ASM Metals HandBook Vol. 8 - Mechanical Testing and Evaluation
Подождите немного. Документ загружается.


3. “Calibration and Testing Laboratory Accreditation Systems—General Requirements for Operation and
Recognition,” ISO/IEC Guide 58, 1993
4. “Guidelines for Auditing Quality Systems, Part 1: Auditing; Part 2: Qualification Criteria for Auditors,”
ISO 10011, 1992
Accreditation of Mechanical Testing Laboratories
Roxanne M. Robinson, American Association for Laboratory Accreditation
Accreditation versus ISO 9000 Certification
The purposes of laboratory accreditation as stated in ISO/IEC Guide 25 (Ref 2) and quality system
certifications such as ISO 9002 (Ref 5) are different, and, thus, examination against them gives different levels
of assurance. The ISO 9000 series of standards provides a generic system for quality management of an
organization, irrespective of the product or service it provides. In contrast, the ISO/IEC Guide 25 is a document
developed specifically to provide minimum requirements to laboratories on both quality management in a
laboratory environment and technical requirements for the proper operation of a laboratory. To the extent that
both documents address quality management, Guide 25 can be considered as a complementary document to
ISO 9002, which is written in terms most understandable by laboratory managers.
Some have expressed the view that the application of ISO 9002 alone is sufficient to ensure the effective
operation of a laboratory and the validity of test data. Several significant differences, however, exist between
laboratory accreditation using ISO/IEC Guide 25 and quality system certifications such as ISO 9002 (Ref 5).
One key difference is that ISO/IEC Guide 25 is intended to ensure the validity of test data, while technical
credibility is not addressed in quality management requirements of standards such as ISO 9002. From the point
of view of the user of test data, quality management systems (ISO 9000) are deficient in that they do not
necessarily provide any assessment of the technical competence of personnel engaged in what can only be
described as a very technical activity, nor do quality management systems necessarily address the specific
requirements of particular products or measurements. The better method of achieving these two objectives is
through laboratory accreditation bodies operating according to an international practice, where laboratories
adopt best practices by working with assessors who are expert in the specific tests in which the customer is
interested.
It is also important to examine the differences in skill and emphasis of assessors involved in quality system
certification and laboratory accreditation assessments. For quality system certification, emphasis is traditionally
placed on the qualifications of the assessor to perform assessment against the systems standard. The systems
assessor (often referred to as the lead assessor) is expected to have a thorough knowledge of the requirements of
the standard. In current practice internationally, a quality system assessment team may or may not include
personnel who have specific technical backgrounds or process familiarity relevant to the organizations being
assessed.
For laboratory accreditation, the assessment team always involves a combination of personnel who have expert
technical knowledge of the test or measurement methodology being evaluated for recognition in a specific
laboratory, together with personnel who have specific knowledge of the policies and practices of the
accreditation body and the general systems applicable to all accredited laboratories. The laboratory
accreditation assessment, thus, includes a technical peer review component plus a systems compliance
component.
Unlike quality system assessment, laboratory accreditation involves appraisal of the competence of personnel as
well as systems. Part of the evaluation of a laboratory includes evaluation of supervisory personnel, in many
cases leading to recognition of specific individuals as part of the laboratory accreditation. The technical
competence and performance of laboratory operators may also be witnessed as part of the assessment process.
The loss of key personnel may affect the continuing accreditation of the laboratory by the accrediting body; for

example, loss of key staff whose absence reduces the technical competence of the laboratory may prompt a
reassessment before it would be normally scheduled.
Quality system certification, on the other hand, is not normally linked to nominated key personnel. The
technical competence of managers and process operators is not a defined activity for quality system assessment
teams. It is through the documented policies, job descriptions, procedures, work instructions, and training
requirements of organizations and through objective evidence of their implementation that quality system
certifiers appraise the personnel component of a system. Staff turnover is not an issue in maintaining
certification.
The final product of a laboratory is test data. In many cases, laboratory accreditation assessments also include
some practical testing of the laboratory through various forms of proficiency testing (interlaboratory
comparisons or reference materials testing). This is another way that accreditation differs from quality system
certification.
