Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

I. The Molecular Design of Life 4. Exploring Proteins 4.5. Three-Dimensional Protein Structure Can Be Determined by NMR Spectroscopy and X-Ray Crystallography
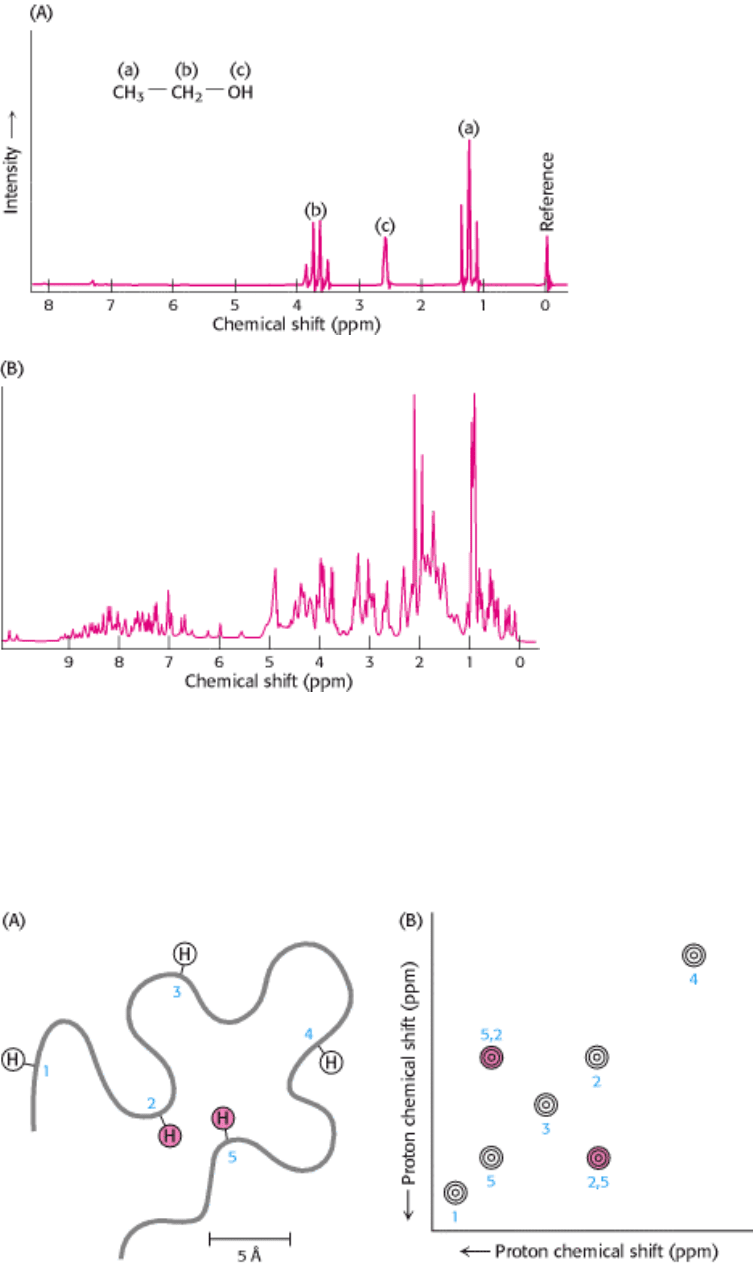
Figure 4.44. One-Dimensional NMR Spectra. (A)
1
H-NMR spectrum of ethanol (CH
3
CH
2
OH) shows that the
chemical shifts for the hydrogen are clearly resolved. (B)
1
H-NMR spectrum from a 55 amino acid fragment of a protein
with a role in RNA splicing shows a greater degree of complexity. A large number of peaks are present and many
overlap. [(A) After C. Branden and J. Tooze, Introduction to Protein Structure (Garland, 1991), p. 280; (B) courtesy of
Barbara Amann and Wesley McDermott.]
I. The Molecular Design of Life 4. Exploring Proteins 4.5. Three-Dimensional Protein Structure Can Be Determined by NMR Spectroscopy and X-Ray Crystallography
Figure 4.45. The Nuclear Overhauser Effect. The nuclear Overhauser effect (NOE) identifies pairs of protons that are
in close proximity. (A) Schematic representation of a polypeptide chain highlighting five particular protons. Protons 2
and 5 are in close proximity (~4 Å apart), whereas other pairs are farther apart. (B) A highly simplified NOESY
spectrum. The diagonal shows five peaks corresponding to the five protons in part A. The peaks above the diagonal and
the symmetrically related one below reveal that proton 2 is close to proton 5.
