Baeckvall J.-E. (ed.) Modern Oxidation Methods
Подождите немного. Документ загружается.

10
Oxidation of Carbonyl Compounds
Eric V. Johnston and Jan-E. B
€
ackvall
10.1
Introduction
Oxidation of carbonyl compounds is an important area of oxidation and involves a
variety of different reaction types. This area has been reviewed by Bolm in Chapter 9
of the book Modern Oxidation Methods (2004), and the present chapter is an update
of this review. We have focused on two areas of carbonyl oxidation where important
developments have occurred: (i) oxidation of aldehydes to carboxylic acids and (ii)
Baeyer-Villiger oxidation.
10.2
Oxidation of Aldehydes to Carboxylic Acids
The transformation of aldehydes to carboxylic acids is a fundamental reaction in
organic synthesis. Many successful methods have been developed for these types of
oxidations [1], but most of them have limitations as they require stoichiometric
amounts of oxidants such as chlorite [2], chromium(VI) reagents [3], potassium
permanganate [4], or peroxides [5]. The use of organic solvents e.g., acetonitrile,
dichloromethane, cyclohexane, formic acid, or benzene is also usually necessary.
Despite the fact that these methods have several disadvantages, such as low selectivity
and production of waste, some of them have been widely used in industry and are still
in use today. The growing awareness of the environment has created a demand for
efficient oxidation processes with environmentally friendly oxidants under mild
conditions (green chemistry) [6].
Molecular oxygen and hydrogen peroxide are desirable oxidants for these trans-
formations, since they are inexpensive and environmentally friendly, with water as
the only by-product. Therefore, various improved methodologies using molecular
oxygen or hydrogen peroxide directly as the oxidants have been explored in recent
years and reported in the literature.
j
353

10.2.1
Metal-Free Oxidation of Aldehydes to Carboxylic Acids
A metal-free general procedure for aerobic oxidation of a variety of aldehydes that
takes place on water was reported by Shapiro and Vigalok [7]. Its use in the
consecutive three-component Passerini reaction both on water and in water was
described. It was found that hydrophobic aliphatic aldehydes (branched and linear),
as well as aromatic aldehydes, undergo facile oxidation upon simply stirring their
aqueous emulsions in air, affording the corresponding carboxylic acids in high yields
(Eq. (10.2)) [7, 8]. The starting materials and products are insoluble in water and can
easily be isolated. The reactions were found to proceed smoothly both on a small scale
(1 mmol) and on a relatively large laboratory scale (50 mmol).
R H
O
R OH
O
AirorO
2
H
2
O
ð10:1Þ
Aromatic and aliphatic aldehydes have been successfully oxidized to their corre-
sponding carboxylic acids by the use of a H
2
O
2
/HCl system in the presence of
hydroxylamine hydrochloride (Eq. (10.2)) [9]. The method is selective and tolerates
the presence of other functional groups such as, carbon-carbon double bonds,
hydroxyl groups, and other heteroatoms.
R CHO
R COOH
H
2
O
2
/HCl/NH
2
OH·HCl
CH
3
CN, reflux
ð10:2Þ
Recently, N-heterocyclic carbene-catalyzed (NHC-catalyzed) oxidative transforma-
tions have gained increasing attention, and this area was reviewed in 2007 [10].
Miyashita [11] has demonstrated that NHCs derived from triazolium and benzimi-
dazolium salts are capable of catalyzing the oxidation of aryl aldehydes, employing
oxidants such as Oxone [12], pyridinium hydrobromide perbromide [13], or per-
oxides [14]. However, these early investigations have mainly focused on ionic
processes [15]. Recently, biomimetic transition metal-free organocatalytic oxidation
methods were developed [16]. One of these methods employs the 2,2,6,6-
tetramethylpiperidine N-oxyl radical (TEMPO) as the oxidant, generating a TEMPO
ester (Eq. (10.3)), which can be hydrolyzed to give the acid. The synthetic potential of
these reactions is far from being fully realized [17], although this type of aldehyde
activation opens up a new field of organocatalysis [18].
