Baeckvall J.-E. (ed.) Modern Oxidation Methods
Подождите немного. Документ загружается.

Pimaricin is an important antifungal agent used in human therapy for the
treatment of fungal keratitis as well as in the food industry to prevent mold
contamination. The enzymes and the gene cluster responsible for their production
by Streptomyces natalensis was identified recently. The cluster contains a P450 that is
responsible for ring decoration [99].
Production of epothilones (potential anticancer agents) was carried out with
recombinant Streptomyces strains. Therefore, the entire epothilone biosynthetic gene
cluster from the myxobacterium Sorangium cellulosum was heterologously expressed
in an engineered Streptomyces venezuelae strain. The resulting strains produced
approximately 0.1 mgL
1
epothilone B as a sole product after 4 days cultivation.
Deletion of a gene encoding a cytochrome P450 epoxidase gave rise to a mutant that
selectively produced 0.4 mgL
1
epothilone D [100].
Biosynthesis of the sesquiterpene antibiotic albaflavenone in Streptomyces coelicolor
A3(2) was studied in detail. The mechanism and stereochemistry of the enzymatic
formation of epi-isozizaene by multistep cyclization of farnesyl diphosphate was
investigated [101] and a P450 (CYP170A1) was identified to carry out two sequential
allylic oxidations to convert epi-isozizaene to an epimeric mixture of albaflavenols
and thence to albaflavenone [102].
While functions of a number of P450s from Streptomyces are known, in the vast
majority of these species the function of P450s so far remains unknown. Efforts are
being made to shed light on this issue. The long-term goal is to identify orphan P450
functions and to establish a strategy for the production of novel secondary metabo-
lites that have new biomedical function [103, 104]. Some crystal structures of P450s
from Streptomyces are already available, for example, that of P450
PikC
from Strepto-
myces venezuelae [105], P450
StaP
(CYP245A1) from Streptomyces sp. TP-A0274 [106],
P450
SU1
(CYP105A1) from Streptomyces griseolus [107], or recently CYP105P1 from
Streptomyces avermitilis [108]. The structural knowledge of these P450s opens up a way
for enzyme optimization and engineering leading to new antibacterial properties.
While Streptomyces produce the majority of known antibiotics, there are also
other bacterial species with interesting activities for pharmaceutical application.
OxyA, OxyB, and OxyC (CYP165A1, CYP165B1, and CYP165C1) from Amycolatopsis
orientalis, for example, are involved in the biosynthesis of the glycopeptide antibiotic
vancomycin [67]. A similar set of P450s with high identity to the oxygenases in
vancomycin biosynthesis (92% to 94%) is involved in the biosynthesis of balhimycin
in Amycolatopsis balhimycina [109].
Another example is P450
eryF
(CYP107A1) from Saccharopolyspora erythraea, which
catalyzes a hydroxylation step leading to the functional molecule in the biosynthesis
of the macrolide antibiotic erythromycin [110]. The crystal structures of OxyB and
OxyC [111, 112] as well as that of P450
eryF
[113] have been published.
Nonrecombinant wild-type strains are often used as biocatalysts for the production
of pharmaceutically relevant metabolites, as in many of the examples described
above. The application of wild-type strains, however, has some drawbacks. These
include product degradation, by-product formation, and catabolite repression [75].
Further, there is little or no possibility of engineering the biocatalyst itself. The
application of recombinant engineered strains, combined with bioprocess and
12.3 Application and Engineering of P450s for the Pharmaceutical Industry
j
433
biocatalyst engineering, provides an attractive alternative. For industrial biocatalysis
E. coli is in general the organism of choice because of the well-developed molecular
biology techniques and high levels of biocatalyst expression. Besides this, high cell
densities can be achieved during fermentation of E. coli [114]. Recombinant Pseu-
domonas strains are of industrial interest as well, since they could be useful for
biotransformations in the presence of toxic organic solvents. A number of solvent-
tolerant P. putida strains have been isolated and used as hosts for the expression of
P450 genes. An excellent review which includes many examples of the use of
recombinant E. coli and P. putida whole-cell biocatalysts on a scale up to 30 L has
been written by Park [75].
Recently construction and application of a novel P450 library, based on about
250 bacterial P450 genes (about 70% originating from actinomycetes), co-expressed
with putidaredoxin and putidaredoxin reductase in E. coli has been reported [115].