I. The Molecular Design of Life 4. Exploring Proteins 4.5. Three-Dimensional Protein Structure Can Be Determined by NMR Spectroscopy and X-Ray Crystallography
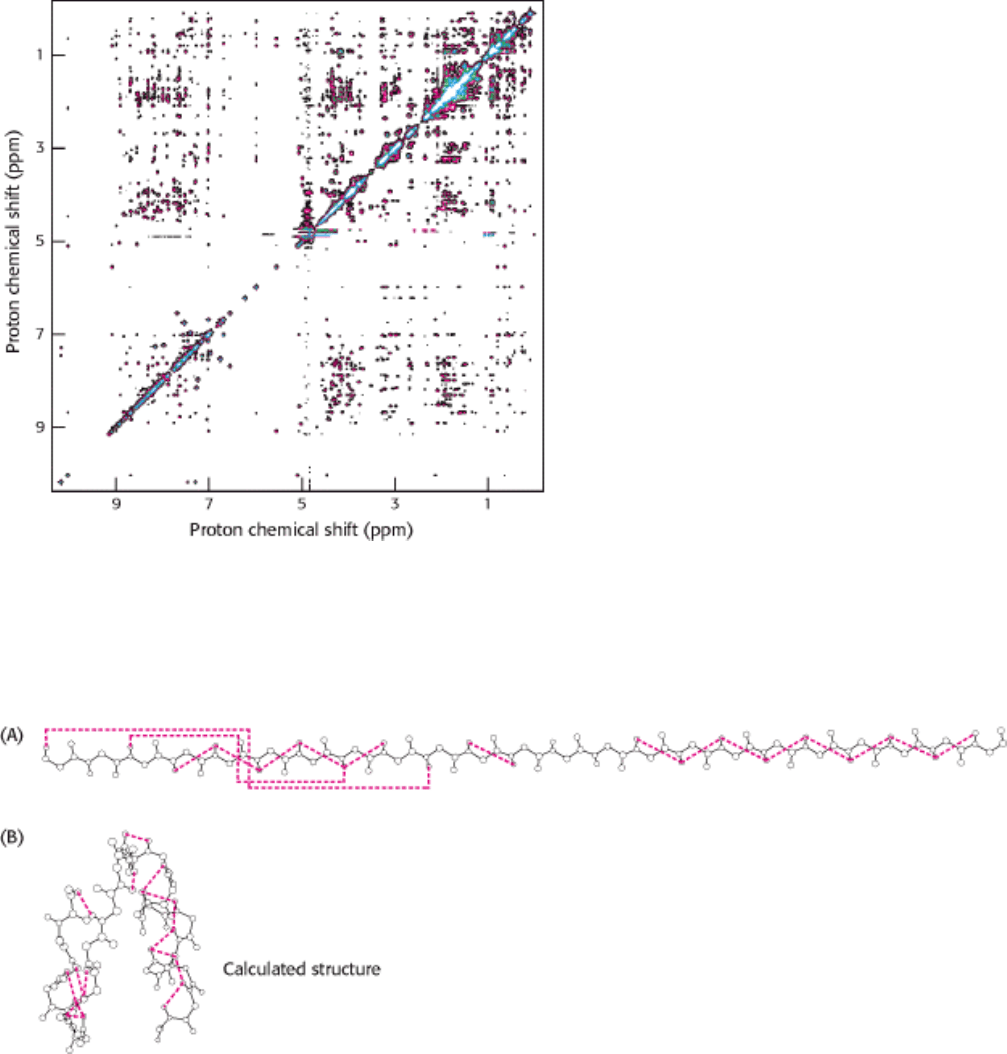
Figure 4.46. Detecting Short Proton-Proton Distances. A NOESY spectrum for a 55 amino acid domain from a
protein having a role in RNA splicing. Each off-diagonal peak corresponds to a short proton-proton separation. This
spectrum reveals hundreds of such short proton-proton distances, which can be used to determine the three-dimensional
structure of this domain. [Courtesy of Barbara Amann and Wesley McDermott.]
