Baeckvall J.-E. (ed.) Modern Oxidation Methods
Подождите немного. Документ загружается.

EPR [31], NMR [32], and UV-Vis [32a] spectroscopic studies have identified that in the
disproportionation of H
2
O
2
both Mn
II
2
and Mn
III
2
oxidation states are involved [33].
The proposed catalase mechanism is depicted in Scheme 11.1. Hydrogen peroxide
decomposition is initiated by (a) the binding of H
2
O
2
to the Mn
III
-Mn
III
dinuclear
center, and this is followed by (b) reduction to the Mn
II
-Mn
II
intermediate and
concomitant oxidation of the peroxide to O
2
[33, 34]. Subsequent binding of a second
molecule of H
2
O
2
to the Mn
II
-Mn
II
species (c) is followed by the reduction of H
2
O
2
to H
2
O and the oxidation of the Mn
II
-Mn
II
species (d), which closes the catalytic
cycle [17].
A series of Mn complexes that mimic the active site have been developed to gain
insight into the mechanisms by which these enzymes operate [34]. Dismukes and
coworkers reported the first functional catalase model that exhibited high activity
toward H
2
O
2
decomposition; even after turnover numbers of 1000, no loss of activity
toward H
2
O
2
decomposition was observed [35]. The dinuclear Mn
II
complex is based
on ligand 1 (Figure 11.1). EPR and UV-Vis spectroscopic investigations indicate that
under conditions of H
2
O
2
decomposition both Mn
III
–Mn
III
and Mn
II
–Mn
II
oxidation
states are present, as was observed for the related manganese catalase enzymes [34].
Sakiyama and coworkers have explored several dinuclear manganese complexes
based on the ligand 2,6-bis[N-(2-dimethylamino)ethyl]iminomethyl-4-methylpheno-
late) (2, Figure 11.1) and related ligands as catalase mimics. Employing UV-Vis and
MS techniques both mono- and di-nuclear Mn
IV
-oxo intermediates could be de-
tected [36]. Notably, the proposed mechanism (Scheme 11.2) is different from that
reported for the manganese catalases and model compounds containing ligand 1
involving high-valent Mn ¼ O species [36].
BH
+
B
BH
+
B
O
O
C
O
C
O
Mn
III
O
Mn
III
O
O
O
C
O
C
O
Mn
III
OH
O
Mn
III
O
Mn
II
O
O
C
O
C
O
Mn
II
OH
OH
2
Mn
II
O
O
C
O
C
O
Mn
II
O
2
H
2
O
H
2
O
2
H
2
O
2
H
2
O
a)
b)
c)
d)
Scheme 11.1 Proposed mechanism for H
2
O
2
decomposition by manganese catalase [17].
11.2 Bio-inspired Manganese Oxidation Catalysts
j
373
Manganese complexes of 1,4,7-triazacyclononane (3, tacn) or 1,4,7-trimethyl-1,4,7-
triazacyclononane (4, tmtacn, Figure 11.1) were studied by Wieghardt and coworkers
as models for the dioxygen-evolving center of photosystem II as well as manganese
catalase [37]. Turnover numbers for the decomposition of H
2
O
2
as high as 1300 are
reached readily [37d]. More recently Krebs and Pecoraro used the tripodal bpia ligand
(bpia ¼ bis-(picolyl)(N-methylimidazol-2-yl)amine) as a Mn catalase model system.
Several Mn complexes based on this ligand were found to be structural mimics for
certain catalase enzymes. Remarkably, the catalytic activity was found to be within 2
to 3 orders of magnitude relative to these enzymes [38]. More recently, dinuclear
manganese-based complexes based on ligand 5, that were developed as an oxidation
Mn
II
Mn
II
Mn
II
Mn
III
O
H
Mn
III
Mn
III
O
H
O
H
Mn
II
Mn
IV
O
Mn
IV
Mn
I
V
OO
0.5 H
2
O
2
H
2
O
2
0.5 H
2
O
2
H
2
O
0.5 H
2
O
2
0.5 O
2
H
2
O
2
2H
2
O
H
2
O
2
O
2
Scheme 11.2 Proposed mechanism of H
2
O
2
decomposition catalyzed by Mn complexes based
on ligand 2 [36].
N
H
N
N
H
N
N
N
H
N
N
H
N
N
OH
1
OHN N
N N
2
N
H
NH
HN
3
N
N
N
4
5
N N
N
OH
Figure 11.1 Ligand sets developed for manganese complexes as catalase mimics.
