Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

Hansbo, S., 1960. Consolidation of clay with special reference to influence of vertical sand
drains. Study in connection with full-scale investigations at Ska
˚
-Edeby. Swedish Geotech-
nical Institute, Proceeding No 18.
Horseman, S. T., Harrington, J. F., 1997. Study of gas migration in MX-80 buffer bentonite.
National Environmental Research Council, British Geological Survey. Report WE/97/7.
Kato, H., Muroi M., Yamada, N., Ishida, H., Sato, H., 1995. Estimation of effective diffu-
sivity in compacted bentonite. In: Murakami, T., Ewing, R. C. (Eds). Scientific Basis for
Nuclear Waste Management XVIII. Materials Research Society Symposium Proceedings
353, 277–284.
Pusch, R., 1994. Waste disposal in rock. Developments in Geotechnical Engineering, 76.
Elsevier Amsterdam.
Pusch, R., Adey, R., 1999. Creep in buffer clay. SKB Technical Report TR-99-32. SKB,
Stockholm.
Pusch, R., Bo
¨
rgesson, L., Erlstro
¨
m, M., 1987. Alteration of isolating properties of dense
smectite clay in repository environment as exemplified by seven pre-Quaternary clays. SKB
Technical Report TR 87-29. SKB, Stockholm.
Yong, R. N., Mohamed, A.M.O., 1992. A study of particle interaction energies in wetting of
unsaturated expansive clays. Canadian Geotechnical Journal 29, 1060–1070.
Chapter 6: Mechanical Properties of Clays and Clay Minerals260
Handbook of Clay Science
Edited by F. Bergaya, B.K.G. Theng and G. Lagaly
Developments in Clay Science, Vol. 1
r 2006 Elsevier Ltd. All rights reserved.
261
Chapter 7
MODIFIED CLAYS AND CLAY MINERALS
F. BERGAYA
a
, B.K.G. THENG
b
AND G. LAGALY
c
a
CRMD, CNRS-Universite
´
d’Orle
´
ans, 1F-45071 Orleans Cedex 2, France
b
Landcare Research, Palmerst on North, New Zealand
c
Institut fu
¨
r Anorganische Chemie, Universita
¨
t Kiel, D-24118 Kiel, Germany
This composite chapter deals with the physical and chemical modification of clays
and clay minerals in the broad sense. Acid activation and therm al treatment (heat-
ing), described in Chapters 7.1 and 7.2, have long been used to produce materials for
certain practical applications. The surface properties and reactivity of clay minerals
may also be modified by adsorption and intercalation of small and polymeric organic
species. Chapter 7.3 reviews the important topic of clay mineral–organic interac-
tions. Clay minerals have been implicat ed in the abiotic origins of life on earth
because of their ability to adsorb, protect, concentrate, and transform biomolecules.
On this basis, the possible role of clay minerals in life’s origins is included here
(Chapter 7.4). In relation to acid activation and thermal treatment, the pillaring of
clay minerals (Chapter 7.5) is a recent method of mo dification. As the term suggest s,
the process commonly involves intercalation of cati onic species acting as ‘pillars’ to
prop the mineral layers apart. Subsequent heating gives rise to a permanently porous
material, useful for organic catalysis and other environmental applications.
DOI: 10.1016/S1572-4352(05)01007-X
Handbook of Clay Science
Edited by F. Bergaya, B.K.G. Theng and G. Lagaly
Developments in Clay Science, Vol. 1
r 2006 Elsevier Ltd. All rights reserved.