I. The Molecular Design of Life 4. Exploring Proteins 4.5. Three-Dimensional Protein Structure Can Be Determined by NMR Spectroscopy and X-Ray Crystallography
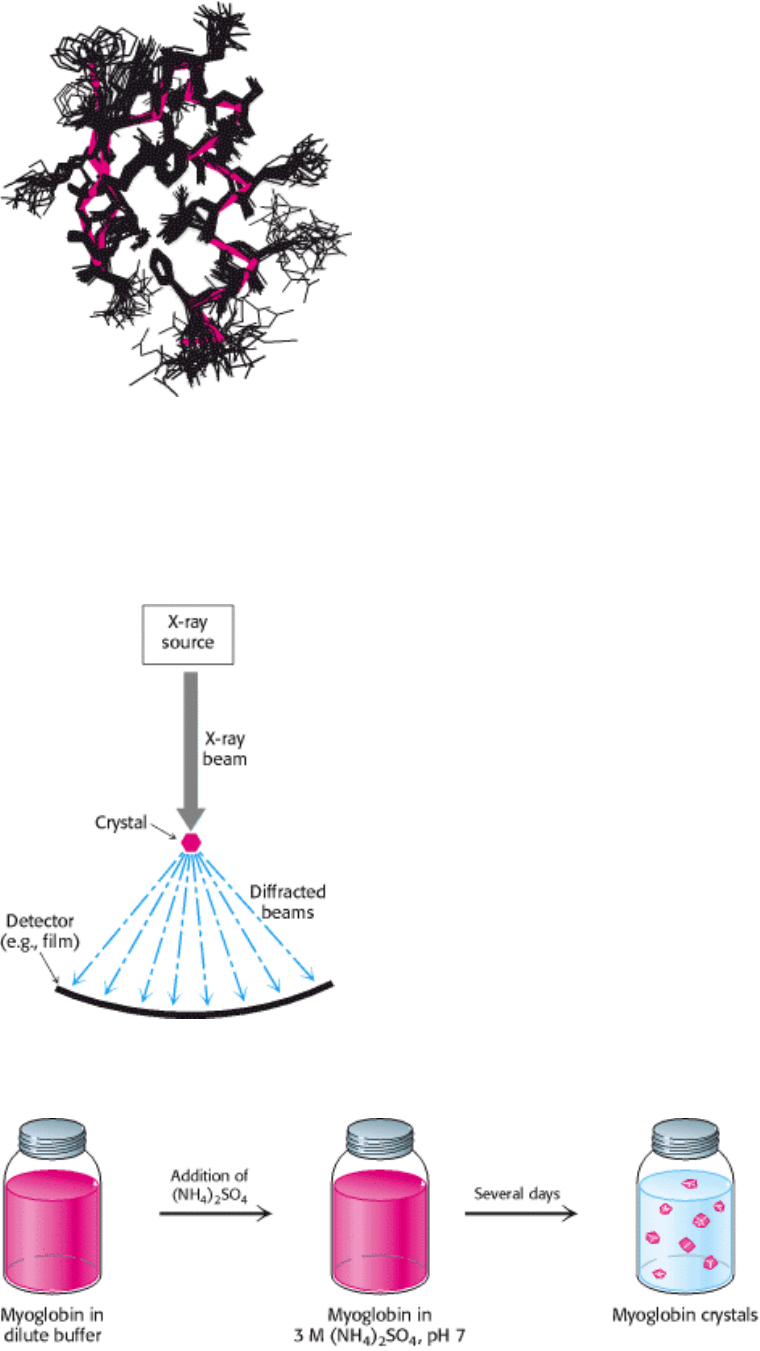
Figure 4.47. Structures Calculated on the Basis of NMR Constraints. (A) NOESY observations show that protons
(connected by dotted red lines) are close to one another in space. (B) A three-dimensional structure calculated with these
proton pairs constrained to be close together.
I. The Molecular Design of Life 4. Exploring Proteins 4.5. Three-Dimensional Protein Structure Can Be Determined by NMR Spectroscopy and X-Ray Crystallography
Figure 4.48. A Family of Structures. A set of 25 structures for a 28 amino acid domain from a zinc-finger-DNA-
binding protein. The red line traces the average course of the protein backbone. Each of these structures is consistent
with hundreds of constraints derived from NMR experiments. The differences between the individual structures are due
to a combination of imperfections in the experimental data and the dynamic nature of proteins in solution. [Courtesy of
Barbara Amann.]
