Basdevant J.-L., Rich J., Spiro M. Fundamentals in Nuclear Physics: From Nuclear Structure to Cosmology
Подождите немного. Документ загружается.

30 1. Basic concepts in nuclear physics
0.6
0.8
1.0
1.2
1.4
1.6
200
400
600
0
counts
30
25
20
15
10
5
0
E (MeV)
26
34
36
38
40
42
44
46
48
50
52
54
56
58
60
γ
(MeV)
22+
30+
40+
50+
60+
30+
40+
0+
10+
20+
prolate
oblate
prolate superdeformed
28
30
32
E
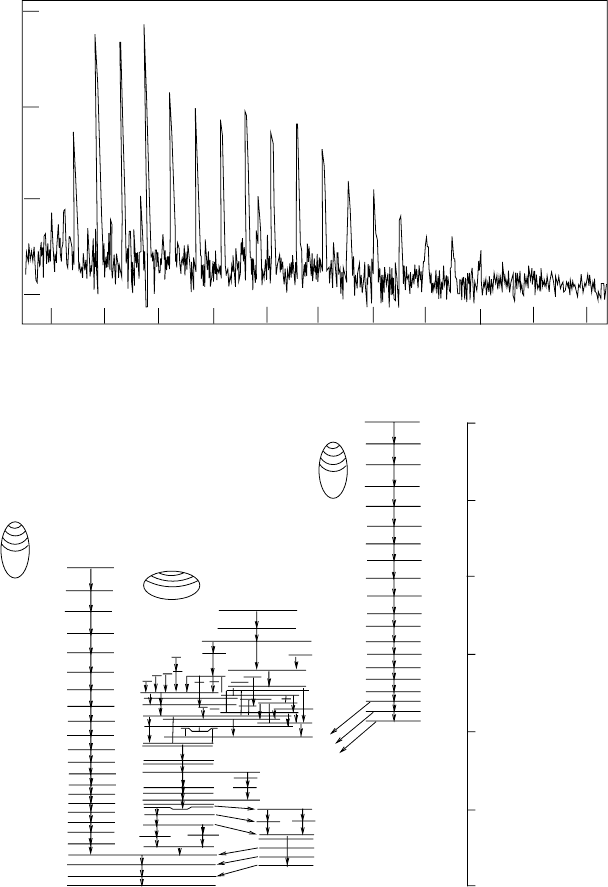
Fig. 1.9. Excited states of
152
Dy. The bottom panel [21] shows the spectrum of
states, including (on the left) the ground state rotation band for a prolate nucleus
and (on the right) a super-deformed band extending from J
P
∼ 22
+
to 60
+
.Nu-
clei in states of a rotation band generally cascade rapidly down the band, giving
coincident photons that are evenly spaced in energy. The top panel [22] shows the
“picket fence” spectrum of photons produced in a cascade of the super-deformed
band.
1.4 Nuclear forces and interactions 31
from first principles. In fact Coulomb’s law (or more generally the equations of
electromagnetism) determines the interactions between electrons and nuclei.
Spin corrections and relativistic effects can be calculated perturbatively to
very good accuracy because of the smallness of the fine structure constant
α = e
2
/4π
0
¯hc ∼ 1/137. Together with the Pauli principle which leads to the
shell structure of electron orbitals, these facts imply that one can calculate
numerically spectra of complex atoms despite the difficulties of the many-
body problem.
Unfortunately, none of this holds in nuclear physics. Forces between nu-
cleons are neither simple nor fully understood. One of the reasons for this
is that the interactions between nucleons are “residuals” of the fundamental
interactions between quarks inside the nucleons. In that sense, nuclear in-
teractions are similar to Van der Waals forces between atoms or molecules,
which are also residual or “screened” Coulomb interactions. For these rea-
sons, forces between nucleons are described by semi-phenomenological forms,
e.g. (1.56), which are only partly deduced from fundamental principles.
Reversing the order of inference, physicists could have derived the form
of Coulomb’s law from the spectrum of bound states of the hydrogen atom.
This is not possible in nuclear physics because there is only one two-nucleon
bound state, the deuteron. In the next subsection, we will find that there is
much to be learned from this fact but it will not be sufficient to derive the
nucleon–nucleon potential in all its detail. To do this, we will need to attack
the more difficult problem of nucleon–nucleon scattering. This will be done
in Chap. 3. When we do this, we will be confronted with the other major
difficulty of nuclear forces: the coupling constants are large so perturbative
treatments do not apply as systematically as in atomic physics.