263
Chapter 7.1
ACID ACTIVATION OF CLAY MINERALS
P. KOMADEL AND J. MADEJOVA
´
Institute of Inorganic Chemistry, Slovak Academy of Sciences, SK-845 36 Bratislava,
Slovakia
One of the most common chemical modifications of clays, used for both industrial
and scientific purposes, is their acid activation. This consists of the treatment of clay
with a mineral acid solution, usually HCl or H
2
SO
4
. The main task is to obtain
partly dissolved material of increased specific surface area, porosity and surface
acidity (Komadel, 2003). The manufactured materials are widely available, relatively
inexpensive solid sources of protons, effective in a number of industrially significant
reactions and processes. Acid attack on clays also occurs naturally, e.g., in the
interaction of acid mine drainage with clay minerals (Gala
´
n et al., 1999; Dubı
´
kova
´
et
al., 2002). Mining waste containing sulphides is the most common and greatest
antropogenic source of acidity. Progressive oxidation leads to the production of
protons and sulphates in leaching waters, which generally also mobilize large
amount of metals by dissolution of minerals. These waters influence the composition
of surface waters but also have an impact on surrounding soils and terrestrial eco-
systems.
From the industrial point of view, the term ‘acid-activated clays’ was reserved
mainly for acid-treated bentonites. Bentonite has always had a multitude of markets
and acid-activated bentonite was a traditional product for many decades. It is usu-
ally a Ca
2+
-bentonite that was treated with inorganic acids to replace divalent cal-
cium ions with monovalent hydrogen ions and to leach out ferric, ferrous,
aluminium and magnesium ions thus altering the layers of smectite and increasing
the specific surface area and porosity. This results in the production of bleaching
earths, clays suitable for a range of bleaching or decolourising applications, in which
they compet e against natural bleaching earths (Siddiqui, 1968 ; Kendall, 1996).
This chapter mainly reviews acid treatment of smectites, the dominant minerals in
bentonites. Other pro ducts include environmentally benign catalysts or their sup-
ports, which are used in various chemical reactions such as Friedel-Crafts alkylation
and acylation, dimerisation and polymerisation of unsatur ated hydrocarbons, etc.
(Adams, 1987; Brown, 1994; see also Chapter 10.2), or colour developers in car-
bonless copying papers (Fahn and Fenderl, 1983). Acid-treated clays pillared with
DOI: 10.1016/S1572-4352(05)01008-1
oxyhydroxyaluminium species are used to prepare clay-modified electrodes (Falaras
et al., 2000a), as adsorbents for oil clarification (Mokaya et al., 1993; Falaras et al.,
2000b; Pagano et al., 2001 ) and as catalysts (Mokaya and Jones, 1994 ; Bovey and
Jones, 1995; Bovey et al., 1996).
7.1.1. PROPERTIES AND AUTO-TRANSFORMATION OF
H
+
-EXCHANGED CLAY MINERALS
The acidity of acid-untreated smectites has two sources: (i) the compensating
cations; these may have a strong polarizing effect on coordinating water molecules,
most of which are in the interlayer spaces and may not be easily accessible; and (ii)
specific site s at the layer edges, where unsaturated ‘‘broken’’ bonds occur; these may
be compensated by OH groups formation, leading to Brønsted acid sites such as
Si–OH and also coordinately unsaturated Al
3+
and Mg
2+
easily formed at the
edges, behave as Lewis acid sites (Lambe rt and Poncelet, 1997).
The first step in acid treatment is that the protons replace the exchangeable
cations and then they attack the layers (C
ˇ
ı
´
c
ˇ
el and Komadel, 1994). The exchange
reaction is fast if there is good contact between acid and smectite, and the quantity of
available protons is sufficient. The substitution rate is independent of the smectite if
the mineral contains only swelling layers. In contrast to smectites saturated with
metal cations, proton-saturated smectites are unstabl e. The layers are attacked by
surface and interlayer hydrated protons, even after drying the separated activated
smectite, similar to what occurs in solution. This process, known as ‘auto-transfor-
mation’, spontaneously changes H
+
-smectites to their (Al
3+
,Fe
3+
,Mg
2+
) -forms
on ageing (Barshad and Foscolos, 1970). In aqueous dispersion at 90 1C the process
is completed within 4 days (Janek and Komadel, 1999).