I. The Molecular Design of Life 4. Exploring Proteins 4.5. Three-Dimensional Protein Structure Can Be Determined by NMR Spectroscopy and X-Ray Crystallography
Figure 4.49. Essence of an X-Ray Crystallographic Experiment: an X-Ray Beam, a Crystal, and a Detector.
I. The Molecular Design of Life 4. Exploring Proteins 4.5. Three-Dimensional Protein Structure Can Be Determined by NMR Spectroscopy and X-Ray Crystallography
Figure 4.50. Crystallization of Myoglobin.
I. The Molecular Design of Life 4. Exploring Proteins 4.5. Three-Dimensional Protein Structure Can Be Determined by NMR Spectroscopy and X-Ray Crystallography
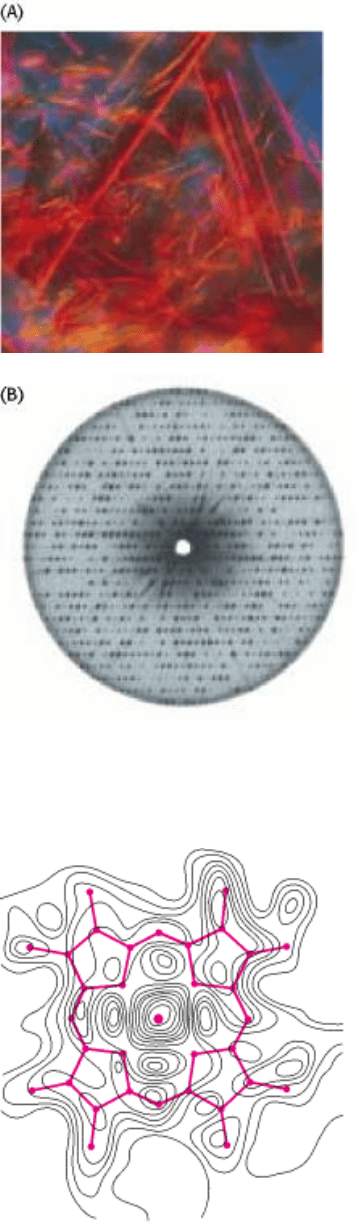
Figure 4.51. Myoglobin Crystal and X-Ray. (A) Crystal of myoglobin. (B) X-ray precession photograph of a
myoglobin crystal. [(A) Mel Pollinger/Fran Heyl Associates.]
I. The Molecular Design of Life 4. Exploring Proteins 4.5. Three-Dimensional Protein Structure Can Be Determined by NMR Spectroscopy and X-Ray Crystallography
Figure 4.52. Section of the Electron-Density Map of Myoglobin. This section of the electron-density map shows the
heme group. The peak of the center of this section corresponds to the position of the iron atom. [From J. C. Kendrew.
The three-dimensional structure of a protein molecule. Copyright © 1961 by Scientific American, Inc. All rights
reserved.]
I. The Molecular Design of Life 4. Exploring Proteins 4.5. Three-Dimensional Protein Structure Can Be Determined by NMR Spectroscopy and X-Ray Crystallography
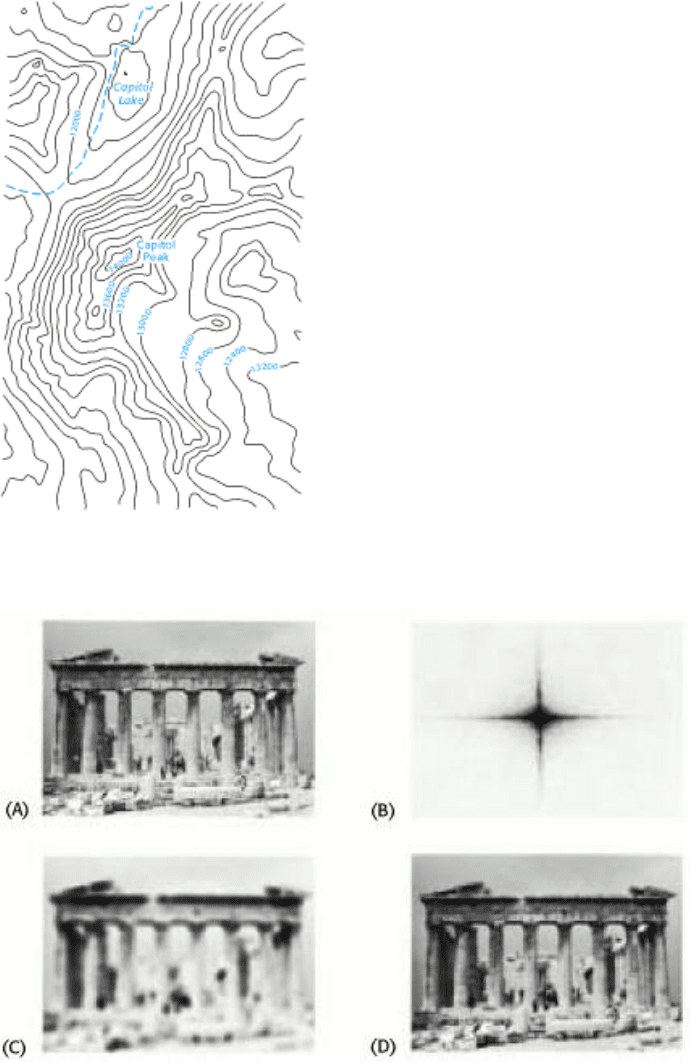
Figure 4.53. Section of a U.S. Geological Survey Map. Capitol Peak Quadrangle, Colorado.