1.4.1 The deuteron
There is only one A = 2 nucleus, the deuteron, and it has no excited states.
Its binding energy, quantum numbers, and magnetic moments are
B(2, 1) = 2.225 MeV J
P
=1
+
µ
d
=0.857µ
N
. (1.42)
Note that B(2, 1) is quite small compared to typical nuclear binding energies,
8 MeV per nucleon.
We also note that to good approximation µ
d
= µ
p
+µ
n
.Thissuggeststhe
the magnetic moment comes only from the spins of the constituents, implying
that the nucleons are in a state of vanishing orbital angular momentum,
l = 0. In fact, this turns out only to be a good first approximation since the
deuteron is slightly deformed, possessing a small quadrupole moment. This
requires that the wavefunction have a small admixture of l = 2. Both l =0
and l = 2 are consistent with the parity of the deuteron since for two-nucleon
states, the parity is −1
l
.
Since the deuteron has spin-1 and is (mostly) in an l = 0 state, the spins
of the nucleons must be aligned, i.e. the total spin, s
tot
= s
n
+ s
p
,must

32 1. Basic concepts in nuclear physics
take on the quantum number s = 1. The other possibility is s = 0. Since the
deuteron is the only bound state, we conclude
n p (s = 1) bound n p (s = 0) unbound .
We conclude that the neutron–proton potential is spin-dependent.
What about the neutron–neutron and proton–proton potentials and the
fact that there are no bound states for these two systems? If the strong inter-
actions do not distinguish between neutrons and protons, the non-existence
of a s = 0 neutron–proton state is consistent with the non-existence of the
analogous pp and nn states:
n n (s = 0) unbound p p (s = 0) unbound ,
i.e. all nucleon–nucleon s =0,l = 0 states are unbound. On the other hand,
the non-existence of pp and nn s =1,l = 0 states is explained by the
Pauli principle. This principle requires that the total wavefunction of pairs
of identical fermions be antisymmetric. Loosely speaking, this is equivalent
to saying that when two identical fermions are at the same place (l = 0),
their spins must be anti-aligned. Thus, the l =0,s = 1 proton–proton and
neutron–neutron states are forbidden.
We make the important conclusion that the existence of a n p bound state
andthenon-existenceofnnandppboundstatesisconsistentwiththestrong
force not distinguishing between neutrons and protons but only if the force
is spin-dependent.
Theexistenceofans = 1 state and non-existence of s = 0 states would
naively suggest that the nucleon–nucleon force is attractive for s = 1 and
repulsive for s = 0. This is incorrect. In fact the nucleon–nucleon force is
attractive in both cases. For s = 1 it is sufficiently attractive to produce a
bound state while for s = 0 it is not quite attractive enough.
We can understand how this comes about by considering the 3-dimensional
square well potential shown in Fig. 1.10:
V (r)=−V
0
r<R V(r)=0 r>R. (1.43)
While it is hardly a realistic representation of the nucleon–nucleon potential,
its finite range, R,anditsdepthV
0
can be chosen to correspond more or less
to the range and depth of the real potential. In fact, since for the moment
we only want to reproduce the deuteron binding energy and radius, we have
just enough parameters to do the job.
The bound states are found by solving the Schr¨odinger eigenvalue equa-
tion
−¯h
2
2m
∇
2
+ V (r)
ψ(r)=Eψ(r) , (1.44)
where m is the reduced mass m ∼ m
p
/2 ∼ m
n
/2. The l = 0 solutions depend
only on r, so we set ψ(r)=u(r)/r and find a simpler equation

1.4 Nuclear forces and interactions 33
V(r)=0
r=R
κ r)
V(r)=−V
r
|u|
2
V(r)
0
u(r) = sin kr
u(r) = exp(−
Fig. 1.10. A square-well potential and the square of the wavefunction ψ(r)=
u(r)/r. The depth and width of the well are chosen to reproduce the binding energy
and radius of the deuteron. Note that the wavefunction extends far beyond the
effective range of the s = 1 nucleon–nucleon potential.