To study the properties of H
+
-clays, maximal saturation by protons and stability
of the product are required. Various preparation methods were tested. The best
results are obtained by passing the clay suspension through a succession of H
+
–OH
–
–H
+
ion-exchange resins. H
+
-forms of o2 mm fractions of ben tonites with
varied Fe
3+
contents are prepared by this method. Potentiometric titrations of pro-
ton-saturated fine fractions of bentonites have been used to characterize the acid sites
at the smectite–water interface in dispersions. The titration curves have revealed that
the number of strong acid sites varies and accounts for 60–95% of the total acidity in
the freshly prepared H
+
-forms (Janek et al., 1997). Layer-charge distributions of all
samples are inhomogeneous. This distribution is changed after oxalate pretreatment
of the samples, due to the removal of readily soluble phases that might have blocked
exchange sites. After auto-transformation, the alkylammonium exchange method
(Lagaly, 1994) reveals inhomogeneous charge density distributions; the fraction of
layers of the highest charge decreases. Comparison of cation exchange capacity
(CEC) obtained from potentiometric curves and the CEC calculated from the mean
layer charge has confirmed that the attack of protons occurs from particle edges.
Chapter 7.1: Acid Activation of Clay Minerals264
However, for several samples the structural attack may also occur from the inter-
layer space. Autotransformation of the H
+
-smectites also decreases the mean layer
charge. Protons preferentially attack the octahedral Mg
2+
during the auto-trans-
formation. A number of strong acid sites decreases and the number of weak acid sites
increases on ageing.
The titration data obtained are used in a thermodynamic calculation of proton
affinity distribution. Numerical solving of an integral adsorption equation reveals a
continuous distribution of proton interaction sites. Proton affinit y distributions
clearly detected up to five different proton interaction sites in all the smectite–water
systems, within accessible experimental range of pHs between 2 and 12. The amoun t
of the strongest acid sites decreases on ageing, while the amount of all weaker acid
sites increases with the progress of autotransformation. The strongest acid sites are
connected with free protons present in the dispersion while the weaker acid sites are
connected with the titration of released structural Al
3+
,Fe
3+
,Mg
2+
cations and/or
their hydrolysed species, and deprotonation of SiOH groups. These results indicate
the sources of acidity in acid activated bentonites (Janek and Komadel, 1993). Hy-
drated aluminium ions in freshly proton-saturated dispersions contribute to a group
of weak acid sites, which also include oligomeric hydroxoaluminium cations. The
amount of these sites increases during auto-transformation. The fres hly prepared
proton-saturated dispersions show low pH values and the particles interact by edge-
to-face contacts. This increases the viscosity in comparison with the sodium forms at
pH close to 7 (Janek and Lagaly, 2001). A kinetic study of proton-promoted dis-
solution of K
+
-montmorillonite in solutions with constant KCl concentrations, us-
ing both titration and batch equilibration experiments, shows that adsorption of H
+
and dissolution of Al
3+
occur (Zysset and Schindler, 1996).
Different clay minerals are treated with HCl or NaOH at room temperature for 2
weeks to obtain information on variable surface charge. Both treatments lead to an
increase of variable surface charge, while the actual charge value increases and
decreases depending on the mineral and the treatment. The heterogeneity of charge-
generating surface groups is observed in natural minerals. Duri ng acid treatment, the
number of weakly acidic surface functional groups increases, while the number of
groups of stronger acidic character decreases. Dissolut ion of Al
3+
prevails over Si
4+
in acid treatments. Illite and kaolinite are most resistant to acid attack. The surface
areas of the minerals computed from both water and nitrogen adsorpt ion isotherms
increase with acid and alkali treatments. The effects of acid and alkali attacks are
controlled by the individual character of the minerals (Jozefaciuk, 2002; Jozefaciuk
and Bowanko, 2002).
7.1.2. METHODS OF INVESTIGATION
The methods being employed to charact erize acid-activated silicates include
chemical analysis, XRD, Mo
¨
ssbauer, Fourier transform infrared (FTIR), and
7.1.2. Methods of Investigation 265
MAS-NMR spectroscopies; Scanning electron microscopy, transmission electron
microscopy (TEM) and high resolution transmission electron microscopy
(HRTEM); acidity, surface area and pore size measurements, etc. Usually a com-
bination of several methods is needed for sufficient characterization of the materials
obtained (C
ˇ
ı
´
c
ˇ
el and Komadel, 1994 ; Vicente et al., 1994; Breen et al., 1995b;
Komadel et al., 19 96b; 1995a; 1995b, 1996b; Gates et al., 2002).