R H
O
N
O
R O
O
N
TEMPO
2
TEMPOH
1) NHC
2) O
2
ð10:3Þ
354
j
10 Oxidation of Carbonyl Compounds

10.2.2
Metal-Catalyzed Oxidation of Aldehydes to Carboxylic Acids
Copper complexes as catalysts have previously been applied in the oxidation
of carbonyl compounds to carboxylic acids [19]. Recently, a CuCl-catalyzed oxidation
of aldehydes to the corresponding carboxylic acids by aqueous tert-butyl hydroper-
oxide in acetonitrile at room temperature was reported by Mannam and Sekar
(Eq. (10.4)) [20]. This new procedure proved to be simple and mild, and works
exceptionally well without any additives. Aromatic, vinylic, and aliphatic aldehydes
were oxidized to the corresponding acids with short reaction times in excellent yields.
Aromatic dialdehydes such as phthaldehyde and terephthaldehyde were also oxi-
dized to their corresponding diacids at room temperature in high yields. Interest-
ingly, aliphatic aldehydes such as cyclohexanecarboxaldehyde, palmitaldehyde and
the aliphatic dialdehyde glutaraldehyde could also be transformed into the corre-
sponding carboxylic acids and diacids, respectively.
R H
O
R OH
O
CuCl(5 mol %)
aq. 70% tBuOOH (1 equiv.)
Me CN, rt
ð10:4Þ
An established procedure for the conversion of an aldehyde to a carboxylic acid
involves the use of Ag
2
O in the presence of NaOH [21, 22]. This has inspired
researchers to employ different forms of Ag(I) compounds as catalysts in combina-
tion with an oxidant. Recently, a mild and selective method for the transformation of
aldehydes into carboxylic acids with a silver catalyst was reported [23]. Thus, in the
presence of 10 mol% AgNO
3
and 5 equiv. of 30% aq. H
2
O
2
in acetonitrile at 50
C,
various aromatic, conjugated, and aliphatic aldehydes were readily oxidized to the
corresponding carboxylic acids in good yields.
The use of 150 nm-size Ag
2
O/CuO and CuO catalysts was found to catalyze the
oxidation of aromatic and aliphatic aldehydes to their corresponding acids by
molecular oxygen in good yields [24]. This method has been used in the industrial
production of furoic acid, although the obvious choice in this case would be CuO
because of easy collection and regeneration of the catalyst.
Over the past decade, the utilization of gold catalysis has emerged as an important
field in oxidation [25]. Gold supported on CeO
2
and TiO
2
nanoparticles has given
promising results with high activity and selectivity in the oxidation of a variety of
aldehydes to carboxylic acids [26, 27].
Oxidation of organic substrates by nickel oxide has been known for more than a
century. The substrate scope of the nickel oxide hydroxide oxidation method is quite
broad, and includes alcohols, aldehydes, phenols, amines, and oximes. However,
an extensive review covering the major part of these reactions demonstrates the
requirement of stoichiometric amounts of nickel oxide hydroxide [28]. Recent
findings have led to a new oxidation method utilizing catalytic amounts of nickel(II)
salts and excess of bleach (5% aqueous sodium hypochlorite) under ambient
10.2 Oxidation of Aldehydes to Carboxylic Acids
j
355
conditions [29]. Notably, the oxidation of aldehydes to the corresponding carboxylic
acids also proceeds without the use of an organic solvent, giving estimated products
in high yields (70–95%) and with high purities (90–100%).
10.3
Oxidation of Ketones
Although there are a number of different reaction types for the oxidation of ketones
(see Chapter 9 of Modern Oxidation Methods by Bolm), the present update chapter
will only deal with the Baeyer-Villiger reaction.
10.3.1
Baeyer-Villiger Reactions
Ever since the discovery by Adolf Baeyer and Victor Villiger [30] that ketones can be
oxidatively converted into the corresponding esters or lactones, substantial progress
has been made in understanding the mechanism and predicting the migratory
preference [31, 32]. However, the classical approach to the Baeyer-Villiger reaction
has a major drawback: a potent oxidant, which in most cases is hazardous, is needed
in stoichiometric amounts. In addition to the fact that these reactive oxidants have to
be handled with care, they are relatively expensive. This has led to an increased
interest in developing more gentle catalytic systems that are enantioselective and
environmentally benign green (chemistry).
10.3.2
Catalytic Asymmetric Baeyer-Villiger Reactions
In the late 20th century, chemists witnessed remarkable advancements in catalytic
asymmetric synthesis. Significant efforts were devoted to the development of
efficient oxidation catalysts, and a range of enantioselective metal-catalyzed reactions
were developed [33]. Despite the long history of the Baeyer-Villiger (BV) oxidation, the
enantioselective version of the process was not studied until recently.