−¯h
2
2m
d
2
dr
2
+ V (r)
u(r)=Eu(r) . (1.45)
The solutions for E<0 oscillate for r<R
u(r<R) ∝ A sin kr + B cos(kr) k(E)=
2m(V
0
+ E)
¯h
, (1.46)
and are exponentials for r>R
u(r>R) ∝ C exp(−κr)+D exp(κr) κ(E)=
√
−2mE
¯h
. (1.47)
We set B = 0 to prevent ψ from diverging at the origin and D =0tomake
the wavefunction normalizable. Requiring continuity at r = R of u(r)and
u
(r) we find the condition that determines the allowed values of E
k(E)cotk(E)R = −κ(E) . (1.48)
We note that for r → 0, the function on the left, k cot kr, is positive and
remains so until kr = π/2. Since the quantity on the left is negative the
requirement for at least one bound state is that there exist an energy E<0
such that
k(E)R>π/2 , (1.49)

34 1. Basic concepts in nuclear physics
i.e. that we can fit at least 1/4 of a wave inside the well. Since k(E) <k(E =
0) the condition is
√
2mV
0
R
2
¯h
>π/2 , (1.50)
i.e.
V
0
R
2
>
π
2
¯h
2
c
2
8mc
2
= 109 MeV fm
2
. (1.51)
The existence of a single s = 1 state and the non-existence of s = 0 states
can be understood by supposing that the effective values of V
0
R
2
are respec-
tively slightly greater than or slightly less than 109 MeV fm
2
. The deuteron
binding energy is correctly predicted if
V
0
R
2
(s = 1) = 139.6MeVfm
2
. (1.52)
This can be verified by substituting it into (1.48) along with the energy
E = −2.225 MeV. Data from neutron–proton scattering discussed in Chap.
3 shows that the s = 0 states just miss being bound
V
0
R
2
(s =0) ∼ 93.5MeVfm
2
. (1.53)
Including scattering data, Sect. 3.6, we can determine both V
0
and R:
s =1: V
0
=46.7MeV R =1.73 fm . (1.54)
The wavefunction in shown in Fig. 1.10. The fact that B(2, 1) is small results
causes the wavefunction to extend far beyond the effective range R of the
potential and explains the anomalously large value of the deuteron radius
(Table 1.1).
The scattering data discussed in Sect. 3.6 allow one to estimate the values
of V
0
and R for s = 0 (Table 3.3). One finds for the proton–neutron system
s =0: V
0
=12.5MeV R =2.79 fm . (1.55)
This potential is quite different from the s = 1 potential.
In summary, the strong interactions have a strength and range that places
them precisely at the boundary between the the interactions that have no
bound states (e.g. the weak interactions) and those that have many bound
states (e.g. the electromagnetic interactions). The phenomenological implica-
tions are of a cosmological scale since the fabrication of heavy elements from
nucleons must start with the production of the one 2-nucleon bound state.
As we will see in Chaps. 8 and 9, if there were no 2-nucleon bound states the
fabrication of multi-nucleon nuclei would be extremely difficult. On the other
hand, the existence of many 2-nucleon states would make it considerably
simpler. The actual situation is that heavy elements can be slowly formed,
leaving, for the time being, a large reserve of protons to serve as fuel in stars.

1.4 Nuclear forces and interactions 35
1.4.2 The Yukawa potential and its generalizations
We want to consider more realistic potentials than the simple potential (1.43).
This is necessary to completely describe the results of nucleon–nucleon scat-
tering (Chap. 3) and to understand the saturation phenomenon on nuclear
binding. The subject of nucleon–nucleon potentials is very complex and we
will give only a qualitative discussion. Our basic guidelines are as follow:
• Protons and neutrons are spin 1/2 fermions, and therefore obey the Pauli
principle. We have already seen in the previous subsection how this restricts
the number of 2-nucleon states.
• Nuclear forces are attractive and strong, since binding energies are roughly
a10
6
times the corresponding atomic energies. They are however short
range forces : a few fm. The combination of strength and short range makes
2-nucleon systems only marginally bound but creates a rich spectrum of
many-nucleon states.
• They are “charge independent.” Nuclear forces are blind to the electric
charge of nucleons. If one were to “turn off” Coulomb interactions, the
nuclear proton–proton potential would be the same as the neutron–neutron
potential. A simple example is given by the binding energies of isobars such
as tritium and helium 3 : B(
3
H) = 8.492 MeV >B(
3
He) = 7.728 MeV. If
the difference ∆B =0.764 MeV is attributed to the Coulomb interaction
between the two protons in
3
He, ∆B = e
2
< 1/r
12
>/4π
0
, one obtains
a very reasonable value for the mean radius of the system : R ≈ 2 fm (this
can be calculated or measured by other means). We shall come back to this
question in a more quantitative way when we discuss isospin.