Chemical analysis of solid and/or liquid reaction products, infrared spectroscopy
(IR) and MAS-NMR spectroscopies are very sensitive to the nature and content of
the octahedral atoms and thus, also to the changes that occur in different stages of
acid attack (Breen et al., 1 995a, 1995b).
IR spectroscopy has been used for identification of the main mineral and the
admixtures in the fine fractions of bentonites (Farmer, 1974; Madejova
´
et al., 1992,
1995 Russell and Fraser, 1994; Madejova
´
and Komadel, 2001). It is also a routine
characterization technique for acid-treated clays, very sensitive to modifications of
the clay structure resulting from acid treatment. As protons penetrate into the clay
layers and attack the OH groups, the resulting dehydroxylation connected with
successive release of the octahedral atoms can be readily followed by changes in the
characteristic absorption bands attributed to vibrations of OH groups and/or oc -
tahedral cations. Comparative IR studies of acid-treated smectites (Madejova
´
et al.,
1998) as well as saponites, sepiolite and palygorskite (Vicente et al., 1996a) have been
published.
Changes in the IR spectra of SWy-1 montmorillonite (with quartz admixture)
after treatment with 6 M HCl (Figure 7.1.1) demonstrate the alteration of the chem-
ical bonds in the structure.
The spectrum of the untreated sample shows an intensive band at 1048 cm
–1
attributed to the Si–O stretching vibrations, and bands at 524 and 466 cm
–1
assigned
to Al–O–Si (octahedral Al) and Si–O–Si bending vibrations, respectively. Three
peaks in the hydroxyl bending region at 917 cm
–1
for Al
2
OH, 886 cm
–1
for AlFeOH,
and 850 cm
–1
for AlMgOH reflect that octahedral Al
3+
is partially replaced by Fe
3+
and Mg
2+
. The doublet at 799 and 779 cm
–1
indicates the presence of quartz im-
purity in the sample, which has been confirmed by XRD.
29
Si MAS-NMR shows
that 12% of total Si
4+
is associated with quartz (Tka
´
c
ˇ
et al., 1994; Madejova
´
et al.,
1998).
After 8 h of HCl treatment, no significant changes are seen in the IR spectra of
SWy-1 montmorillonite except for a slight upward shift of the Si–O stretching band
(Dn ¼ 6cm
–1
) together with a weak decrease in the intensities of the OH and
Al–O–Si bending bands. This reflects the partially diminishing content of octahedral
cations due to acid attack.
As the treatment time progresses, a more pronounced decrease is observed in the
intensities of the bands related to octahedral cations. Changes in the Si
4+
environ-
ment with prolonged acid treatment are reflected in both the position and the shape
of the Si–O stretching band. In addition to the tetrahedral Si–O band near
1058 cm
–1
, the IR spectrum of the sample treated for 12 h shows a pronounced
Chapter 7.1: Acid Activation of Clay Minerals266
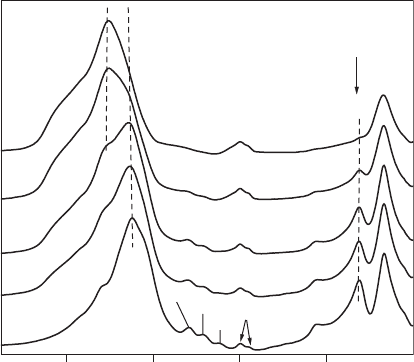
absorption near 1100 cm
–1
. This absorption band is assigned to Si–O vibrations of
amorphous silica with a three-dimensional framework formed during acid treatment.