A good example demonstrating the exquisite regioselectivity of P450s, which is
impossible to reach by chemical oxidations, is presented by a screening of this library
with testosterone. Within the screening 24 bacterial P450s were identified, which
monohydroxylate testosterone regio- and stereoselectively at the 2a-, 2b-, 6b-, 7b-,
11b-, 12b -, 15b-, 16a- and 17-positions [116]. Most of these hydroxylations are
common for both prokaryotic and human P450s. Thus, the identified bacterial
candidates will be applied for the production of drug metabolites on a preparative
scale.
A screening among 1800 bacterial strains identified the Mycobacterium sp. strain
HXH-1500, catalyzing regio- and stereoselective hydroxylation of limonene at
carbon-atom C7 to yield the anticancer drug ()-perillyl alcohol. The biocatalytic
production of ()-perillyl alcohol from limonene was performed using the
Mycobacterium sp. P450 alkane hydroxylase (CYP153 family) recombinantly
expressed in P. putida cells [117]. The whole-cell process was performed in a two-
phase system resulting in 6.8 g L
1
of product in the organic phase.
12.3.2
Application of Mammalian P450s for Drug Development
An ongoing field of research in drug discovery and drug development is the use of
recombinant human P450s. Human P450s expressed in baculovirus-infected insect
cell lines have been in use for quite some time now to identify human P450s involved
in drug metabolism [118, 119]. They are also used to synthesize drug metabolites in
order to assess the toxicity of potential pharmaceuticals [120–122]. Recent achieve-
ments in the expression of recombinant human P450s in Saccharomyces cerevisiae
[76, 123, 124], Yarrowia lipolytica [125] and Pichia pastoris [126, 127] facilitate their use
for the synthesis of drug metabolites.
Besides the achievements in the expression of mammalian P450s in eukaryotic
organisms, E. coli is an attractive expression system and is of particular interest for
industrial applications (see also Section 12.3.1). Efforts to express human P450s in E.
coli have been made [128–130]; in this case, however, mammalian cDNAs have to be
modified before they can be expressed [131].
434
j
12 Biooxidation with Cytochrome P450 Monooxygenases
12.3.2.1 Enhancement of Recombinant Expression in E. coli
Initial attempts to enhance the expression of human P450s in E. coli involved
modification of the N-terminal sequence, since this region has been proposed to
be important in determining protein yield [132, 133]. This strategy was applied to
express a number of mammalian P450s including CYP 2E1 [134], 4A4 [135],
6A1 [136], 1A1 [137], as well as recently 20A1 [138] and 4X1 [139]. A different
approach was pursued by truncating the N-terminal hydrophobic region – which is
responsible for the interaction with the membrane – to a greater or lesser extent [140–
142]. Three human P450s (3A4, 2C9, and 1A2) with truncated N-termini were each
co-expressed with human CPR in E. coli using a bicistronic expression system. Intact
E. coli cells and membranes containing P450 and CPR were used in the preparative
synthesis of drug metabolites. The optimized biooxidation conducted on a 1-L scale
yielded 59 mg 6b-hydroxytestosterone (32), 110 mg 4-hydroxydiclofenac (33), and
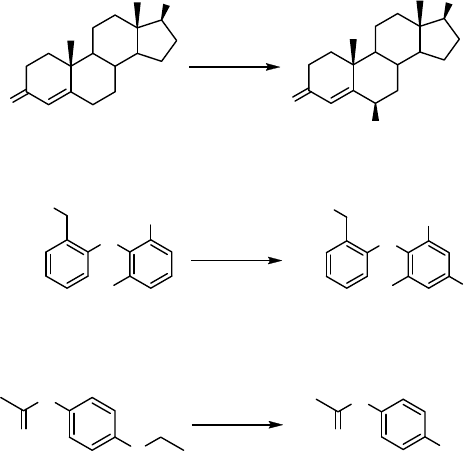
88 mg acetaminophen (34) [130] (Scheme 12.11).