• Nuclear forces saturate. As we have already mentioned, this results in the
volumes and binding energies of nuclei being additive and, in first approx-
imation, proportional to the mass number A.Thisisaremarkablefact
since it is reminiscent of a classical property and not normally present in
quantum systems. It appears as if each nucleon interacts with a given fixed
number of neighbors, whatever the nucleus.
The theoretical explanation of the saturation of nuclear forces is subtle.
The physical ingredients are the short range attractive potential (r ∼ 1fm),
a hard core repulsive force at smaller distances r<0.5 fm, and the Pauli
principle. Being the result of these three distinct features, there is no simple
explanation for saturation. It is simple to verify that the Pauli principle alone
cannot suffice, and that any power law force does not lead to saturation
(Exercise 1.9).
Many properties of nuclear forces can, be explained quantitatively by the
potential proposed by Yukawa in 1939:
V (r)=g
¯hc
r
exp(−r/r
0
) . (1.56)
The factor ¯hc is present so that the coupling constant g is dimensionless. As
we will see in the next section, forces between particles are due, in quantum

36 1. Basic concepts in nuclear physics
field theory, to the quantum exchange of virtual particles.Theranger
0
of a
force is the Compton wavelength ¯h/mc of the exchanged particle of mass m.
Yukawa noticed that the range of nuclear forces r
0
1.4 fm, corresponds to
the exchange of a particle of mass 140 MeV. This is how he predicted the
existence of the π meson. The discovery of that particle in cosmic rays was a
decisive step forward in the understanding of nuclear forces.
When applied to a two-nucleon system, the potential (1.56) is reduced
by a factor of (m
π
/2m
p
)
2
∼ 10
−2
because of spin-parity considerations. A
dimensionless coupling constant of g 14.5 explains the contribution of the
π meson to the nucleon–nucleon force.
It is necessary to add other exchanged particles to generate a realistic
potential. In fact, the general form of nucleon–nucleon strong interactions
can be written as a linear superposition of Yukawa potentials :
V (r)=
i
g
i
¯hc
r
exp(−µ
i
r) (1.57)
where the sum is over a discrete or continuous set of exchanged particles with
masses given by µ
i
= m
i
c/¯h.
However, we need some more elements to explain saturation and prevent
a nuclear “pile-up” where all nucleons collapse to an object of the size of the
order of the range of the strong interactions.
First, one must add a strong repulsive shorter range potential, called a
“hard core”interaction. This potential is of the form (1.56) with a negative
coupling constant g and a range r
0
0.3 fm. The physical origin of the
repulsive core is not entirely understood but it certainly includes the effect of
the Pauli principle that discourages placing the constituent quarks of nucleons
near each other.
Second, spin effects and relativistic effects must be taken into account.
One writes the potential as the sum of central potentials with spin-dependent
coefficients:
V = V
C
(r)+Ω
T
V
T
(r)+Ω
SO
V
SO
(r)+Ω
SO2
V
SO2
(r) , (1.58)
where V
C
is a pure central potential and the other terms are spin dependent.
The most important is the tensor potential V
T
with
Ω
T
=[3(σ
1
· r/r)(σ
2
· r/r) − σ
1
· σ
2
] (1.59)
where σ
1
and σ
2
are the Pauli spin matrices for the two nucleon spins. Figure
1.11 shows how this term has the important effect of inducing a correlation
between the position and spin of the two nucleons. This results in a permanent
quadrupole moment for the deuteron. It is also the dominant force in binding
the deuteron. However, it averages to zero for multi-nucleon systems where
it plays a minor role.