The spectrum of the sample treated for 18 h confirms a high degree of structural
decomposition. The Si–O band of amorphous silica at 1103 cm
–1
dominates the Si–O
stretching region and only inflections related to OH bending bands are present in the
950–800 cm
–1
region. Anothe r characteristic band of amorphous silica near 800 cm
–1
increases in intensit y with treatment time and gradually overlaps the doublet of
quartz. The band near 524 cm
–1
, which is most sensitive to the presence of residual
Al
3+
in the octahedral sheets, decreases stepwise in intensity with increasing layer
decomposition.
After 30 h of treatment, the small band near 524 cm
–1
in the spectrum of the 18 h
treated sample, is changed to an inflection. This reflects a very high, but yet incom-
plete dissol ution of montmorillonite in 6 M HCl (Madejova
´
et al., 1998).
7.1.3. ACID DISSOLUTION OF SMECTITES
Treatment of clays with strong inorganic acids is frequently called ‘‘acid disso-
lution’’ or ‘‘acid activation’’ of clays. Depending on the extent of acid activation, the
resulting solid product also contains unaltered layers and amorphous three-dimen-
sional cross-linked silica, while the ambient acid solution contains ions according to
the chemical composition of the smectite and acid used.
1200 1000 800 600
30 h
18 h
12 h
8 h
0 h
Al-O-Si
Q
524
917
886
850
799
1103
1048
Absorbance
Wavenumbers / cm
-1
Fig. 7.1.1. IR spectra of SWy-1 montmorillonite treated with 6 M HCl at 95 1C for 0, 8, 12, 18
and 30 h. Q is quartz. (From Madejova
´
et al., 1998.)
7.1.3. Acid Dissolution of Smectites 267
Early acid-dissolution studi es of dioctahedral smectites in HCl by Osthaus (1954,
1956), based on solution analysis, indicated faster dissolution of octahedral than
tetrahedral sheets. Assays of solid reaction products employing advanced spec-
troscopic techniques provided experimental evidence that acid treatments dissolve
central atoms from the tetrahedral and octahedral sheets at similar rates (Luca and
MacLachlan, 1992; Tka
´
c
ˇ
et al., 1994).
Luca and MacLachlan (1992) studied the dissolution in 10% HCl of two non-
tronites from Garfield and Hohen–Hagen, by Mo
¨
ssbauer spectroscopy. They fit the
spectra either with two octahedral Fe
3+
doublets or with two octahedral and one
tetrahedral Fe
3+
doublet. Isomer shift and quadrupole splitting values obtained
from the two-doublet models correspond to octahedral Fe
3+
and not to tetrahedral
Fe
3+
as Osthaus (1954) has suggested. W hen a tetrahedral Fe
3+
doublet is included
in the model used to fit the Mo
¨
ssbauer spectra of acid-treated Garfield nontronite
samples, a slight increase in the intensity of that doublet occurs with increasing
dissolution. This is much lower than Osthaus (1954) indicated. No trend in the
intensity of the tetrahedral Fe
3+
doublet is observed for acid-treated Hohen-Hagen
nontronite samples. Therefore, acid treatment appears to remove octahedral and
tetrahedral Fe
3+
from the nontronite structure at about the same rate. Mo
¨
ssbauer
and IR spectroscopies, and XRD indicate that the undissolved part of nontronite
remaining after acid treatment is structurally similar to the untreated nontronite.
27
Al and
29
Si MAS NMR spectroscopic studies on removal of tetrahedral and oc-
tahedral Al
3+
from montmorillonite by 6 M HCl, yield very similar conclusions
(Tka
´
c
ˇ
et al., 1994). The rates of dissolution of tetrahedral and octahedral Al
3+
are
also comparable for montmorillonite. Three different types of structural units have
been identified in acid-treated samples, including (SiO)
3
SiOH units, remaining as a
result of poor-ordering of the framework without the possibility of cross-linking.
