Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

the case for only a small fraction of the pore water. The interlayer water is strongly
adsorbed and ap pears to be ‘‘immobile’’ at normal hydraulic gradients. As a result,
the hydraulic conductivity of dense smectites is very low, irrespective of the type of
exchangeable cation. This means that consolidation under an applied external load,
and expansion under a reduced load, are very slow.
Swelling beyond complete interlayer saturation is associ ated with double-layer
interactions between external surfaces of particles, and hence is an osmotic phe-
nomenon. The extension of the double-layers is less under marine than under fresh-
water conditions. Thus, the clay mineral expand s to larger volume in the latter case.
At high densities, the space between clay particles and aggregates is smaller and
the repulsion between the negatively charged surface is greater. As a result, the
swelling pressure increases.
At low and moderate densities, the electrolyte concentration has a substantial influ-
ence on the distance between particles. The particles forming a network with rather much
free space can coagulate at high electrolyte concentration. The effect of changes in pore
water composition on the micro-structural constitution is obvious. When a Na
+
-smectite
is put in contact with water rich in Ca
2+
ions, partial interlayer dehydration takes place
because the stacks of layers contract and the pore spaces between the particles become
larger and more continuous. This leads to a drop in shear strength and swelling pressure
under constant bulk volume conditions, and to an increased hydraulic conductivity.
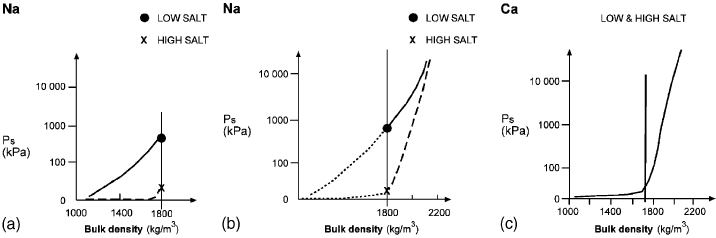
Fig. 6.3 shows the relationship between pore water salinity, bulk density and
swelling pressure for Na
+
- and Ca
2+
-smectites. For Ca
2+
-smectite, the influence of
the salt content on the swelling pressure is app reciably smaller than for the Na
+
-
smectite particularly at low and intermediate bulk densities (o1800 kg/m
3
). The
difference in swelling behaviour between the two smectites may be explained in terms
of their microstructure; there are few contacts between thicker particles of Ca
2+
-
smectite than between thin ones of Na
+
-smectite.
Like natural clays, artificially ‘‘prepared’’ clays maintain a significant degree of
micro-structural heterogeneity. The softer pa rts of the clay matrix, i.e. the gels in the
Fig. 6.3. Relationship between swelling pressure and density, at water saturation, at low and
high salt concentrations for Na
+
-montmorillonite (a and b) and Ca
2+
-montmorillonite (c).
Chapter 6: Mechanical Properties of Clays and Clay Minerals250
pore spaces between stable and dense particles or aggregates, are particularly sen-
sitive to pore water chemistry.
6.1.3. Hydraulic Conductivity
Bulk density and swelling pressure have a direct effect on hydraulic conductivity .
High density and low electrolyte content of the clay mineral give rise to a very low
conductivity for Na
+
-smectite. The conductivity of Ca
2+
-smectite is only slightly
higher because of the dense particle arrangement. However the difference between
the two becomes obvious at low densities. Indeed, for densities lower than
1600–1800 kg/m
3
Ca
2+
-smectite becomes very conductive because of the lack of
micro-structural continuity and coherence.
A general picture of the importance of the mineralogical composition of clays to
hydraulic conductivity is shown in Fig. 6.4, illustrating the dependence of hydraulic
conductivity on density at fluid saturation for different clay minerals.