The last two terms in (1.58) are spin-orbit interactions:
¯hΩ
SO
=(σ
1
+ σ
2
) · L (1.60)
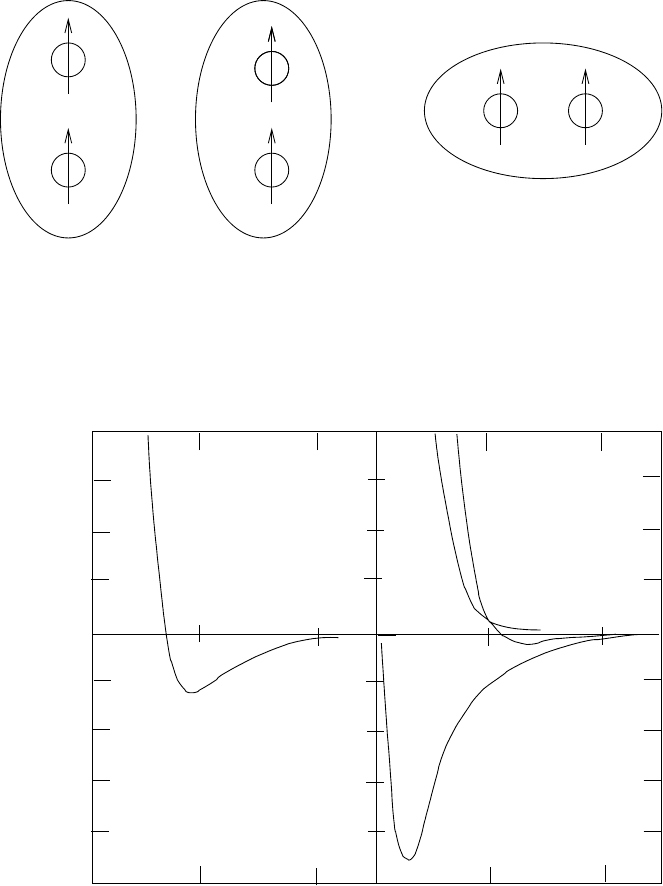
1.4 Nuclear forces and interactions 37
n
p
n
p
np
Fig. 1.11. The tensor potential for the s = 1 state (1.59) makes the configuration
on the right (σ ·r = 0) have a different potential energy than the two configurations
on the left (σ · r = 0). This results in the permanent quadrupole moment of the
deuteron.
S
=
0
p−p n−n p−n
p−n
V
50
−200
−150
−100
−50
0
100
150
V(r) (MeV)
r (fm)
121
r (fm)
S=1
V
C
V
SO
V
C
T
2
Fig. 1.12. The most important contributions to the nucleon–nucleon potentials in
the s = 0 state (left) and the s = 1 state (right) (the so-called Paris potential).
The two central potentials V
C
depend only on the relative separations. The tensor
potential V
T
is of the form (1.59) is responsible for the deuteron binding and for
its quadrupole moment. The spin-orbit potential is V
SO
.

38 1. Basic concepts in nuclear physics
¯h
2
Ω
SO2
=(σ
1
· L)(σ
2
· L)+(σ
2
· L)(σ
1
· L) (1.61)
where L is the orbital angular momentum operator for the nucleon pair.
Figure 1.12 shows the most important contributions to the nucleon–
nucleon potential [23]. For spin-anti-aligned nucleons (s = 0), only the central
potential contributes.
Finally, we note that to correctly take into account the charge indepen-
dence of nuclear forces the formalism of isospin (Sect. 1.7) must be used.
1.4.3 Origin of the Yukawa potential
The form (1.56), which is derived from quantum field theory, can be under-
stood quite simply. Consider a de Broglie wave
ψ =exp
− i(Et − p.r)/¯h
. (1.62)
The Schr¨odinger equation in vacuum is obtained by using E = p
2
/2m and
then taking the Laplacian and the time derivative. Assume now that we use
the relativistic relation between energy and momentum :
E
2
= p
2
c
2
+ m
2
c
4
(this is how Louis de Broglie proceeded initially). By taking second-order
derivatives of (1.62) both in time and in space variables we obtain the Klein—
Gordon equation :
1
c
2
∂
2
ψ
∂t
2
−∇
2
ψ + µ
2
ψ = 0 (1.63)
wherewehavesetµ = mc/¯h. Originally, this equation was found by
Schr¨odinger. He abandoned it because it did not lead to the correct rela-
tivistic corrections for the levels of the hydrogen atom.
5
It was rediscovered
later on by Klein and Gordon.