References cited in this section
2. “General Requirements for the Competence of Calibration and Testing Laboratories,” ISO/IEC Guide
25, 1990
5. ISO 9002, Quality Systems—Model for Quality Assurance in Production and Installation, 1994
Accreditation of Mechanical Testing Laboratories
Roxanne M. Robinson, American Association for Laboratory Accreditation
Requirements of ISO/IEC Guide 25 (1990)
For international acceptance, accredited laboratories are required to comply with ISO/IEC Guide 25 (Ref 2). In
this guide, attention is paid to the activities of both calibration and testing laboratories, and account is taken of
other requirements for laboratory competence such as those of the Organization of Economic Cooperation and
Development (OECD) Code of Good Laboratory Practice (GLP) (Ref 6) and the ISO 9000 series of quality
assurance standards. Additional program requirements (specific criteria) for specific fields of testing (e.g.,
mechanical) or product requirements (e.g., fastener quality) (Ref 7) may also complement general requirements
in particular areas.
ISO Guide 25 is recognized on an international level as the appropriate standard for determining the
competency of a laboratory to perform specific tests or types of tests, or calibrations. Guide 25 is a balanced
standard that addresses quality system requirements of ISO 9000 and the technical requirements needed to
perform testing or calibration. The following criteria are included in ISO Guide 25:
• Organization and management
• Quality system, audit, and review
• Personnel
• Accommodation and environment
• Equipment and reference materials
• Measurement traceability and calibration
• Calibration and test methods
• Handling calibration and test items
• Records
• Certificates and reports
• Subcontracting calibration or testing
• Outside support and services
• Complaints
The mechanical testing accreditation program offered by accrediting bodies may differ in the breadth of testing
each program covers. By the same token, a mechanical testing laboratory can request to be accredited for only a
portion of, or the entire, testing capability of the laboratory. The scope of accreditation is based on the
information provided by the laboratory to describe in detail the kinds of products tested, the test technologies
used, and the specific test methods performed.
References cited in this section
2. “General Requirements for the Competence of Calibration and Testing Laboratories,” ISO/IEC Guide
25, 1990
6. “Good Laboratory Practice Principles,” Organization for Economic Cooperation and Development
(OECD), 1998
7. Fastener Quality Act, H. R. 3000 or PL 101–592, Federal Register, 26 Sept 1996
Accreditation of Mechanical Testing Laboratories
Roxanne M. Robinson, American Association for Laboratory Accreditation
Accreditation Process
Application. A laboratory applies for accreditation by obtaining the application package from the accrediting
body headquarters and completing appropriate application sheets. All applicants may have to agree to the set of
conditions for accreditation, pay the appropriate fees, and provide detailed supporting information on the
following:
• Scope of testing in terms of field(s) of testing, testing technologies, test methods, and relevant standards
(Table 1)
• Organization structure
• Proficiency testing
Table 1 Examples of items that can be specified in the scope of accreditation for a mechanical testing
laboratory
Category
Examples
Products tested
Abrasives; adhesives and sealants; aircraft and automotive components; ceramics;
coatings; fasteners; films; packaging; furniture; gaskets; glass and glass products;
gypsum and gypsum products; leather; metals and alloys; packaging and containers;
paper, paperboard, and pulp; plastic and polymers; pipes, hoses, valves, and fittings;
pressure vessels; rubber and rubber products; safety tests on motor vehicles, toys,
helmets; textiles; tools; windows and doors; and wood and wood products
Mechanical testing, including tensile, compression, hardness, shear, torsion, ductility,
stress rupture; fracture (Charpy, Izod, etc.) and fatigue testing
Metallography, including preparation, microstructure, inclusion content, grain size,
hydrogen embrittlement, macroetching and microetching, depth of decarburization,
and case depth
Testing
technologies used
Environmental simulation, including acceleration, altitude, durability, explosion,
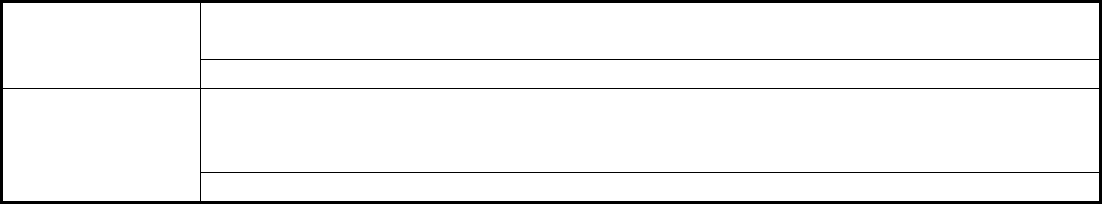
fungus, high/low temperature, high pressure, humidity, radioactivity, salt spray, sea
water immersion, shock, and sun exposure simulation
Dimensional inspection
(a)
Consensus methods such as those issued by standards organizations such as ASTM,
SAE
(b)
, and ISO
(c)
, or by companies such as General Motors Corp. and Ford Motor
Co.
