., montmorillonite, vermiculite), the surface CCL-clay should contain
1:1 phyllosilicates (e.g., kaolinite) as index minerals. The 2:1 minerals enable retar-
dation by adsorption and a high degree of impermeability to be obtained, while the
1:1 clays of the surface sealing unit, combined with a sand/silt matrix, are practically
impermeable but have a low-adsorption potential. Because of the small particle size,
a sand–silt–kaolinite admixture for the mineral surface sealing can have a very low
permeability (k
f
¼ 10
28
210
212
cm=s).
Fig. 11.3.3. Adsorption isotherms of Cu
2+
on natural and Na
+
clinoptilolite in deionised
water and 0.01 M CaCl
2
solution (1 g samples in 40 mL copper salt solutions, contact time
96 h, at 20 1C). The CEC of clinoptilolite is 720 mmol(+)/g and of Na
+
–clinoptilolite is
900 mmol(+)/g.
Chapter 11.3: Clay Liners and Waste Disposal698
11.3.4. CONCLUSIONS
For hazardous industrial wastes and toxic sanitary landfills, we have to locate a
site that functions primarily as a geologic barrier with k
f
o10
–6
m/s and at least 3 m in
thickness. In case of inert (non-toxic) wastes a geologic barrier is not necessary. It is
essential, however, to follow the multibarrier concept and to add on top of the
geologic barrier a system of engineered barriers and drainage layers. The engineer ed
barriers comprise as a core unit the combined CCL and geomembrane double layer.
A similar multibarrier system has to be constructed for the cover sealing. The dif-
ference is expressed in the type of GCL and the drainage layers. The GCL should not
contain expanding 2:1 clay minerals, such as montmorillonite or vermiculite, because
they tend to dry out and form desiccation cracks. Therefore non-swelling 1:1 clay
waste
waste
drainage
geomembrane
clay liner
geologic
barrier
drainage
clay liner
(a)
(b)
Fig. 11.3.4. (a) Base liner system for inert waste. Two CCL without geomembrane (b) Base
liner system for domestic waste. Three CCL combined with a geomembrane.
11.3.4. Conclusions 699
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
~
30cm 30cm
75cm
150cm
Base Liner System
Domestic waste
German regulation
Industrial waste
German regulation
Category I
Category II
Multimineral barrier
Waste
Drainage layer 30cm
Mineral barrier 50cm
Geologic barrier
Geo-
membrane
Sorption
layer
Clay,
Na-bentonite
optimized
Clay,
kaolinite
optimized
Im
pervious
layer
Fig. 11.3.5. Comparing different base liner systems. The multibarrier system consists of two
clay units: an adsorbing bentonite unit and a sealing kaolinite unit.
(a)
(b)
Fig. 11.3.6. Environmental Protection Agency (EPA) recommendation for surface liner sys-
tems in the USA. Clay barriers are combined with geomembranes (a) The optional system (b)
comprises additional filler systems: a biotic filter on top of the sealing unit and a gas collection
layer below.
Chapter 11.3: Clay Liners and Waste Disposal700
minerals (e.g., kaolinite) or tectosilicates (e.g., zeolites) should be the index minerals
for the sand–silt–clay surface GCL. In case of untreated household waste, a gas
drainage layer has to be provided in order to divert the methanol that develops as the
waste decomposes.
REFERENCES
Bradshaw, A.D., Southwood, R., Warner, F., 1992. The Treatment and Handling of Wastes.
Chapman & Hall, London.
Caldwell, J.A., Reith, C.C., 1993. Principles and Practice of Waste Encapsulation. Lewis
Publishers, Boca Raton, FL.
Czurda, K.A., 1992. Deponie und Altlasten. EF-Verlag fu
¨
r Energie- und Umwelttechnik
GmbH, Berlin.
Deponieverordnung, 2002. Verordnung u
¨
ber Deponien und Langzeitlager, Bun-
desministerium fu
¨
r Umwelt, Naturschutz und Reaktorsicherheit, Berlin.