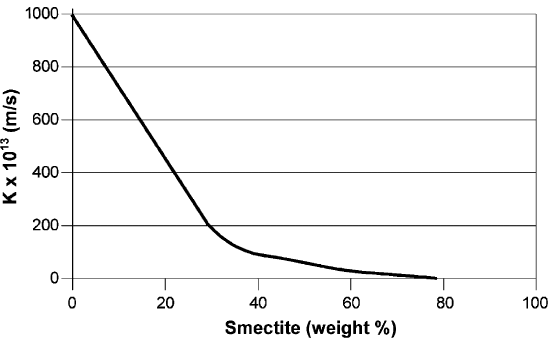
Fig. 6.5 shows the approximate relationship between the smectite content and the
hydraulic conductivity for mechanically undisturbed clay with a high percentage of
clay-sized particles, low-electrolyte pore water and a bulk density at saturation of
about 2000 kg/m
3
. The conductivity is very low even when the smectite content drops
to a few percent.
If the hydraulic gradient is high, as in many laboratory oedometer tests, the
particles can also move and this affects the hydraulic conductivity. Thus, particles
and aggregates that are set free can be transported by flowing pore water to narrow
parts of the pore spaces and cause clogging (Hansbo, 1960).
6.1.4. Gas Penetrability
Permeation of gas through clay is a phenomenon of considerable practical impor-
tance for the clay sealing of underground facilities. It is equally important for the
understanding of gas prospection and exploitation in areas like the North Sea.
However, it is not yet fully understood.
In practice one can assume gas conductivity to be about a thousand times higher
than that of water. Once gas has made its way through buffer clay and further out
through even more permeable geological units, its rate of flow is more dependent on
the availability of pressurized gas than on the gas conductivity. Therefore, the gas
pressure that yields penetration through the buffer clay, i.e. the ‘‘critical gas pres-
sure’’, is the most impor tant factor. The solubility of the gas such as air, hydrogen or
organic gases is also important. The bubbles of temporarily stagnant gas contained
in pore water of the clay shrink and the dissolved gas diffuses out of the system.
According to current hypotheses micro-structural heterogeneity has a decisive
influence on the critical gas pressure. Gas makes its way along continuous channels
of neighbouring larger spaces, where it finds least resistance. Here capillary retention
is at minimum and the bonds between adjacent particles are overcome (Pusch, 1994;
6.1. Physico-chemical Behaviour of Clay Minerals 251
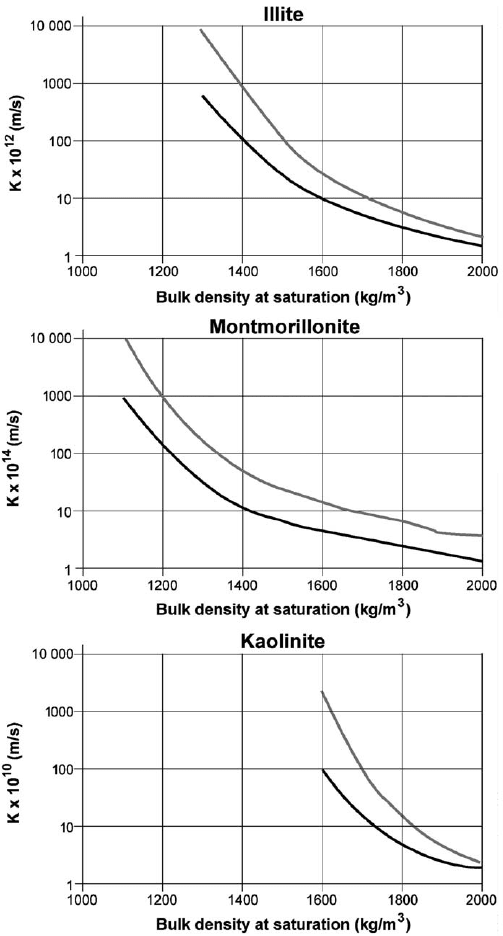
Fig. 6.4. Hydraulic conductivity K of artificially prepared mono-mineral clays as a function
of density at fluid saturation (literature survey). The upper curve in each diagram represents
saline (ocean-type) pore water, while the lower represents saturation and percolation with
distilled water (Pusch, 1994).