(d)
Applicable
standards or test
methods
In-house test methods
(e)
(a) Additional information concerning the range of measurement for each parameter and best measurement
capability may have to be determined and included in the scope of accreditation.
(b) SAE, Society of Automotive Engineers.
(c) ISO, International Organization for Standardization.
(d) Usually, the laboratory must identify standard methods by designation and short title (e.g., ASTM B 117
Salt Spray).
(e) Accredited in-house methods are nonproprietary and must be made available to public persons with a valid
reason to make such a request.
Applicants may also need to provide their quality manual or quality manual references, which address the
documentation requirements of ISO/IEC Guide 25 and provide a matrix of the technical training of their
laboratory personnel.
On-Site Assessment. Once the application information is completed and the appropriate fees are paid,
headquarters staff identifies and tentatively assigns one or more assessors to conduct an on-site assessment. The
laboratory has the right to ask for another assessor if it objects to the original assignment. Assessments may last
from one to several days.
Assessors are given an assessor guide and checklists to follow in performing an assessment. These documents
are intended to ensure that assessments are conducted as uniformly and completely as possible among the
assessors and from laboratory to laboratory.
Before the assessment is conducted, the assessor team requests copies of the quality manual and related
documentation (i.e., standard operating procedures related to Guide 25 requirements) in order to prepare for the
assessment. The quality manual and related documentation must be reviewed by the assessor team before the
on-site assessment can begin. Ideally, this review is done before the assessment is scheduled. Upon review of
submitted documentation, the assessor(s) may ask the laboratory to implement corrective action to fill any
documentation gaps required by Guide 25 before scheduling the assessment. A preassessment visit may be
requested by the laboratory as an option at this point to enhance the success of the full assessment. Prior to
scheduling the full assessment, the assessor reviews the scope of the draft to determine the tests to possibly
witness and checks on the availability of the technical personnel who perform the tests. The assessor provides
an assessment agenda.
The full assessment generally involves the following activities:
• An entry briefing with laboratory management
• Audit of the quality system to verify that it is fully operational and that it conforms to all sections of
ISO/IEC Guide 25, including documentation
• Interviews with technical staff
• Demonstration of selected tests including, as applicable, tests done at representative field locations
• Examination of equipment and calibration records
• A written report of assessor findings
• An exit briefing including the specific written identification of any deficiencies
The objective of an assessment is to establish whether or not a laboratory complies with the requirements for
accreditation and can competently perform the types of mechanical tests for which accreditation is sought.
However, when accreditation is required to demonstrate compliance with additional criteria that may be
imposed by other authorities, such as in the case of the Fastener Quality Act, the assessment will include such
additional criteria. Assessors may also provide advice, based on observations or in response to questions, in
order to help the laboratory improve its performance.
Deficiencies. During the assessment, assessors may observe deficiencies. A deficiency is any nonconformity to
accreditation requirements including the following:
• The inability of a laboratory to perform a test or type of test for which the laboratory seeks
accreditation.
• The nonconformance of a laboratory quality system to a clause or section of ISO/IEC Guide 25,
inadequate documentation of a quality system, or a quality system that is not completely operational
• The nonconformance of a laboratory to any additional requirements of the accrediting body or specific
fields of testing or programs necessary to meet particular needs
At the conclusion of an assessment, the assessor prepares a report of findings identifying deficiencies that, in
the assessor's judgment, the laboratory must resolve in order to be accredited. The assessor holds an exit
briefing with top management of the laboratory. The assessor goes over the findings and presents the list of
deficiencies (deficiency report). The authorized representative of the laboratory (or designee) is asked to sign
the deficiency report to attest that the deficiency report has been reviewed with the assessor. The signature does
not imply that the laboratory representative concurs that the individual item(s) constitute a deficiency. The
laboratory is requested to respond promptly after the date of the exit briefing, detailing either its corrective
action or why it does not believe that a deficiency exists. The corrective action response should include a copy
of any objective evidence (e.g., calibration certificates, lab procedures, paid invoices, packaging slips, and
training records) to indicate that the corrective actions have been implemented/completed.