After the pioneering work by the groups of Bolm [34] and Strukul [35] in 1994, a
number of chiral metal complexes and organic molecules were developed as catalysts
for the BV reaction of various ketones [36]. Although the use of aqueous hydrogen
peroxide as an environmentally benign oxidant has been a long-sought goal for the
BV oxidation, there are only a few cases in which catalytic reactions can be carried out
with this oxidant [34, 37]. Impressive results have been achieved for the catalytic
enantioselective BV reaction in the work reported by the groups of Bolm [34, 36],
Katsuki [38], and Murahashi [37b]. However, the development of asymmetric BV
reactions has been rather slow when compared to the rapid development of other
catalytic asymmetric transformations [39], and significant efforts are required in this
area to meet the strong demand for the development of more efficient catalytic
methods where environmentally friendly oxidants are employed.
356
j
10 Oxidation of Carbonyl Compounds
10.3.2.1 Chemocatalytic Versions
At present, high conversions together with high enantioselectivity can be achieved
either with chiral enantiopure organocatalysts [37b,40] or with chiral metal complexes.
The BV transformations with chiral metal complexes are based on either transition
metals such as Cu [37b, 40, 41], Pt [42, 43], Co [44], Pd [45], Zr [46], Hf [47] or
nontransition metals such as Mg [48] and Al [49]. For example, Ko
covsk
y and
coworkers have developed a series of new terpene-derived pyridinephosphine
ligands, whose complexes with Pd(II) have been proven to catalyze the BV oxidation.
Prochiral cyclobutanones (1) were oxidized at low temperature (40
C), with
5 mol % catalyst loading and the urea-H
2
O
2
complex as the stoichiometric oxidant
to give lactones 2 in good yields and up to 81% ee (Eq. (10.5)) [50].
O
R
O
O
R
(NH
2
)
2
CO-H
2
O
2
(1.3 equiv.)
(PhCN)
2
PdCl
2
(5 mol%)
L* (5.5 mol%)
AgSbF
6
(10 mol%)
THF, -40 ºC, 15 h
2
(
81% ee
)
N
Ph
2
P
L*
(5)
1
ð10:5Þ
All these complexes are active towards meso or chiral cyclobutanone substrates 1,
which are significantly more reactive than the larger cyclic ketones. Oxidation of the
latter were only successful with Pt(II) [42, 46, 51, 52] or Cu(II) [53] catalysts, resulting
in moderate to good enantiomeric excess.
Recently, the first example of an environmentally benign enantioselective BV
oxidation, utilizing a strong chiral Brønsted acid as catalyst was reported [42]. With a
chiral phosphoric acid and 30% aqueous H
2
O
2
as the oxidant, various of 3-substituted
cyclobutanones were converted into their corresponding c -lactones in excellent yields
and enantioselectivities up to 93% ee.
Among the synthetic catalysts used for cyclohexanones, the organocatalyst devel-
oped by Peris and Miller is noteworthy [54]. Recently, an efficient system for the
oxidation of 4-substituted cyclohexanones (3) to lactones (4) was reported by Strukul
and coworkers [55], in which they use a chiral enantiopure Pt(II) catalyst in water with
added surfactant and hydrogen peroxide as the terminal oxidant (up to 92% ee),
(Eq. (10.6)). The surfactant enables solubilization of the otherwise insoluble chiral
catalyst and enhances the enantioselectivity of the reaction because of tighter
catalyst–substrate interactions favored by the micellar supramolecular aggregate.
O
O O
O
O
H
2
O
2
,Cat*
H
2
O, Surfactant,RT
P
P
Ar
Ar
Ar
Ar
Pt
OH
2
OH
2
2+
2CF
3
SO
3
-
Cat*
3
4a
4b
ð10:6Þ
10.3 Oxidation of Ketones
j
357
10.3.2.2 Biocatalytic Versions
Many of todays target molecules in oxidation are asymmetric and require enantio-
selective reactions for their preparation. An obvious approach is to use a biocatalyst
together with molecular oxygen as the oxidant. The rapid development of molecular
engineering during the past decade has enabled biocatalysis to become a well-
established field of research for chemists, and has provided access to a wide range of
interesting bioreagents.
The first indication of the existence of so-called Baeyer-Villiger monooxygenases
(BVMOs) was reported in the late 1940s [56]. It was observed that certain fungi were
able to oxidize steroids via a BV reaction [56], but two decades elapsed before the first
BVMOs were isolated and characterized [57, 58]. All characterized BVMOs contain a
flavin cofactor that is vital for the catalytic activity of the enzyme, Furthermore,
NADH or NADPH cofactors are needed as electron donors. Careful inspection of all
available biochemical data on BVMOs has revealed that at least two discrete classes of
BVMOs exist, types I and II [59].