Further optimization could be achieved by fusions of a bacterial signal peptide (for
example a modified ompA-leader sequence) to human P450 cDNAs [122]. This leader
is removed during P450-processing, and the native P450 is released. Following this
strategy, 14 recombinant human P450s co-expressed with NADPH P450 reductase in
H
N
Cl
Cl
HOOC
H
N
Cl
Cl
HOOC
OH
diclofenac 33
CYP2C9
H
N
O
O
H
N
OH
O
CYP1A2
phenacetin
34
O
OH
testosterone
O
OH
32
OH
CYP3A4
Scheme 12.11 Oxidation of testosterone, diclofenac, and phenacetin to yield 6b-
hydroxytestosterone (32), 4-hydroxydiclofenac (33) and acetaminophen (34), respectively.
12.3 Application and Engineering of P450s for the Pharmaceutical Industry
j
435
E. coli have been used by several pharmaceutical companies, both as biocatalysts for
the preparation of metabolites of drug candidates and for high-throughput P450
inhibition screenings. Up to 300 mg of different metabolites could be obtained using
permeabilized recombinant E. coli cells expressing human P450s [143].
12.3.2.2 Enhancement of Activity and Selectivity and Engineering of Novel Activities
Since structure-function relationships for mammalian (and other eukaryotic) P450s
are far less clear than for bacterial ones, most protein engineering efforts conducted
on mammalian P450s so far focus on the identification of key residues involved in
substrate binding. Nevertheless, as more crystal structures of mammalian P450s are
becoming available, the structural knowledge can also be used to engineer novel
enzyme properties.
Recent examples include the engineering of CYP2B1 by a combination of random
and site-directed mutagenesis to accept the anti-cancer prodrugs cyclophosphamide
and ifosfamide [144]. Homology modeling and rational design performed on
CYP2B1 allowed the regioselectivity of progesterone 16-a-hydroxylation to switch
to 21-hydroxylation [145].
The ability of human CYP1A2 to catalyze O-demethylation of 7-methoxyresorufin
was improved by three rounds of mutagenesis. The triple-mutant E163K/V193M/
K170Q exhibited turnover rates more than five times faster than wild-type
CYP1A2 [146].
TheGillamgroupconstructedachimericlibraryofCYP1A1andCYP1A2employing
restriction enzyme-mediated DNA family shuffling. They observed different activity
profiles toward various luciferin derivatives among active clones, including improved
specific activity, novel activities, and broadening of substrate range [147, 148].
The substrate spectrum of human CYP2D6 was expanded toward bulky indole
derivatives, like 4- and 5-benzyloxyindoles, by random mutagenesis and site-directed
mutagenesis [149].
12.3.2.3 Construction of Artificial Self-Sufficient Fusion Proteins
A very effective mode to eliminate the reconstitution procedure of redox chains is
to tailor self-sufficient electron transport chains emulating natural fusion enzymes.
A large variety of these artificial fusion enzymes have been reported. Fusions of P450s
with a diflavin (FAD- and FMN-containing) reductase, P450s with flavodoxins, P450s
with ferredoxins and ferredoxin reductases, P450s with dioxygenase reductase-like
enzymes, and even semisynthetic heme-containing flavoproteins that can act as
a monooxygenase have been described [70, 150]. Genes of various mammalian P450s
and their redox partners were used to generate functional multi-domain proteins
with designed properties (molecular Lego) beyond the restrictions imposed by the
naturally occurring protein domains [151].
For example, the N-terminal human CYP2D6 sequence was linked to the
C-terminal human cytochrome reductase module, resulting in a self-sufficient
enzyme exhibiting activity toward a wide range of pharmaceutical compounds such
as bufuralol, metoprolol, or dextromethorphan [152]. Other examples are fusion
proteins of human CYP1A1 with rat CPR [153], human CYP2C9 with BMR [154], and
436
j
12 Biooxidation with Cytochrome P450 Monooxygenases
human CYP3A4 with rat or human CPR [155]. As cytochrome b
5
was detected to
remarkably enhance the coupling efficiency if co-expressed with the CYP3A4-fusion
systems [156], recombinant three-component systems with CYP3A4/CPR/
cytochrome b
5
were tailored, maintaining even higher oxidation activity for testos-
terone and nifedipine [157].
12.4
Application of P450s for Synthesis of Fine Chemicals
Another valuable application for P450s, aside from the production of pharmaceu-
tically relevant metabolites, is the fabrication of sought-after fine chemicals and
flavors that are of interest for the food industry [158]. P450s are interesting
biocatalysts for such syntheses, since chirality of the products is often very important.