I. The Molecular Design of Life 4. Exploring Proteins 4.5. Three-Dimensional Protein Structure Can Be Determined by NMR Spectroscopy and X-Ray Crystallography
Figure 4.54. Resolution Affects the Quality of an Image. The effect of resolution on the quality of a reconstructed
image is shown by an optical analog of x-ray diffraction: (A) a photograph of the Parthenon; (B) an optical diffraction
pattern of the Parthenon; (C and D) images reconstructed from the pattern in part B. More data were used to obtain
image D than image C, which accounts for the higher quality of image D. [(A) Courtesy of Dr. Thomas Steitz. (B)
Courtesy of Dr. David DeRosier).]

I. The Molecular Design of Life 4. Exploring Proteins
Summary
The rapid progress in gene sequencing has advanced another goal of biochemistry
elucidation of the proteome. The
proteome is the complete set of proteins expressed and includes information about how they are modified, how they
function, and how they interact with other molecules.
The Purification of Proteins Is an Essential Step in Understanding Their Function
Proteins can be separated from one another and from other molecules on the basis of such characteristics as solubility,
size, charge, and binding affinity. SDS-polyacrylamide gel electrophoresis separates the polypeptide chains of proteins
under denaturing conditions largely according to mass. Proteins can also be separated electrophoretically on the basis of
net charge by isoelectric focusing in a pH gradient. Ultracentrifugation and gel-filtration chromatography resolve
proteins according to size, whereas ion-exchange chromatography separates them mainly on the basis of net charge. The
high affinity of many proteins for specific chemical groups is exploited in affinity chromatography, in which proteins
bind to columns containing beads bearing covalently linked substrates, inhibitors, or other specifically recognized
groups. The mass of a protein can be precisely determined by sedimentation equilibrium measurements or by mass
spectrometry.
Amino Acid Sequences Can Be Determined by Automated Edman Degradation
The amino acid composition of a protein can be ascertained by hydrolyzing it into its constituent amino acids in 6 N HCl
at 110°C. The amino acids can be separated by ion-exchange chromatography and quantitated by reacting them with
ninhydrin or fluorescamine. Amino acid sequences can be determined by Edman degradation, which removes one amino
acid at a time from the amino end of a peptide. Phenyl isothiocyanate reacts with the terminal amino group to form a
phenylthiocarbamoyl derivative, which cyclizes under mildly acidic conditions to give a phenylthiohydantoin-amino acid
and a peptide shortened by one residue. Automated repeated Edman degradations by a sequenator can analyze sequences
of about 50 residues. Longer polypeptide chains are broken into shorter ones for analysis by specifically cleaving them
with a reagent such as cyanogen bromide, which splits peptide bonds on the carboxyl side of methionine residues.
Enzymes such as trypsin, which cleaves on the carboxyl side of lysine and arginine residues, also are very useful in
splitting proteins. Amino acid sequences are rich in information concerning the kinship of proteins, their evolutionary
relations, and diseases produced by mutations. Knowledge of a sequence provides valuable clues to conformation and
function.