Forgetting about the exact meaning of ψ in this context, (1.63) is the
propagation equation for a relativistic free particle of mass m.Inthecase
m = 0, i.e., the photon, we recover the propagation equation for the electro-
magnetic potentials :
1
c
2
∂
2
ψ
∂t
2
−∇
2
ψ =0 . (1.64)
The classical electrostatic potential produced by a point charge is obtained
as a static, isotropic, time-independent solution to this equation with a source
term added to represent a point-like charge at the origin, i.e.,
∇
2
V (r)=−
q
0
δ(r) , (1.65)
5
Schr¨odinger did not have in mind spin and magnetic spin-orbit corrections. These
effects are accounted for by the Dirac equation, the relativistic wave equation
for a spin 1/2 particle
1.4 Nuclear forces and interactions 39
whose solution is
V (r)=
q
4π
0
r
. (1.66)
Similarly, by looking for static, isotropic solutions of the Klein–Gordon
equation 1.63 with a point-like source at the origin :
∇
2
V (r) − µ
2
(r)=−
g(¯hc)
4π
δ(r) , (1.67)
one can readily check that the Yukawa potential (1.56)
V (r)=g(¯hc/r) exp(−µr) , (1.68)
satisfies this equation. The solution V (r)=g(¯hc/r) exp(+µr) is discarded
since it diverges at infinity.
1.4.4 From forces to interactions
We have emphasized that, in quantum field theory, forces between particles
are described by the exchange of virtual particles. The interactions can be
described by (Feynman) “diagrams” like those shown in Fig. 1.13. Each dia-
gram corresponds to a scattering amplitude that can be calculated according
to the rules of quantum field theory. As we will see in Chap. 3, the effective
potential is Fourier transform of the amplitude.
In quantum electrodynamics, the exchange of massless photons leads to
the Coulomb potential. The exchange of massive particles leads to Yukawa-
like potentials. One example is the exchange of pions as shown in the first
two diagrams in Fig. 1.13. These diagrams contribute to the nucleon–nucleon
potential of Fig. 1.12 and lead to the binding of nucleons and to nucleon–
nucleon scattering.
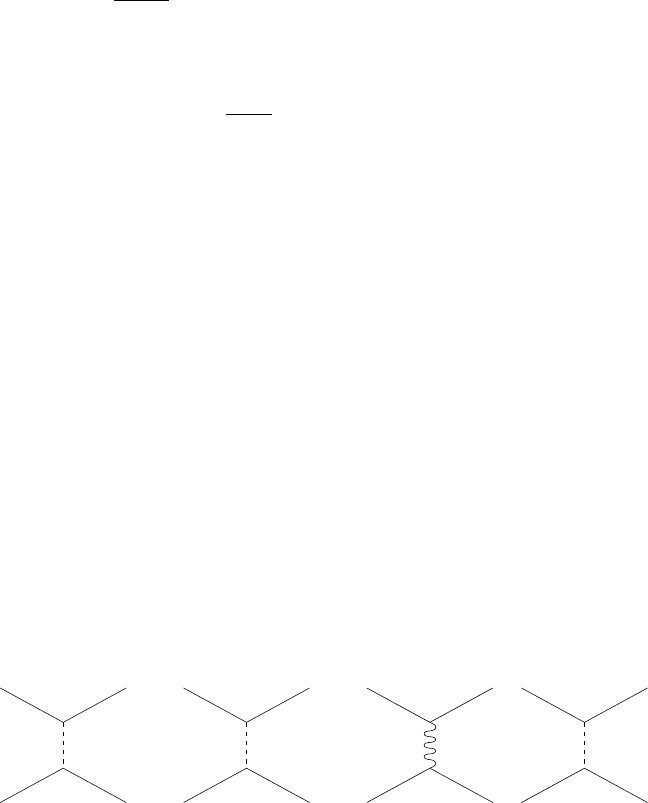
π
N
N’ N’
N
π
n
pn
p
γ
Z
NNNN
N
’
N
’
N
’
N
’
+−
0
Fig. 1.13. Diagrams contributing to the nucleon–nucleon interaction due to the
exchange of a pion, a photon and a Z
0
. In these diagrams N and N
represent either
a proton or a neutron. The two first diagrams with the exchange of a neutral or
charged pion generates the long-range part of the nucleon–nucleon potential. The
photon exchange diagram generates the Coulomb interaction between protons and
the magnetic interaction between any nucleons. The diagram with Z exchange is
negligible for the nucleon–nucleon interactions but dominates for neutrino-neutron
scattering since the neutrino has no strong interactions.