374
j
11 Manganese-Catalyzed Oxidation with Hydrogen Peroxide
catalyst, were found to be highly efficient in H
2
O
2
decomposition both in solution
and when immobilized on solid supports [39]. Several of the manganese oxidation
catalysts which were developed from these systems will be discussed in the following
sections in their application to oxidation catalysis with H
2
O
2
.
11.3
Manganese-Catalyzed Bleaching
Bleaching processes in the paper industry and the bleaching of stains on textiles have
been studied intensively over the last century. Conventional bleaching procedures for
laundry cleaning employ H
2
O
2
and high temperatures [6, 34]. Several catalysts have
been investigated to achieve bleaching at lower temperatures (i.e., 40–60
C) and even
under ambient conditions [34, 40]. Manganese complexes based on 1,4,7-trimethyl-
1,4,7-triazacyclononane, that is, [Mn
2
O
3
(tmtacn)
2
](PF
6
)
2
(6) (Figure 11.2), were
studied extensively by Unilever Research in the 1990s as bleach catalysts for stain
removal at reduced temperatures [41]. Unfortunately, following press releases on
textile damage as a result of using this catalyst for laundry cleaning, this catalyst is
no longer used in laundry detergent products [41]. Nevertheless, this and related
catalysts have seen new life in bulk processes including wood pulp and raw cotton
bleaching and in dishwasher formulations, areas where multi-wash textile damage
is less relevant [6, 42].
11.4
Epoxidation and cis-Dihydroxylation of Alkenes
Epoxides are an important and extremely versatile class of organic compounds, and
the development of new methods for the selective epoxidation of alkenes continues
to be a major challenge [2, 12, 43]. The epoxidation of alkenes can be achieved by
N
N
N
Mn
N
N
N
Mn
O
O
O
IVIV
(PF
6
)
2
Figure 11.2 The complex [Mn
2
O
3
(tmtacn)
2
](PF
6
)
2
(6).
11.4 Epoxidation and cis-Dihydroxylation of Alkenes
j
375
applying one of a number of oxidants including peroxycarboxylic acids [44], dioxir-
anes [45], alkylhydroperoxides [46], hypochlorite [47], iodosylbenzene [47], dioxy-
gen [48], and hydrogen peroxide [12,43c,46]. With a few exceptions, most of the
oxidants have the disadvantage that, in addition to the oxidized products, stoichio-
metric amounts of waste products are formed which have to be separated from the
often sensitive epoxides. The use of H
2
O
2
in combination with Mn complexes offers
several advantages including the high reactivity of the catalytic systems, although the
oxidant is often partially destroyed by the catalase-type activity typically associated
with Mn catalysts [34]. It should be noted also that nonselective side reactions
can occur because of the formation of hydroxyl radicals formed by homolytic cleavage
of H
2
O
2
[49].
11.4.1
Manganese Salts
Ligand-free epoxidation systems are attractive, particularly in the context of the
development of green oxidation procedures and in terms of cost [3, 50]. A remarkably
simple and effective ligand-free Mn-based epoxidation system, using 0.1–1.0 mol%
of MnSO
4
and 30% aqueous H
2
O
2
as the oxidant in the presence of bicarbonate,
was introduced by Burgess and coworkers [12, 51]. Bicarbonate and H
2
O
2
form the
actual oxidant peroxy monocarbonate (Scheme 11.3), which is proposed to react with
the Mn ion to generate the active epoxidation catalyst, as was indicated by EPR
studies [51, 52].
A series of cyclic alkenes and aryl- and trialkyl-substituted alkenes are converted
into their corresponding epoxides in high yields using 10 equiv. of H
2
O
2
. Notably,
monoalkyl alkenes were unreactive with this system. A range of additives were tested
in an effort to increase the H
2
O
2
efficiency by enhancing the activity for epoxidation
and suppressing H
2
O
2
decomposition. The use of 6 mol% of sodium acetate in
t
BuOH or 4 mol% of salicylic acid in DMF as solvent resulted in an improved
D
M
F
o
r
t
B
u
O
H
p
H
8
.
0
1mol%
Mn
II
X
n
R
1
R
R
3
R
2
R
1
R
R
3
R
2
O
H
2
O
H
2
O
2
HCO
4
-
HCO
3
-
Scheme 11.3 MnSO
4
-catalyzed epoxidation with bicarbonate/hydrogen peroxide, where X
n
is
an undefined ligand.