Type I and Type II BVMOs Type I BVMOs consist of only one polypeptide of about
500 amino acids with the cofactor flavin adenine dinucleotide (FAD) tightly bound
into the structure, and is dependent on NADPH for activity [60]. Most of the presently
known BVMOs are of this type, and are typically soluble proteins located in the cytosol
of bacteria or fungi, which is in contrast to many other monooxygenase systems that
often are found to be membrane bound or membrane associated. They contain two
Rossmann sequence motifs, GxGxxG, which indicate that these enzymes bind the
two cofactors (FAD or NADPH) using separate dinucleotide binding domains [61].
Type II BVMOs on the other hand are typically composed of two different subunits,
and use flavin mononucleotide (FMN) as flavin cofactor and NADH as electron
donor. At the time of the preliminary classification, the respective N-terminus
sequences did not provide any clue concerning the structure of these two-component
monooxygenases. However, sequence data suggest a relationship with the flavin-
dependent luciferases [62]. In contrast to the Type I BVMOs, for which the genes have
been frequently reported, Type II BVMOs have only been explored to a limited extent,
and so far no cloning of Type II genes has been described in the literature. In fact
only one Type II BVMO sequence (limonene monooxygenase, gi47116765) has been
deposited in the database.
Other BVMOs Recent findings of several enzymes that display Baeyer-Villiger
activity, although they do not resemble the above-mentioned BVMOs, suggest that
at least two other BVMO classes exist [63].
A flavoprotein monooxygenase (MtmOIV) involved in the biosynthesis of mithra-
mycin, and a heme-containing BVMO belonging to the cytochrome P450 super-
family have recently been reported [64]. Both enzymes were shown to catalyze BV
oxidations [65]. In addition, a plant enzyme turned out to be capable of modifying
steroids via BV oxidation [66]. Further studies will reveal more mechanistic details
concerning these newly identified BVMOs that may be useful for the development of
new biocatalytic applications in the future.
358
j
10 Oxidation of Carbonyl Compounds
Cyclohexanone Monooxgenase (CHMO
Acineto
) Most biochemical and biocatalytic
studies have been performed on Type I BVMOs [67], since they represent relatively
uncomplicated monooxygenase systems, and several expression systems have been
developed for a number of Type I BVMOs.
The isolation and characterization of cyclohexanone monooxygenase (CHMO)
from Acinetobacter sp. NCIB 9871 was reported in 1976 [68]. In addition to
cyclohexanone and cyclopentanone, CHMO was shown to be able to catalyze the
oxidation of a variety of cyclic ketones to the corresponding lactones [69]. This
attracted the attention of several other groups, which led to investigations of its
mechanism [70, 71] as well as sequencing and cloning [72]. It is by far the most
extensively studied BVMO, and it has been used as a model system for up-scaling
BVMO-mediated biocatalysis.
A comprehensive list of ketonic substrates was first published in 1998 and has
grown significantly in recent years [73]. Substrate profiling studies have shown that
BVMOs have wide substrate specificity that often overlap. In 2002 it was reported that
over 100 different substrates could be converted by CHMO [74], including cyclic,
bicyclic, tricyclic, and heterocyclic ketones with a variety of substitution pat-
terns [73, 75]. The oxidizing capacity of CHMO is not only limited to Baeyer-Villiger
reactions, but has also been successfully employed in the enantioselective oxidation
of a wide range of sulfides [76, 77], dithienes, dithiolanes [78], and in the conversion of
tertiary amines, secondary amines, and hydroxylamines to their N-oxides, hydro-
xylamines, and nitrones, respectively [79].
Isolated Enzymes versus Designer Organisms In the early 1990s, biotransformations
were performed with either isolated enzymes or whole-cell native organisms. All type
I BVMOs are strictly dependent on cofactors, which complicates their utilization
in organic synthesis. In particular, the consumption of stoichiometric quantities of
NAD(P)H requires appropriate recycling strategies in order to allow for a cost-
effictive process on an industrial scale [80].
To overcome this obstacle, two different approaches have been exploited, both
possessing benefits and disadvantages. For the isolated enzyme, a closed-loop
system has been developed, where an auxiliary substrate has been added to
regenerate NAD(P)H. The second approach is based on whole-cell-mediated
biotransformations.