Chapter 6: Mechanical Properties of Clays and Clay Minerals252
Horseman and Harrington, 1997). These hypotheses imply that the critical gas
pressure is of the same order of magnitude as the prevailing effective pressure.
6.1.5. Ion Diffusivity
The transport rate of dissolved ions and molecules in clays depends on their diffu-
sivity under the influence of concentration gradients. Diffusion transport capacity is
expressed by the ‘‘effective’’ diffusion coefficient. This parameter refers to the actual
‘‘effective’’ porosity and gives information on the ion transport at the micro-struc-
tural level. On the other hand, the ‘‘apparent’’ diffusion coefficient derives directly
from recording of the concentration profile in the clay. Thus, the importance of
porosity is emphasised by the fact that cation diffusion takes place in several ways: in
continuous water-filled voids, along particle surfaces with electrical double-layers,
and through the interlayer space in smectites. The latter two mechanisms involve
ion-exchange for which a sorption parameter, K
d
, is used.
In practice, the ion transport capacity can be predicted by applying Fick’s law,
using the relevant values for the coefficient D
e
of the density-related ‘‘effective’’
diffusion. Table 6.1 gives typical literature-derived data on this parameter for
montmorillonite-rich bentonite with a density at saturation of 2000 kg/m
3
, which
represents the approximate density of clay embedding canisters with highly radio-
active waste.
The density of the clay plays a rather important role in ion diffusion except for
clays exchanged with monovalent cations. Fig. 6.6 implies that the D
e
of the anion
Fig. 6.5. Approximate relationship between the content in weight percent of smectite clay
minerals and the hydraulic conductivity K for natural, mechanically undisturbed clay with a
high percentage of clay-sized particles, mostly illite and chlorite, and saturated and permeated
by low-electrolyte pore water.
6.1. Physico-chemical Behaviour of Clay Minerals 253
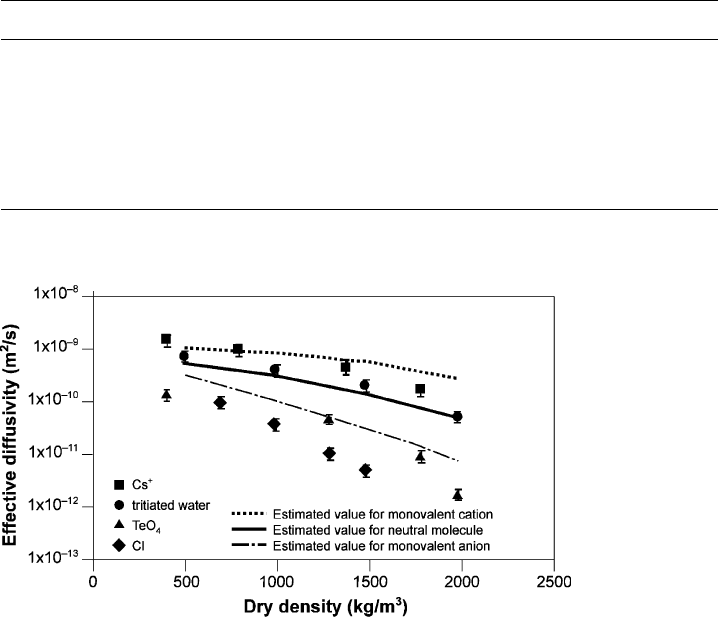
chloride becomes one order of magnitude lower if the dry density is increased from
500 to 1500 kg/m
3
. At fluid saturation the density is 1950 kg/m
3
and D
e
is 10
11
m
2
/s.
The curve for neutral molecules is also approximately valid for water.
The diffusive anion transport capacity is proportional to the pore space/smectite
particles ratio since the anions are excluded from the interlayer space by the Donnan
effect. With increasing density there is a strong reduction of the available space for
migration, causing the diffusion coefficient of anions to drop significantly. Many
cations move by both pore and surface diffusion. This includes respectively the
interlayer space and the vicinity of the particles where electrical double-layers with
dominant cationic population are present. Hence, increasing the density has a small
effect on retarding the diffusion of cations, especially for monovalent ions.