Immunology Provides Important Techniques with Which to Investigate Proteins
Proteins can be detected and quantitated by highly specific antibodies; monoclonal antibodies are especially useful
because they are homogeneous. Enzyme-linked immunosorbent assays and Western blots of SDS-polyacrylamide gels
are used extensively. Proteins can also be localized within cells by immunofluorescence microscopy and
immunoelectron microscopy.
Peptides Can Be Synthesized by Automated Solid-Phase Methods
Polypeptide chains can be synthesized by automated solid-phase methods in which the carboxyl end of the growing
chain is linked to an insoluble support. The α -carboxyl group of the incoming amino acid is activated by
dicyclohexylcarbodiimide and joined to the α -amino group of the growing chain. Synthetic peptides can serve as drugs
and as antigens to stimulate the formation of specific antibodies. They can also be sources of insight into relations
between amino acid sequence and conformation.
Three-Dimensional Protein Structure Can Be Determined by NMR Spectroscopy and
X-Ray Crystallography
Nuclear magnetic resonance spectroscopy and x-ray crystallography have greatly enriched our understanding of how
proteins fold, recognize other molecules, and catalyze chemical reactions. Nuclear magnetic resonance spectroscopy
reveals the structure and dynamics of proteins in solution. The chemical shift of nuclei depends on their local
environment. Furthermore, the spins of neighboring nuclei interact with each other in ways that provide definitive
structural information.
X-ray crystallography is possible because electrons scatter x-rays; the way in which the scattered waves recombine
depends only on the atomic arrangement. The three-dimensional structures of thousands of proteins are now known in
atomic detail.
Key Terms
proteome
assay
homogenate
salting out
dialysis
gel-filtration chromatography
ion-exchange chromatography
affinity chromatography
high-pressure liquid chromatography (HPLC)
gel electrophoresis
isoelectric point
isoelectric focusing
two-dimensional electrophoresis
sedimentation coefficient (Svedberg units, S)
matrix-assisted laser desorption- ionization-time of flight spectrometry (MALDI-TOF)
dabsyl chloride
dansyl chloride
Edman degradation
phenyl isothiocyanate

cyanogen bromide (CNBr)
overlap peptides
diagonal electrophoresis
antibody
antigen
antigenic determinant (epitope)
monoclonal antibodies
enzyme-linked immunosorbent assay (ELISA)
Western blotting
fluorescence microscopy
green fluorescent protein (GFP)
solid-phase method
nuclear magnetic resonance (NMR) spectroscopy
x-ray crystallography
I. The Molecular Design of Life 4. Exploring Proteins
Problems
1.
Valuable reagents. The following reagents are often used in protein chemistry:
Which one is the best suited for accomplishing each of the following tasks?
(a) Determination of the amino acid sequence of a small peptide.
(b) Identification of the amino-terminal residue of a peptide (of which you have less than 0.1 µg).
(c) Reversible denaturation of a protein devoid of disulfide bonds. Which additional reagent would you need if
disulfide bonds were present?
(d) Hydrolysis of peptide bonds on the carboxyl side of aromatic residues.
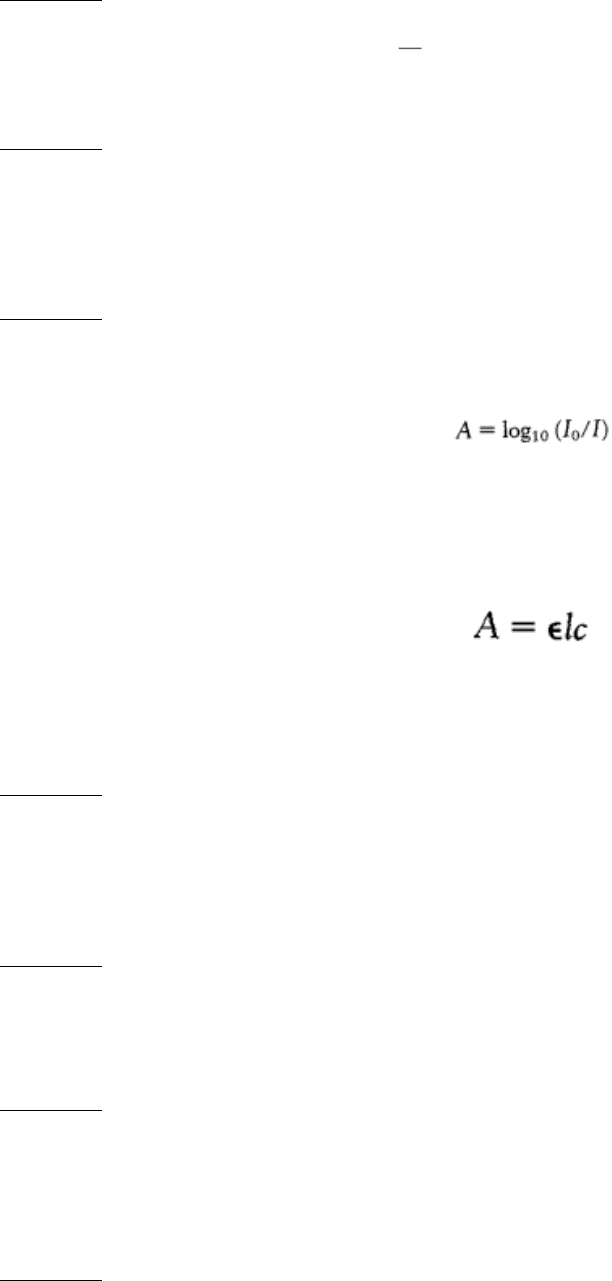
(e) Cleavage of peptide bonds on the carboxyl side of methionines.