A comparative study of the isolated CHMO enzyme with that overexpressed in
E. coli has shown that they are practically identical. The slight difference in pH and
thermal stability was due to the differences in trace impurities with proteolytic
enzymes present in the E. coli host [81]. At present, the main focus has been on using
intact cells expressing CHMO as a biocatalyst. Intact cells of designer bioreagents
are more accessible and more chemical friendly than the isolated enzymes used for
biotransformations.
Biocatalytic Properties of Available Recombinant BVMOs The construction of recom-
binant overexpression systems for CHMO in E. coli and their success in regio- and
enantioselective oxidation has inspired the search for other BVMOs. Several new
10.3 Oxidation of Ketones
j
359
BVMOs have been overexpressed in E. coli., and the list of reported Type I BVMOs has
grown significantly during recent years (Table 10.1).
They include several cyclohexanone monooxygenases [83, 84], a steroid mono-
oxygenase (SMO) [85], and cyclodecanone monoxygenase (CDMO). The latter was
the first characterized enzyme to catalyze BVoxidation of large cyclic compounds [86].
In 2001, overexpression systems in E. coli were engineered for 4-hydroxyacetophe-
none monooxygenase (HAPMO) [87] and for cyclopentanone monooxygenase
(CPMO) [88, 89]. Quite recently, BVMOs that readily accept phenylacetone deriva-
tives (PAMO) have been described [90]. Also, variants specific for acetone
(ACMO) [91], linear aliphatic ketones (AKMO) [92], and for the large cyclic ketone
cyclopentadecanone (CPDMO) [93] were reported.
Substrate-profiling studies suggest that BVMOs have a rather broad specificity and
often display overlapping substrate specificities. However, catalytic efficiencies and
regio- and/or enantioselectivities can differ significantly when comparing BVMOs.
Comparative biocatalytic studies using highly similar enzymes have revealed
that all studied CHMOs and CPMOs cover a similar substrate range [94–96],
although it was observed that CPMOs and CHMOs often display opposite
enantioselectivities [96].
Although BVMOs display broad substrate specificity, each type of BVMO has a
certain preference for a specific type of substrate. CHMO and CPMO are highly active
with a range of smaller cyclic aliphatic ketones, whereas HAPMO and PAMO prefer
aromatic substrates [87, 90, 97–99].
Directed Evolution of Enantioselective Enzymes for Catalysis in Baeyer-Villiger Reactions
Reetz and co-workers have demonstrated that the methods of directed evolution can
be applied successfully to the creation of enantioselective cyclohexanone monoox-
ygenases (CHMOs) as catalysts in Baeyer-Villiger reactions of several different
substrates, for which the enantioselectivity ranges between 90–99% [100]. Ketone
5 gives a very poor enantioselectivety (9% ee, R-selective) with the wild-type CHMO.
The enantioselectivety for 5 was significantly improved by directed evolution, and an
S-selective variant gave 79% ee (Scheme 10.1).
Recently, Reetz has devised a new strategy in directed evolution in order to
construct a robust experimental platform for asymmetric Baeyer-Villiger reactions
based on the thermostable phenylacetone monooxygenase (PAMO) [101]. Unfortu-
nately, the substrate scope of the wild-type (WT) PAMO is very limited, only accepting
phenylacetone and structurally similar linear phenyl-substituted ketones. By exploit-
ing bioinformatics data derived from sequence alignment of eight different BVMOs,
in conjunction with the known X-ray structure of PAMO, this problem could be
circumvented. Their goal was to expand the substrate scope, to increase the reaction
rate, and to reach high enantioselectivity without compromising thermostability.
Mutants were evolved which showed unusually high activity and enantioselectivity in
the oxidative kinetic resolution of a variety of structurally different 2-substituted aryl-
and alkylcyclohexanone derivatives and of a structurally unrelated bicyclic ketones.