Table 6.1. Effective diffusion coefficient D
e
for elements migrating in MX-80 bentonite with a
density at saturation of about 2000 kg/m
3
(Brandberg and Skagius, 1991)
Species D
e
,m
2
/s
C-14 10
10
I-129 2 10
12
Sr-90 2 10
8
Cs-137 2 10
9
Na-22 2 10
9
Pu-238 10
10
Am-243 10
10
Fig. 6.6. Measured and calculated effective diffusivities for smectite-rich clay (Kato et al.,
1995).
Chapter 6: Mechanical Properties of Clays and Clay Minerals254
6.2. MECHANICAL CHARACTERISTICS OF CLAYS
Textbooks on soil mechanics specify the parameters used in applied foundation
engineering and the ways of determining them in the laboratory and under field
conditions. The physico-chemical background, with special reference to processes at
the micro-structural level is usuall y not a major issue in applied engineering geology,
but is the focus of this section.
6.2.1. Swelling and Consolidation Properties
Smectitic soils give a clear picture of the physical performance of clay minerals, and
the following is focused on such clay materials. The expandability of clay is best
expressed in terms of swelling pressure, which is strongly dependent on density and,
at low densities, on the salinity and major type of exchangeable cation.
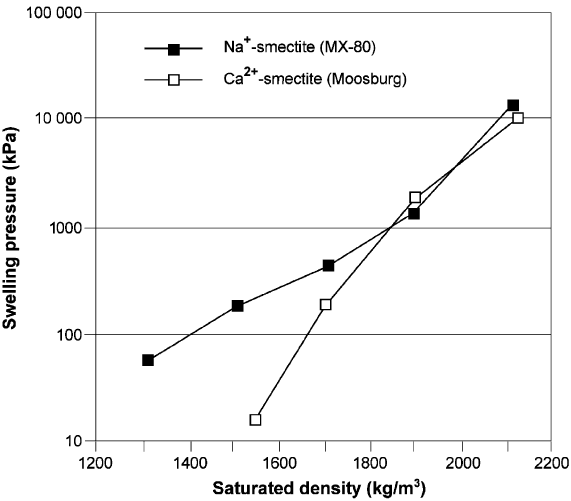
Density is the major factor, as illustrated in Fig. 6.7, which refers to MX-80
(Na
+
-bentonite) and a commercial Ca
2+
-bentonite from Moosburg, Germany (Su
¨
d-
Chemie). The difference in swelling pressure between the two clays is due to different
Fig. 6.7. Generalized diagram showing swelling pressure versus density at saturation, with
distilled water of a Na
+
-bentonite (MX-80) and a Ca
2+
-bentonite with similar smectite con-
tent (bentonite from Bavaria, Germany) ( Pusch, 1994).
6.2. Mechanical Characteristics of Clays 255
microstructures and is negligible at densities higher than about 2000 kg/m
3
. Under
these conditions, the particles have been forced together so that their micro-stru c-
tural patterns are similar.
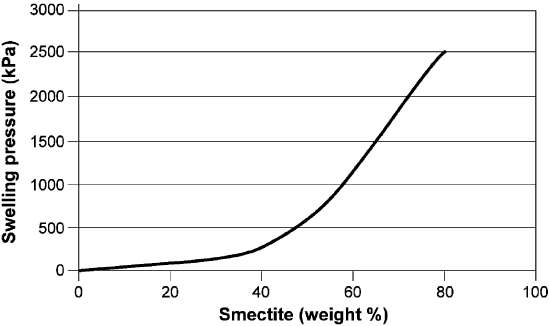
Fig. 6.8 shows the relationship between swelling pressure and the smectite content
of mechanically undisturbed clays. Saturation is made wi th low-electrolyte pore
water, and bulk density at fluid saturation is about 2000 kg/m
3
. This figure indicates
that the influence on the expandability, and particularly on the swelling pressure, of
the content of expandable minerals becomes significant when this content exceeds
about 30%. At lower bulk densities most of the expandable minerals consist of
relatively soft gels in pore spaces between non-expandable minerals. When the con-
tent exceeds about 70%, which is typical for many commercially exploited bent-
onites, the expandable minerals form a continuous matrix with a density similar to
the bulk density. For densities below 1800 and 1900 kg/m
3
, clay with illite as the
dominant clay mineral has a swelling pressur e of 5–20% of that of smectites. At
higher densities this percenta ge can be much larger. Common sedimentary clays of
pre-Ordovician age ha ve low smectite contents and the swelling pressure is therefore
not significant.