(f) Hydrolysis of peptide bonds on the carboxyl side of lysine and arginine residues.
See answer
2.
Finding an end. Anhydrous hydrazine (H
2
N NH
2
) has been used to cleave peptide bonds in proteins. What are the
reaction products? How might this technique be used to identify the carboxyl-terminal amino acid?
See answer
3.
Crafting a new breakpoint. Ethyleneimine reacts with cysteine side chains in proteins to form S-aminoethyl
derivatives. The peptide bonds on the carboxyl side of these modified cysteine residues are susceptible to hydrolysis
by trypsin. Why?
See answer
4.
Spectrometry. The absorbance A of a solution is defined as
in which I
0
is the incident light intensity and I is the transmitted light intensity. The absorbance is related to the
molar absorption coefficient (extinction coefficient) ε (in M
-1
cm
-1
), concentration c (in M), and path length l (in
cm) by
The absorption coefficient of myoglobin at 580 nm is 15,000 M
-1
cm
-1
. What is the absorbance of a 1 mg ml
-1
solution across a 1-cm path? What percentage of the incident light is transmitted by this solution?
See answer
5.
A slow mover. Tropomyosin, a 93-kd muscle protein, sediments more slowly than does hemoglobin (65 kd). Their
sedimentation coefficients are 2.6S and 4.31S, respectively. Which structural feature of tropomyosin accounts for its
slow sedimentation?
See answer
6.
Sedimenting spheres. What is the dependence of the sedimentation coefficient S of a spherical protein on its mass?
How much more rapidly does an 80-kd protein sediment than does a 40-kd protein?
See answer
7.
Size estimate. The relative electrophoretic mobilities of a 30-kd protein and a 92-kd protein used as standards on an
SDS-polyacrylamide gel are 0.80 and 0.41, respectively. What is the apparent mass of a protein having a mobility of
0.62 on this gel?
See answer
8.
A new partnership? The gene encoding a protein with a single disulfide bond undergoes a mutation that changes a
serine residue into a cysteine residue. You want to find out whether the disulfide pairing in this mutant is the same as
in the original protein. Propose an experiment to directly answer this question.
See answer
9.
Sorting cells. Fluorescence-activated cell sorting (FACS) is a powerful technique for separating cells according to
their content of particular molecules. For example, a fluorescence-labeled antibody specific for a cell-surface protein
can be used to detect cells containing such a molecule. Suppose that you want to isolate cells that possess a receptor
enabling them to detect bacterial degradation products. However, you do not yet have an antibody directed against
this receptor. Which fluorescencelabeled molecule would you prepare to identify such cells?
See answer
10.
Column choice. (a) The octapeptide AVGWRVKS was digested with the enzyme trypsin. Would ion exchange or
molecular exclusion be most appropriate for separating the products? Explain. (b) Suppose that the peptide was
digested with chymotrypsin. What would be the optimal separation technique? Explain.
See answer
11.
Making more enzyme? In the course of purifying an enzyme, a researcher performs a purification step that results in
an increase in the total activity to a value greater than that present in the original crude extract. Explain how the
amount of total activity might increase.