Celik et al. have described an example of substrate hydroxylation by P450s with
a high degree of regio- and diastereose lectivity [159]. The authors used recombinant
E. coli whole-cell systems, in which cytochromes P450
SU1
, P 450
SU2
, and P450
SOY
were over-expressed with their cognate ferrodoxins for hydroxylation of a-ionone
(35)andb-ionone (38) to their corresponding mono-hydrox ylated derivatives. For
a-ionone the reaction was diastereoselective, yielding only the anti-isomers
(3S,6S)- and (3R,6R)-3-hydrox y-a-ionone (36 and 37). For b-ionone an enantiomeri c
excess of 35% in favor of the (S)-enantiomer (39) was observed (Scheme 12.12).
In another screening of the recombinant P450-library described in Ref. [115] (see
Section 12.3.1), CYP109B1 was identified to be capable of regioselective hydroxyl-
ation of ( þ )-valencene (48) at allylic C2-position, thereby producing ( þ )-nootkatone
– a sought after fragrance for flavoring that has a strong grapefruit odor and bitter
taste [160, 161] – via nootkatol. By product formation, which accounted for 35% of the
total product during biooxidation with recombinant E. coli in an aqueous milieu, was
significantly reduced when aqueous-organic two-liquid-phase systems were set up
O O O
HO
HO
O O
OH
6353
37
38
39
P450
+
P450
Scheme 12.12 Regio- and diastereoselective hydroxylation of a-ionone (35) and b-ionone (38).
12.4 Application of P450s for Synthesis of Fine Chemicals
j
437
leading to accumulation of nootkatol and ( þ )-nootkatone of up to 97% of the total
product. Up to 120 mg L
1
of nootkatol and ( þ )-nootkatone were produced within
8 h employing this system [162].
Functional expression of tricistronic constructs consisting of P450
cam
from
P. putida and the auxiliary redox partners putidaredoxin reductase and putidaredoxin
in E. coli has been reported. The transformed whole-cells efficiently oxidized (1R)-
( þ )-camphor (10) to 5-exo-hydroxycamphor (11) and limonene to ()-perillyl
alcohol [163]. Since these bioengineered e-cells possess a heterologous self-sufficient
P450 catalytic system, they may have advantages in terms of low cost and high yield
for the production of fine chemicals.
12.5
Engineering of P450s for Biocatalysis
12.5.1
Cofactor Substitution and Regeneration
The application of cytochrome P450 monooxygenases requires functional and
efficient electron suppliers and is dependent on the availability of the costly cofactors
NAD(P)H. Thus, various approaches have been made to substitute or regenerate
NAD(P)H and to increase the coupling efficiency for both in vitro and in vivo
applications of P450s. These attempts include direct chemical or electrochemical
reduction of P450s, the use of cheap chemicals to directly replace NAD(P)H, the
development of (enzymatic) cofactor recycling systems [164], and the engineering
of artificial fusion proteins [150].
12.5.1.1 Cofactor Substitution In Vitro
Direct chemical reduction can be achieved by the use of organometallic complexes
such as Co
II
sepulchrate trichloride [165, 166] by addition of Ti
III
citrate [167] or by the
strong reducing agent sodium dithionite [168] – an inexpensive agent routinely used
to produce the ferrous-carbonyl form of P450. The limitations of these approaches
are, however, low system efficacy, low sensitivity of mediators to molecular oxygen,
and the production of reactive oxygen species or mediator aggregation.
Peroxides that directly convert the heme iron of P450s to a ferric hydroperoxy
complex by the peroxide shunt (e.g., hydrogen peroxide, cumene peroxide, or tert-
butyl oxide) could be useful for oxidation of various substrates. The essential problem
in utilizing the peroxide shunt for P450 biocatalysis seems to lie in the time-
dependent degradation of the heme and in oxidation of the protein [169, 170].
Methods of directed evolution, like random and site-specific mutagenesis, were
applied to evolve P450s to enhance the efficiency of the peroxide shunt
pathway [171].