Consolidation–expansion of smectite-rich clays are almost reversible at least over
a limited density range since the process depends primarily on the hydration po-
tential. Some hysteresis is involved but in principle the same swelling pressure is
obtained by compression of soft smectite clay to a certain density as when a
dense clay of this type is allowed to expand to this same density. This is not the case
for non-expandable clays because the change in micro-structure induced by
Fig. 6.8. Approximate relationship between the content in weight percent of smectite min-
erals and the swelling pressure for natural, mechanically undisturbed clay with a high per-
centage of clay-sized particles, mostly illite and chlorite, and saturated and permeated by
distilled water. The bulk density at saturation is around 2000 kg/m
3
(Pusch et al., 1987).
Chapter 6: Mechanical Properties of Clays and Clay Minerals256
consolidation is largely permanent. Thus, when the consolidation pressure of a typ-
ical soft natural illitic clay is exceeded, larger pores become compressed first, re-
sulting in shearing of weak aggregates in the clay matrix. Further increase in effective
pressure also starts compression of smaller pores and the shear-strain of the clay
matrix becomes significant.
The effect of unloading after consolidation of strongly compressed illitic clay is
similar to that of smectitic clays. Expansion occurs when compressed, and partly
dehydrated, particles are rehydrated. This expansion is much smaller for illitic than
for smectitic clays, because the number of restored hydrated layers in smectite is
greater. The compressed pore spaces in illite are not re-established.
6.2.2. Rheological Properties
The rheological properties of clays are expressed in the form of parameters defining
stress- and time-dependent strain. The maximum shear stress that clay can resist
under defined boundary conditions is referred to as ‘‘strength’’. This can be ex-
pressed in terms of the unconfined, undrained compressive strength and a number of
traditional strength and strain parameters. These parameters are determined in the
laboratory using ordinary laboratory compression devices such as triaxial test
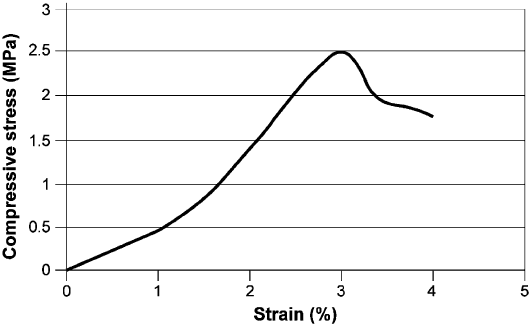
equipment or a simple uniaxial compression machine. A typical picture of the stress/
strain behaviour of natural dense bentonite clay is shown in Fig. 6.9. Because of
slight cementation in nature, this material is somewhat stronger than an artificially
altered clay. Failure occurs at an axial strain of about 3% for a compressive stress of
2.5 MPa.
Fig. 6.9. Example of uniaxial testing of natural bentonite clay from Burgsvik, Sweden. The
density of the saturated clay is about 2100 kg/m
3
.