376
j
11 Manganese-Catalyzed Oxidation with Hydrogen Peroxide
epoxidation system with higher epoxide yields, decreased reaction times, and amount
of H
2
O
2
used (5 equiv., Table 11.1) [51].
The Mn-salt/bicarbonate system is also catalytically active in ionic liquids. Epox-
idation of a range of alkenes with 30% aqueous H
2
O
2
can be accomplished with
Table 11.1 Epoxidation of alkenes using MnSO
4
/salicylic acid catalyst [51].
R
3
R
R
1
R
2
R
3
R
R
1
R
2
O
1 mol% MnSO
4
4 mol% salicylic acid
H
2
O
2
,
DMF
0.2 M, pH 8.0, NaHCO
3
buffer
Alkene Epoxide Equiv. H
2
O
2
Yield (%)
O
2.8 96
O
589
OH
OH
O
591
O
597
O
595
Ph
Ph
O
595
n
Pr
n
P
r
n
Pr
n
P
r
O
25 75
n
Pr
n
P
r
n
Pr
n
P
r
O
25 75
a)
a) Approximately 1 : 1 cis/trans mixture.
11.4 Epoxidation and cis-Dihydroxylation of Alkenes
j
377
catalytic amounts of MnSO
4
in combination with TMAHC (tetramethylammonium
hydrogen carbonate) in the ionic liquid [bmim][BF
4
] (1-butyl-3-methylimidazolium
tetrafluoroborate). Moderate to excellent yields are obtained for internal alkenes, and
the ionic liquid can be reused at least 10 times when fresh amounts of the Mn salt and
bicarbonate are added [53].
11.4.2
Porphyrin-Based Catalysis
Metallo-porphyrins and several other metal porphyrin complexes, in particular those
of Mn, Fe, and Cr, have been studied extensively as catalysts in the epoxidation of
alkenes [47, 48]. Although the terminal oxidants iodosylarenes, alkylhydroperoxides,
peracids, and hypochlorite have received the most attention, reactions using H
2
O
2
have been reported also [47, 48]. The earliest porphyrin-based catalysts were limited
by rapid deactivation due to oxidative degradation of the ligand. Substantial im-
provements were achieved in catalyst robustness and activity in both alkene epox-
idation and alkane hydroxylation through the introduction of halogen substituents
on the porphyrin ligands [54]. Nevertheless, porphyrin-based epoxidation catalysts
suffer general disadvantages compared with other systems in regard to their
synthesis, and purification, which is often tedious.
Initial attempts to use H
2
O
2
as an oxidant for alkene epoxidation with porphyrin-
based catalysts were unsuccessful due to dismutation of H
2
O
2
into H
2
O and O
2
,
leading to rapid depletion of the oxidant. Introduction of bulky groups on the
porphyrin ligand enabled the use of aqueous H
2
O
2
, albeit with only low conversions
being achieved. It was demonstrated, however, that this catalytic system could be
improved by performing the oxidation reaction in the presence of excess imidazole
[55, 56]. The role of the imidazole is proposed to be twofold: (a) in acting as
a stabilizing axial ligand, and (b) in promoting the formation of the Mn
V
¼ O
intermediate (the oxygen transfer agent) through heterolysis of an Mn
III
-OOH
intermediate. This catalytic system provides epoxides in yields of up to 99%.
The excess in axial ligand could be reduced significantly through addition of a
catalytic amount of carboxylic acid [57, 58]. Under two-phase reaction conditions
with addition of benzoic acid the oxidation reaction was accelerated significantly,
and high conversions could be obtained in less than 10 min at 0
C (Scheme 11.4,
Table 11.2) [57].
The carboxylic acids and nitrogen-containing additives are generally considered to
facilitate the heterolytic cleavage of the OO bond in the manganese hydroperoxy
intermediate to provide a catalytically active manganese(V)-oxo species [59]. DFT
(Density Functional Theory) calculations reported by Balcells et al. have highlighted
the potential importance of the axial ligand in determining the activity of the Mn
V
-oxo
species formed in engaging in CH abstraction [60]. However, competing homolytic
cleavage of the OO bond leads to the formation of hydroxyl radicals and nonse-
lective oxidation reactions – a serious challenge encountered in general in using
H
2
O
2
in metal-catalyzed oxidations [48]. The proposed catalytic cycle for epoxidation of
alkenes using manganese porphyrin 7 begins with conversion to the well-established
378
j
11 Manganese-Catalyzed Oxidation with Hydrogen Peroxide
Mn
V
-oxo species (Scheme 11.5) [49a,61]. Subsequently, the oxygen atom is transferred
to the alkene via a concerted- (path a) or stepwise- (path b) pathway followed by release
of the Mn
III
-species and formation of the epoxide. In the stepwise route b, which
involves a neutral carbon radical intermediate, rotation around the former double
bond can result in cis/trans isomerization leading to trans-epoxides from cis-alkenes,
as is observed experimentally [61].