See answer
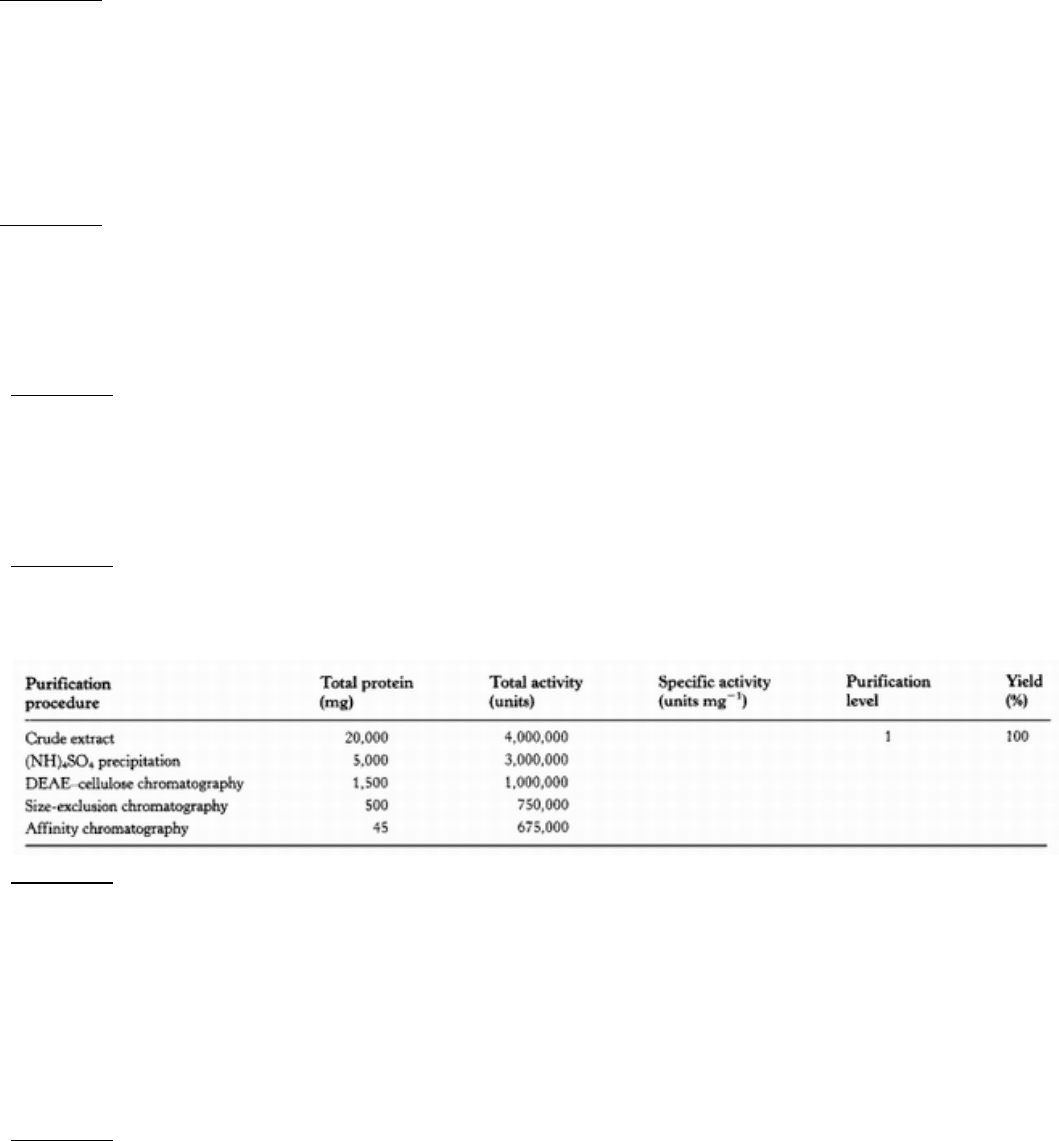
12.
Protein purification problem. Complete the table below.
See answer
Chapter Integration Problems
13.
Quaternary structure. A protein was purified to homogeneity. Determination of the molecular weight by molecular
exclusion chromatography yields 60 kd. Chromatography in the presence of 6 M urea yields a 30-kd species. When
the chromatography is repeated in the presence of 6 M urea and 10 mM β-mercaptoethanol, a single molecular
species of 15 kd results. Describe the structure of the molecule.
See answer