The extent of the dissolution reaction depends on both clay mineral type and
reaction conditions, such as the acid/ clay ratio, acid concentration, time and tem-
perature of the reaction. The composition of the clay layers substantially affects their
stability against acid attack; trioctahedral layers dissolve much faster than their
dioctahedral counterparts (Vicente et al., 1994, 1995b; Breen et al., 1995a; Komadel
et al., 1996b). Higher substitutions of M g
2+
and/or Fe
3+
for Al
3+
in dioctahedral
smectites increase their dissolution rate in acids (Breen et al., 1995b). For 15 di-
octahedral smectites, there is a good correlation of the Mg
2+
and Fe
3+
contents with
the half time of dissolution in 6 M HCl at 96 1C(Nova
´
k and C
ˇ
ic
ˇ
el, 1978). Effects of
smectite type, acid concentration and temperature on the ha lf time of dissolution in
0.2 L HCl/g smectite acid/clay ratio in closed systems (no substances being add ed or
removed) are summarized in Table 7.1.1.
The rate of dissolution of various atoms, obtained from chemical analysis of the
liquid reaction products, indicates the presence of different phases in bentonite.
Readily soluble octahedral and tetrahedral constituents, and ‘‘insoluble’’ portions of
constituent atoms can be calculated from the dissolution curves. This provides in-
formation on the distribution of atoms in the sample (C
ˇ
ı
´
c
ˇ
el and Komadel, 1994).
Chapter 7.1: Acid Activation of Clay Minerals268
Readily soluble portions include exchangeable cations and easily soluble admixtures
such as goethite (Komadel et al., 1993) and calcite (Komadel et al., 1996b). The most
common ‘‘insoluble’’ phases found in the fine fractions of bentonites include kao-
linite, quartz, anatase and volcanic glass. Halloysite is the most decomposed mineral
after treatment in sulphuric acid of different concentrations, followed by montmo-
rillonite, pyrophyllite and kaolinite (Kato et al., 1966). Low octahedral substitution
is one reason for the observed low dissolution rate of pyrophyllite compared with
montmorillonite. The other reason is the presence of collapsed, non-swelling inter-
layers in pyrophyllite.
A series of reduced-charge montmorillonites is prepared via Li
+
fixation at ele-
vated temperatures (Hofmann–Klemen effect) to explore how the expandability of
the interlayer spaces influences the extent of dissolution. As the negative layer charge
of these samples decreases substantially, pyrophyllite-like features appear in both IR
(Madejova
´
et al., 1996) and
29
Si MAS NMR (Gates et al., 2000 ) spectra. The in-
creased content of non-swelling interlayer spaces is confirmed by both XRD and
HRTEM images (Komadel et al., 1996a). The dissolution of reduced-charge mont-
morillonites in HCl indicates that pyrophyllite -like layers surrounded by non-swell-
ing interlayer spaces dissolve much more slowly than montm orillonite layers of
similar chemical composition located between swelling interlayer spaces (Figure 7.1.2).
This clearly shows that protons attack the layers not only from the particle edges but
also from the swollen interlayer spaces. Non-swelling illite and kaolinite are also
more resistant to HCl attack than montmorillonite or vermiculite (Jozefaciuk and
Bowanko, 2002).