Improvement in the stereoselectivity of the oxidation of cis-stilbene was observed
by increasing the number of substituents on the aryl groups of the porphyrin ligand,
pointing to an enhanced preference for a concerted pathway. In general, it should be
noted, however, that trans-alkenes are poor substrates for these catalysts [57, 62].
Enhanced epoxidation rates were observed using a Mn-porphyrin complex 8
in which the carboxylic acid and imidazole groups are both linked to the ligand
N
N
N
N
Cl
Cl
Cl Cl
Cl
Cl
ClCl
Mn
III
Cl
-
7
N
N
(CH
2
)
5
CH
3
O
OH
O
0.5 mol% 0.5 mol%
CH
2
Cl
2
/ H
2
O
2 equiv. H
2
O
2
(aq 30%)
0.5 mol% 7
Scheme 11.4 Manganese porphyrin complex 7 – an effective catalyst in the epoxidation of alkenes
with H
2
O
2
[57].
Table 11.2 Oxidation of alkenes with Mn
III
-porphyrin complex 7 [57].
Substrate Conversion (%) Epoxide (%) Reaction time (min)
Cyclooctene 100 100 10
Dodec-1-ene 96 92 15
a-Methylstyrene 100 100 7
cis-Stilbene 90 85 (cis)20
trans-Stilbene 0 0 300
trans-4-Octene 75 54 (trans)15
11.4 Epoxidation and cis-Dihydroxylation of Alkenes
j
379
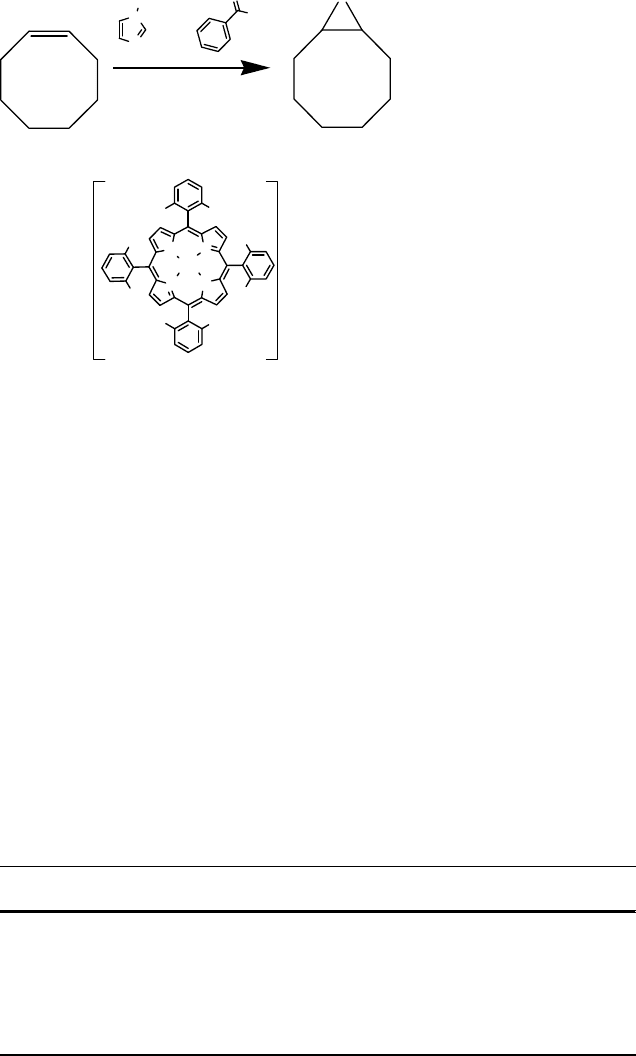
covalently (Scheme 11.6) [63]. When 0.1 mol% of the Mn complex and 2 equiv. of
H
2
O
2
were employed, cyclooctene was converted in only 3 min to the corresponding
epoxide with 100% conversion and selectivity. Analogous results were obtained for
alkenes such as a-methylstyrene, p-chlorostyrene, a-pinene, and camphene, with
turnover numbers of up to 1000. The proposed mechanism is similar to oxidation
reactions with porphyrin-based catalysts in the presence of the imidazole and
carboxylic acid co-catalysts, with a prominent role played by the pendent carboxylic
acid group in heterolysis of the hydroperoxide (Scheme 11.6) [63].