Electrochemical reduction of P450s by using an electrode seems to be the most
convenient way of fabricating bioreactors [172] and has been studied in detail for
more than 15 years. However, heme reduction on a cathode is complicated and most
438
j
12 Biooxidation with Cytochrome P450 Monooxygenases
often insufficient for biocatalysis, which can partly be attributed to the deeply buried
heme iron and partly to the instability of the enzyme upon interaction with the
electrode surface. Improvements to this approach include the modification of the
electrode surface [150].
12.5.1.2 Cofactor Regeneration In Vitro
One of the most common approaches to overcome the stoichiometric need for
NAD(P)H involves enzymatic regeneration systems. Strategies for the regeneration
of NADPH-dependent P450s are based on
D/L-isocitrate dehydrogenase [173], an
engineered formate dehydrogenase from Pseudomonas sp. 101 [174], glucose-6-
phosphate dehydrogenase [175], or alcohol dehydrogenase from Thermoanaerobium
brockii [176]. Although these systems work reasonably well, further reduction of costs
for enzymatic oxyfunctionalization can be achieved by engineering of NADPH-
dependent P450s to accept NADH [177, 178]. NADH has the advantage that it is about
ten times less expensive and more stable than NADPH. Furthermore, more NAD
þ
-
dependent enzymes at lower prices are available for cofactor regeneration. Our group
used homology modeling and site-directed mutagenesis to construct P450
BM3
mutants showing altered cofactor specificity from NADPH to NADH. The best
mutant, W1046S/S965D, demonstrated high turnover rates (k
cat
¼ 14600 min
1
)
during cytochrome c reduction and low K
M
for NADH. It had equal affinity to both
NADH and NADPH and displayed a 2.6-times higher activity when NADH was
used [179].
12.5.1.3 Cofactor Regeneration in Whole-Cells
Cofactor concentration within the cell may become a bottleneck for the overall
process if concentrations of the recombinant P450 biocatalyst and/or their activities
reach a very high level [180]. Strategies to overcome this limitation employ the use
of artificial cofactor recycling systems supporting the endogenous ones and the
employment of native electron transfer systems to increase the coupling efficiency of
P450s and reduce metabolic stress for the host organism.
The catalytic efficiency of P450
cam
expressed i n E. coli could be increased up to
25-fold by the coupling of its native electron transfe r syst em (putidaredoxin
reductase and putidaredoxin) to enzymatic NADH regeneration catalyzed by
a recombinantly expresse d glycerol dehydrogenase. This whole-cell s ystem was
applied for camphor hydroxylation in aqueous solution, as well as in a biphasic
system with isooctane [181].
A recent approach in this field is the construction of a novel E. coli whole-cell
biocatalyst with improved intracellular cofactor regeneration driven by external
glucose [182]. In this system, additional recombinant intracellular NADPH regen-
eration takes place through co-expression of a glucose facilitator from Zymomonas
mobilis for uptake of unphosphorylated glucose and an NADP
þ
-dependent glucose
dehydrogenase from Bacillus megaterium that oxidizes glucose to gluconolactone.
When a quintuple mutant of P450
BM3
that oxyfunctionalizes a-pinene (49) – a cheap
waste product of wood industry – to yield a-pinene oxide, verbenol and myrtenol [183]
was expressed in this system, the engineered strain showed a 9-fold increased initial
12.5 Engineering of P450s for Biocatalysis
j
439
a-pinene oxide formation rate and a 7-fold increased a-pinene oxide yield in the
presence of glucose compared to glucose-free conditions. Further bioprocess engi-
neering addressed the low water solubility and the toxicity of a-pinene by setting up
an aqueous-organic two-phase bioprocess with diisononyl phthalate as a biocom-
patible organic carrier solvent. With an aqueous-to-organic phase ratio of 3: 2 and
30% (v/v) of a-pinene in the organic phase, a total product concentration of over
1gL
1
was achieved [184]. This process marks a promising step toward a future
application of recombinant microorganisms for the selective oxidation of terpenoids
to value-added products.
12.5.2
Construction of Artificial Fusion Proteins
As in the case of human P450s (described in Section 12.3.2.3), a variety of arti ficial
fusion enzymes have also been generated for bacterial P450s aiming to increase
enzyme activity and coupling efficiency.