6.2. Mechanical Characteristics of Clays 257
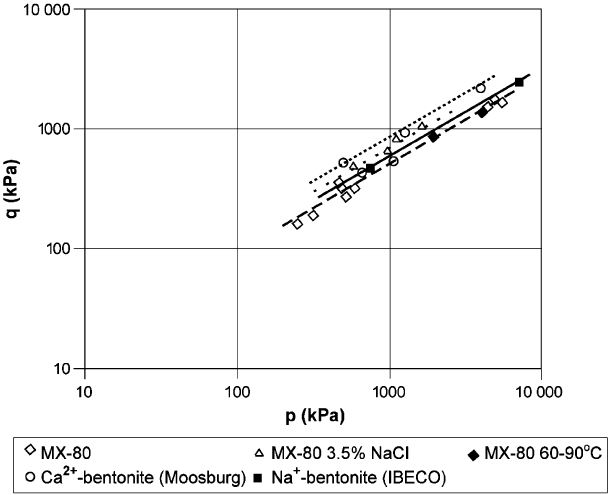
Equation 6.1 gives a generalized expression of the shear strength, q, as a function
of the mean effecti ve stress, p:
q ¼ ap
b
ð1Þ
where a ¼ q for p ¼ 1 kPa; and b is the slope of log p/log q curve (Fig. 6.10). The key
parameter, a, is a particularly good measure of how shear strength depends on the
microstructure. Thus the value of a, is much higher for coarse- than fine-grained
clays. This is illustrated by the facts that this value is twice higher for Ca
2+
than for
Na
+
-smectite, and that strengthening by coagulation is induced by salt water.
Creep is the expression for time-dependent strain and is caused by shearing at the
micro-structural level both in con solidation (compression under drained conditions)
and by macro-strain under undrained conditions.
Based on a conceptual model that takes strain processes at the micro-structural
level into consideration, and applying thermodynamics and stochastic mechan ics
Fig. 6.10. Triaxial tests on bentonite as buffer materials (Bo
¨
rgesson and Hernelind, 1999).
q ¼ shear stress, p ¼ average effective normal stress.
Chapter 6: Mechanical Properties of Clays and Clay Minerals258
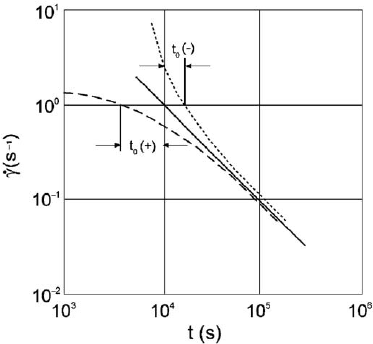
(Pusch and Adey, 1999), the impact of time, t, on the shear strain can be expressed by
Equation 6.2:
dg=dt ¼ b TDlnðtÞð2Þ
where g is the angular strain; b is a strain parameter evaluated from undrained
triaxial tests (3.10
10
to 2.10
8
kPa for the density range of 1900–2100 kg/m
3
for
MX-80); D is the deviat or stress (s
1
–s
3
) in kPa and T is the absolute temperature in
Kelvin.
Typical generalised curves for primary creep, implying strain attenuation, are
shown in Fig. 6.11. This type of creep usually prevails when bulk shear stress is in the
interval 1/3–2/3 of the conven tionally determined shear strength. For lower stresses
the attenuation is stronger; for higher stresses failure cannot be avoided (secondary
creep). Creep will occur when critical strain has been reached, i.e. when compre-
hensive breakdown of the microstructure has taken place.
REFERENCES
Bo
¨
rgesson, L., Hernelind, J., 1999. Coupled thermo-hydro-mechanical calculations of the
water saturation phase of a KBS-3 deposition hole. SKB Technical Report TR-99-41. SKB,
Stockholm.
Brandberg, F., Skagius, K., 1991. Porosity, sorption and diffusivity data compiled for the
SKB 91 study. SKB Technical Report TR 91-16, SKB, Stockholm.
Fig. 6.11. Typical clay creep curves. The term t
o
describes the form of the creep onset, i.e. the
initial part of the creep strain rate
_
g at stress application, assuming that
_
g is ultimately pro-
portional to log time. Negative t
0
indicates cementation of the clay matrix by precipitated
matter.
References 259