IR spectroscopy in both middle and near regions was applied for the investigation
of acid dissolution of reduced-charge montmorillonites. Reduction of the layer
Table 7.1.1. Effects of smectite type, acid concentration and temperature on half time of
dissolution in 0.2L HC1/g smectite in closed systems
Smectite HC1 (M) T(1C) t
0.5
(h)
Effect of smectite type
Nontronite 6.0 95 0.16
Mg-rich montmorillonite 6.0 95 6.2
Al-rich montmorillonite 6.0 95 8.0
Effect of acid concentration
Hectorite 0.25 20 4.6
Hectorite 0.50 20 2.6
Hectorite 1.00 20 1.7
Effect of temperature
Fe
3+
-beidellite 6.0 50 12.0
Fe
3+
-beidellite 6.0 60 6.0
7.1.3. Acid Dissolution of Smectites 269
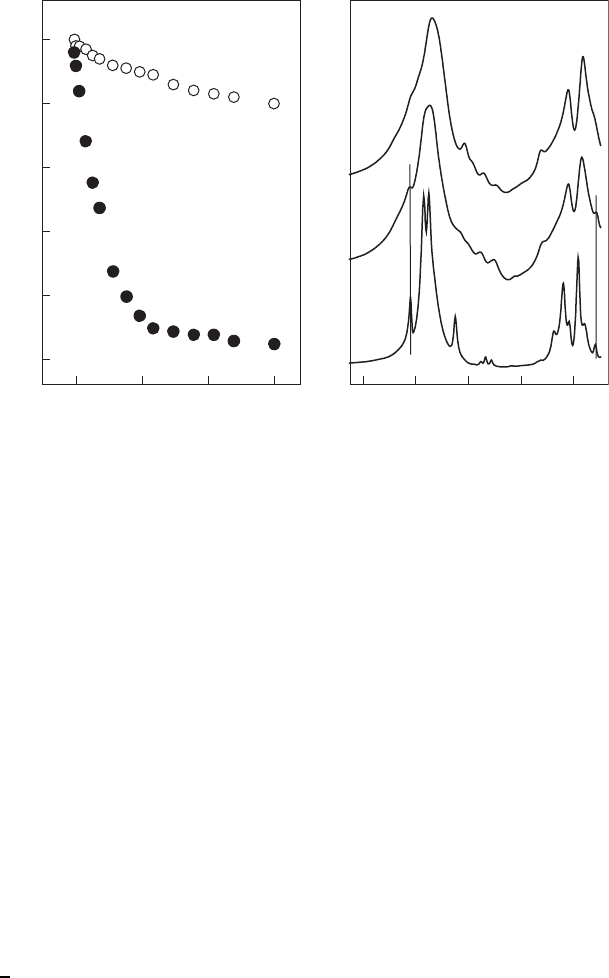
charge connected with development of non-swelling interlayer spaces substantially
decreases the extent of the dissolution of SAz-1 montmorillonite in HCl. New bands
at 3744 and 7314 cm
–1
are assigned to the vibrations of Si–OH groups formed by acid
treatment. These bands are indicative of the extent of acid attack, even when no
differences are observed in the 1300–400 cm
–1
spectral region, commonly used to
follow this process. Both the IR spectra and solut ion analysis reveal that non-swell-
ing interlayers develop on heating, causing a marked reduction in dissolution rate.
The results obtained confi rm that acid attack of the smectite structure occurs at both
interlayer surfaces and edges. However, if the accessibility of the layers to protons is
low, due to non-swelling interlayer spaces, the dissolution is reduced and takes place
mainly from the particle edges (Pa
´
lkova
´
et al., 2003).
An in situ observation by AFM shows that the dissolution of hectorite and non-
tronite particles in acid solutions occurs inward from the edges; the basal surfaces
appear to be unreactive during the timescale of the experiments. The hectorite (0 1 0)
faces appear to dissolve about six times more slowly than the lath ends, usually
involving ‘‘broken’’ bonds at the edge surfaces. The edges visibly dissolve on all
sides, and appear to roughen somewhat. On the other hand, the (0 1 0), (1 1 0), and
(1
1 0) faces on nontronite particles are exceptionally stable. Thus, dissolution fronts
originating at broken edges or defects would quickly become fixed along these faces,
after which no more dissolution is observable. These observations can be explained
10% S
100% S
100% S
10% S
1.0
0.8
0.6
0.4
0.2
0.0
0
10
20 30
Undissolved portion
Dissolution time (h)
1300 1100 900 700 500
419
pyrophylite
1121
Absorbance
Wavenumbers (cm
-1
)
Fig. 7.1.2. Ca
2+
-saturated 100% smectite and a reduced-charge smectite with about 10%
swelling interlayers. Left: dissolution of Al
3+
in 6 M HCl at 95 1C; Right: pyrophyllite-like
features in the IR spectra. From Komadel et al. (1996a).
Chapter 7.1: Acid Activation of Clay Minerals270