Concerning P450
cam
, the hemoprotein was fused to its natural electron donors,
putidaredoxin and putidaredoxin reductase in different orders of the individual parts
and variable linkers between them. However, despite increased coupling, the catalytic
activity of the engineered assembly was only 30% of that of the reconstituted wild-type
mixture, reconstituted in a 1 : 1 : 1 ratio with the separate redox partners [185]. The
catalytic activity of the fusion enzyme toward
D-( þ )-camphor (10) could be increased
10-fold by the development of an enzymatic cross-linking of putidaredoxin with a
peptide fragment between putidaredoxin reductase and P450
cam
, accommodating a
reactive glutamyl residue prone to attack by transglutaminase [186].
The putidaredoxin reductase-like unit of the self-sufficient CYP116B2 from Rho-
dococcus sp. (P450RhF) [187] was ligated to the heme domain of P450
cam
, CYP153A, or
CYP203A. The chimeric proteins showed catalytic activity against a variety of
compounds like n-alkanes,
D-( þ )-camphor (10), and 4-hydroxybenzoate [188].
12.5.3
Engineering of New Substrate Specificities
In this chapter focus exclusively on the two best characterized P450s: P450
cam
(CYP101A1) from P. putida [189] and P450
BM3
(CYP102A1) from B. megaterium.
Their structure, catalytic mechanism, and biochemistry have been studied in detail
extensively [10]. Mutagenesis studies on these P450s have led to significant improve-
ments in our understanding of general aspects of P450 catalytic function, and
numerous approaches have been undertaken to engineer new substrate specificities
for these enzymes.
12.5.3.1 P450
cam
from Pseudomonas putida
For P450
cam
it was demonstrated that mutations of the active site residue Y96 to more
hydrophobic ones considerably increased its activity toward the oxidation of hydro-
phobic molecules smaller than camphor (10), like styrene (40) and alkanes [190, 191],
440
j
12 Biooxidation with Cytochrome P450 Monooxygenases
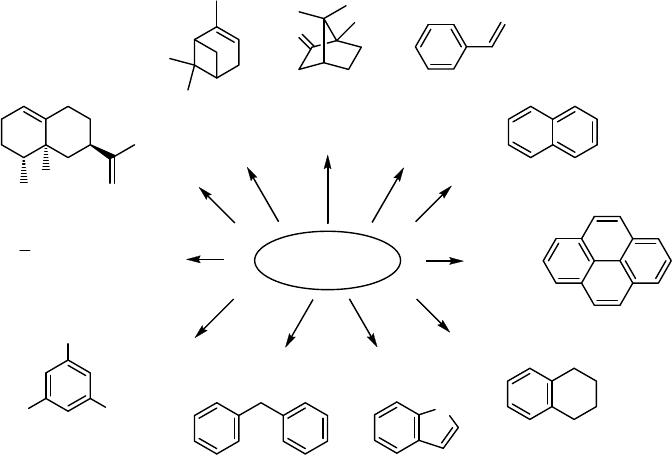
or larger than camphor, such as naphthalene (41 ) and pyrene (42) [192, 193]. The
Y96Fmutant was capable of regio- and stereoselective hydroxylation of tetralin (43)to
the 1-(R)-alcohol [194]. Using saturation mutagenesis at positions Y96 and F87,
several mutants were constructed with activity toward indole (44) and diphenyl-
methane (45) [195]. Some mutants have been engineered for biodegradation of
environmentally harmful pollutants, such as di-, tri-, tetra-, penta-, and hexachlor-
obenzene (46) [196, 197] (Figure 12.3).
P450
cam
was further engineered to an alkane hydroxylase by step-by-step adap-
tation of the enzyme to smaller n-alkanes beginning with hexane [198], then
proceeding to butane and propane [199], and finally to ethane (47) [200]. The
best mutant, with eight substitutions, oxidized propane at 500 min
1
with 86%
coupling, which was comparable with that of the wild-type enzyme toward camphor
(10) [200].
Successful attempts to engineer mutants for selective oxidation of cheap terpenes to
expensive oxidized derivatives have also been accomplished. Rational mutants
of P450
cam
exhibited activity toward the sesquiterpene ( þ )-valencene (48) and pro-
duced > 85% of ( þ )-trans-nootkatol and ( þ )-nootkatone [201]. The monoterpene
( þ )-a-pinene (49) was oxidized by P450
cam
mutants to 86% ( þ )-cis-verbenol or to a
mixture of ( þ )-verbenone and ( þ )-cis-verbenol (Figure 12.3) [202]. Verbenol and
verbenone are active pheromones against various beetle species.