It is interesting to note that WT PAMO favors the formation of 8 as the (1S,5R )-
enantiomer and also produces some 9 as the (1S,5R)-enantiomer, whereas the
360
j
10 Oxidation of Carbonyl Compounds
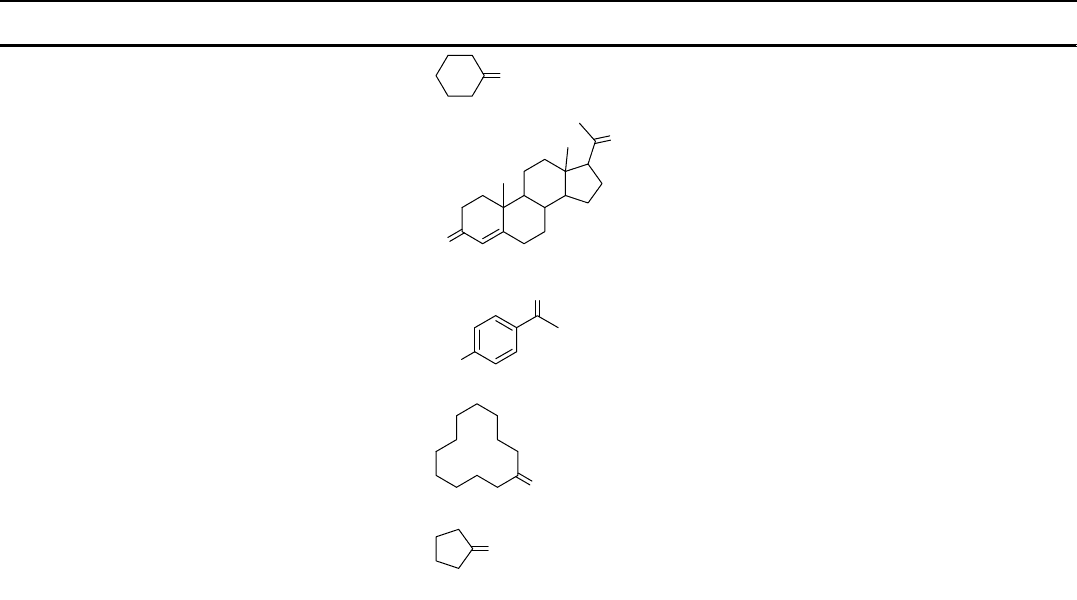
Table 10.1 List of BVMOs that have been overexpressed in E. coli. [82].
BVMO Abbreviation Primary substrate Origin Year of cloning
Cyclohexanone
monooxygenase
CHMO
O
Acinetobacter sp. NCIMB
9871
1988
Steroid monooxygenase STMO
O
O
Rhodococcus rhodochrous 1999
4-Hydroxyacetophenone
monooxygenase
HAPMO
HO
O
Pseudomonas fluorescens
ACB
2001
Cyclododecanone
monooxygenase
CDMO
O
Rhodococcus ruber 2001
Cyclopentanone
monooxygenase
CPMO
O
Comamonas sp. NCIMB
9872
2002
(Continued)
10.3 Oxidation of Ketones
j
361
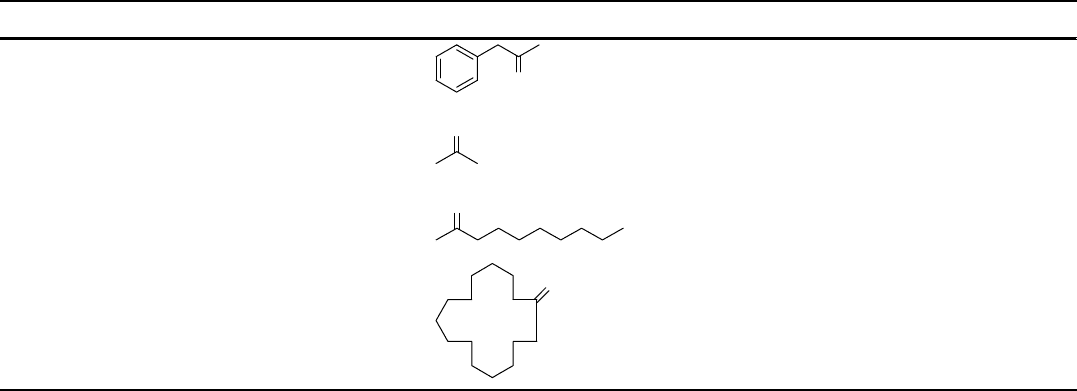
Table 10.1 (Continued)
BVMO Abbreviation Primary substrate Origin Year of cloning
Phenylacetone
monooxygenase
PAMO
O
Thermobifida fusca 2005
Acetone monooxygenase ACMO
O
Gordonia sp. TY-5 2006
Alkyl ketone
monooxygenase
AKMO
O
Pseudomonas fluorescens 2006
Cyclopentadecanone
monooxygenase
CPDMO
O
Pseudomonas sp. strain
HI-70
2006
362
j
10 Oxidation of Carbonyl Compounds