Following the first report by Groves and Myers [72] on asymmetric oxidation using
a chiral metalloporphyrin, a wide range of porphyrin ligands linked to chiral
functional groups have been reported [64]. Although high enantioselectivities were
observed with iodosylbenzene as terminal oxidant, with H
2
O
2
only moderate en-
antioselectivity has been achieved to date [65]. For a discussion of the design of chiral
ligands and the stereochemical issues involved, the reader is referred to detailed
reviews on the topic [64, 66].
The immobilization of homogeneous Mn-porphyrin epoxidation catalysts on
silica to achieve facile catalyst recovery has been realized through anchoring of
the porphyrin ligand A [67] or the axial imidazole ligand B (Figure 11.3) [68]. The
advantages of the supported catalysts are to some extent lost, however, because of
reduced epoxidation activity compared to the analogous homogeneous system.
Mn
III
L
Mn
V
L
O
a
b
or
O
Mn
V
L
O
Mn
V
L
O
+
Scheme 11.5 The proposed (a) concerted pathway, (b) stepwise pathway in the epoxidation of
alkenes by manganese-porphyrin complexes.
380
j
11 Manganese-Catalyzed Oxidation with Hydrogen Peroxide
Recently, Hulsken et al. have employed STM to probe the mechanism by which
manganese porphyrin complexes achieve epoxidation of alkenes with dioxygen [69].
Importantly, this study demonstrates the positive role of solid-support immobiliza-
tion in precluding the formation of inactive oxido-bridged catalyst dimers (a common
deactivation pathway).
11.4.3
Salen-Based Systems
Following the seminal report by Kochi and coworkers on the use of Mn-salen
complexes as epoxidation catalysts [70], considerable attention has been directed
N
N
N
N
Cl
Cl
Cl Cl
Cl
Cl
YX
Mn
III
Cl
-
8
N
N
(CH
2
)
6
O-
X =
Y =
-O(CH
2
)
5
-CO
2
H
Im = imidazole
S
SO
8
Mn
III
Im
H
+
H
2
O
2
-H
3
O
+
9
10
11
O
O
(H
2
C)
6
(CH
2
)
5
Mn
III
Im
O
O
(H
2
C)
6
(CH
2
)
5
HO
O
H
2
O
+
O
Mn
III
Im
O
O
(H
2
C)
6
(CH
2
)
5
O
O
O
Mn
V
Im
O
O
(H
2
C)
6
(CH
2
)
5
HO
O
O
O
HO
Scheme 11.6 Mn-porphyrin complex 6 with tethered carboxylate and imidazole groups and their
proposed role in catalyzed oxidation [63].
11.4 Epoxidation and cis-Dihydroxylation of Alkenes
j
381
toward this family of catalysts, as they offer synthetic advantages over the porphyrin
systems described in the previous section. The breakthroughs made by the groups of
Jacobsen [71a], Katsuki [71b], and coworkers in Mn-catalyzed alkene epoxidation by
the introduction of a chiral diamine functionality in the salen ligand (Figure 11.4)
have paved the way for effective enantioselective epoxidation catalysts.
Compared to chiral manganese porphyrin complexes [72], in general the use of the
Mn-salen catalysts can provide enantioselectivities greater than 90% and yields
exceeding 80% [47, 73]. A wide range of oxidants, including hypochlorite [73],
iodosylbenzene [73], or m-chloroperbenzoic acid (m-CPBA), can be applied [74].
Excellent enantioselectivities are observed in the epoxidation of cis-disubstituted
alkenes and trisubstituted alkenes catalyzed by the Mn-salen complexes 13 and 14,
Mn
Cl
NN
O
HH
O
NN
OO
R
R
Ph H
HPh
Mn
OAc
NN
OO
Ph
Ph
COO
-
Mn
13 1514
Figure 11.4 Chiral manganese complexes introduced by Jacobsen (13) and Katsuki (14, 15) for
asymmetric epoxidation of nonfunctionalized alkenes [71].
Cl
ClH
3
COCHN
(A)
Mn
N
Mn
Cl
Cl
3
HN
Si
O
O
O
Mn
=Porphyri
n
(B)
12
7
N (CH
2
)
3
Si
O
O
O
O
Figure 11.3 Immobilized Mn-porphyrin epoxidation catalysts.
382
j
11 Manganese-Catalyzed Oxidation with Hydrogen Peroxide