10
O
P450
cam
WT
H
N
40
47
41
42
44
45
Cl
ClCl
48
46
49
43
Y96A
Y96
V247
F87 Y96
L244 V247
Y96F
F87W Y96F T101L
T185M V247L
G248AL294M
L1244M L1358P
F87 Y96
F87W Y96F
V247L
F87 Y96
Y96F
F87 Y96
H
3
C
CH
3
Figure 12.3 Mutations of P450
cam
leading to altered substrate specificities. WT: wild-type enzyme
without mutations.
12.5 Engineering of P450s for Biocatalysis
j
441
12.5.3.2 P450
BM3
from Bacillus megaterium
P450
BM3
is an obvious target enzyme for the development of biotechnological
applications, since it is a self-sufficient single-component protein with a high catalytic
activity. The turnover rates of P450
BM3
toward fatty acids are among the highest
activity values reported for P450s [203].
The mutant A74G/F87V/L188Q, designed by saturation mutagenesis, was shown
to oxidize indole, n-octane, highly branched fatty acids and fatty alcohols, poly-
chlorinated dibenzo-p-dioxins, polyaromatic hydrocarbons, styrene, and many other
chemical compounds [204 –208]. The monoterpene geranylacetone was converted by
P450
BM3
(R47L/Y51F/F87V) with high activity (2080 min
1
) and stereoselectivity
(97% ee) to a single product, namely 9,10-epoxygeranylacetone [209]. The mutant
A74E/F87V/P386S exhibited an 80-fold improved activity toward b-ionone (42)
compared to the wild-type enzyme and produced the flavoring (R)-4-hydroxy-b-io-
none as the only product [210].
Using a combination of directed evolution and site-directed mutagenesis Arnold
and coworkers altered the selectivity of P450
BM3
from hydroxylation of dodecane
(C12), first to octane (C8) and hexane (C6) and further on to gaseous propane (C3) and
ethane (C2) [211–214]. Some mutants were found with high stereoselectivity, leading
either to (R)- or to (S)-2-octanol [215].
In our group, a systematic analysis of the structures of 29 P450s and 6379 P450
sequences, with the aim of identifying selectivity- and specificity-determining
residues, led to identification of a positively charged heme-interacting residue in
the SRS5, which was present in about 98% of the sequences analyzed. This residue is
located in close vicinity to the heme center and restricts the conformation of the
SRS5. It is preferentially located at position 10 or 11 after the conserved ExxR motif (in
about 95% of the sequences). Replacing this residue by hydrophobic residues
of different size has been shown to change substrate specificity and regioselectivity
for P450s of different superfamilies [216]. Based on this analysis, a minimal P450
BM3
mutant library of only 24 variants plus wild-type was constructed by combining five
hydrophobic amino acids (alanine, valine, phenylalanine, leucine, and isoleucine) in
positions 87 and 328. The library was screened with four terpene substrates
geranylacetone, nerylacetone, (4R)-limonene, and ( þ )-valencene (52). Eleven var-
iants demonstrated either a strong shift or improved regio- or stereoselectivity during
oxidation of at least one substrate as compared to P450
BM3
wild-type [217].
Although wild-type P450
BM3
is not able to metabolize any drug-like compound
tested so far, it has been turned by protein design and directed evolution into an
enzyme that oxidizes human drugs [218]. The R47L/F87V/L188Q mutant was shown
to metabolize testosterone, amodiaquine, dextromethorphan, acetaminophen, and
3,4-methylenedioxymethylamphetamine [219]. Several mutants were obtained by
means of directed evolution which are able to convert propranolol, a multi-function
beta-adrenergic blocker [220]. Another mutant was capable of stereo- and regiose-
lective hydroxylation of the peptide group of buspirone to yield (R)-6-hydroxybuspir-
one – an anti-anxiety agent – with > 99.5% ee [221].
Recent approaches in this field reported mutants with applicability as biocatalysts
in the production of reactive metabolites from the drugs clozapine, diclofenac, and
442
j
12 Biooxidation with Cytochrome P450 Monooxygenases