Drescher, J., 1997. Deponiebau. Alphabet KG, Berlin.
EU-Richtlinie, 1999. Richtlinie u
¨
ber Abfalldeponien, Rat der Europa
¨
ischen Union. Amtsblatt
der Europa
¨
ischen Union, Bru
¨
ssel.
Gassner, E., 1991. Gesamtfassung der Zweiten allgemeinen Verwaltungsvorschrift zum Ab-
fallgesetz 1. Teil. Verlag Franz Rehm, Mu
¨
nchen.
Hiltmann, W., Stribrny, B., 1998. Tonmineralogie und Bodenphysik. Springer-Verlag, Berlin.
Huttenloch, P., Roehl, K.E., Czurda, K.A., 2001. Sorption of nonpolar aromatic contam-
inants by chlorosilane surface-modified natural minerals. Environmental Science and
Technology 35, 4260–4264.
Rowe, R.K., Quigley, R.M., Booker, J.R., 1995. Clayey Barrier Systems for Waste Disposal
Facilities. Chapman & Hall, London.
TA Siedlungsabfall, 1993. Technische Anleitung zur Verwertung, Behandlung und sonstigen
Entsorgung von Siedlungsabfa
¨
llen, Ministerium fu
¨
r Umwelt, Rheinland-Pfalz. Bundesan-
zeiger, Ko
¨
ln.
References 701
Handbook of Clay Science
Edited by F. Bergaya, B.K.G. Theng and G. Lagaly
Developments in Clay Science, Vol. 1
r 2006 Elsevier Ltd. All rights reserved.
703
Chapter 11.4
CLAYS AND NUCLEAR WASTE MANAGEMENT
R. PUSCH
Geodevelopment AB, Ideon, S-22370 Lund, Sweden
Eliminating the risk of transportation of radionuclides from radioactive waste
stored underground to the biosphere is the goal set by all countries that make use of
nuclear power. Different countries plan ‘‘geologic disposal’’ in rocks, salt and clay
sediments. The design principle is that highly rad ioactive waste, like burnt-out fuel
and products from processed waste, is enclosed in containers—canisters—isolated by
smectitic (smectite-rich) clay. These canisters are placed in vertical boreholes or long
horizontal bored or blasted tunnels at a depth of 300–800 m. Low- and medium-level
waste has shorter lifetimes and can be stored at smaller depth with less effective
isolation.
Selection of smectitic clays with suitable bulk density to embed the canisters, is
based on the fact that this type of ‘‘clay buffer’’ has the following valuable prop-
erties: (i) very low hydraulic conductivity; (ii) very low anion diffusion capacity and
fairly low transport capacity of positively charged radionuclides; (iii) a high swelling
potential for self-sealing of gaps and openings in the buffer and its contacts with the
rock and canisters; (iv) favourable rheological properties, such as sufficient bearing
capacity to minimise settlement of the heavy canisters, and sufficient softness to
avoid transfer of high tectonically induced shear stresses to the canisters; (v) suf-
ficient thermal conductivity to transfer heat caused by the radioactive decay to the
rock without being too hot; (vi) colloid filtering capacity; and (vii) capacity to filter
microbes.
The optimum bulk density at fluid saturation should be in the region of
1900–2100 kg/m
3
to meet the criteria; this density cannot be achieved by on-site
compaction. Instead, very dense blocks of highly compacted smectitic clay powder
are placed around the canisters, embedding them tightly. The required tightness is
attained when the clay material swells after taking up water from the surrounding
rock. Suggested design principles for repositories are illustrated in Fig. 11.4.1. The
design of a repository for low- and medium-radioact ive waste is illustrated in
Fig. 11.4.2. This type of repository is located about 150 km north of Stockholm and
has been in operation for 15 years (Pusch, 1994).
DOI: 10.1016/S1572-4352(05)01023-8