It is entirely possible that the laboratory will disagree with the findings that one or more items are deficiencies.
In that case, the laboratory is requested to explain in its response why it disagrees with the assessor.
If the laboratory fails to respond in the agreed time frame, it may be treated as a new applicant subject to new
fees and reassessment should it wish to pursue accreditation after that time.
Proficiency testing is a process for checking actual laboratory testing performance, usually by means of
interlaboratory test data comparisons. For many test methods, results from proficiency testing are very good
indicators of testing competence. Proficiency testing programs may take many forms, and standards for
satisfactory performance can vary depending on the field. An accredited laboratory must participate in method-
specific proficiency testing related to its field(s) of accreditation if such programs are available. There are
commercially available proficiency testing programs that cover a wide array of mechanical testing procedures.
Proficiency testing is available for plastics, rubber, textiles, paper, metals, and fasteners. Where proficiency
testing programs are not available or suitable to the accredited testing, the laboratories often devise their own
round-robin testing with a limited number of similar laboratories. Data from these round-robin studies are
acceptable alternatives to proficiency testing program participation. When neither proficiency testing nor
round-robin testing is available, internal performance-based data can substitute.
Accreditation Decisions. Before an accreditation decision ballot is sent to the person or group making the
accreditation decision, the laboratory staff may review the deficiency response, including objective evidence of
completed corrective action, for adequacy and completeness. If there is any doubt about the adequacy or
completeness of any part of the deficiency response, the response may be submitted to the assessor(s) for
additional review. The laboratory may then be asked to respond further to ensure a successful accreditation
decision. The accreditation body then reviews the assessment record and any corrective action response to
render a decision. Any concerns or negative decisions are relayed back to the laboratory for further response
until the issue is resolved in a satisfactory way for final accreditation of the laboratory.
When accreditation is granted, the laboratory is issued a certificate and scope of accreditation for the
mechanical field of testing and any special testing program. The laboratory should keep its scope of
accreditation available to show clients or potential clients the testing technologies and test methods for which it
is accredited. The scopes of accreditation are also used by the accrediting body to respond to inquiries and to
prepare the directory of accredited laboratories.
Annual Review. Accreditation is generally established for a certain period of time before a reassessment is
required. However, at set intervals between this established accreditation period, each laboratory would likely
pay annual fees and undergo some type of surveillance activity that could include a one-day surveillance visit
by an assessor. This surveillance visit is performed to confirm that the quality system of a laboratory and
technical capabilities remain in compliance with the accreditation requirements. Other possible surveillance
activities may include submission of updating information by the laboratory on its organization, facilities, and
key personnel, and the results of any proficiency testing. Objective evidence of completion of the internal audit
and management review may also be required. If the laboratory does not promptly provide complete annual

review documentation, or significant changes to the facility or organization have occurred, a one-day
surveillance visit and payment of the associated assessor fees may be required.
Reassessment and Renewal of Accreditation. Full on-site reassessments of all accredited laboratories are
conducted at intervals determined by the period of accreditation. Reassessments are also conducted when
evaluations and submissions from the laboratory or its clients indicate significant technical changes in the
capability of the laboratory have occurred.
The accredited laboratory is sent some type of renewal prompt, well in advance of the expiration date of its
accreditation, to allow sufficient time to complete the renewal process. A successful on-site reassessment must
be completed before accreditation is extended.
If deficiencies are noted during the renewal assessment, the laboratory is asked to respond in a timely fashion
with a corrective action. All deficiencies must be resolved before accreditation is renewed.
Accreditation of Mechanical Testing Laboratories
Roxanne M. Robinson, American Association for Laboratory Accreditation
References
1. “General Terms and Their Definitions Concerning Standardization and Related Activities,” ISO/IEC
Guide 2, 1993
2. “General Requirements for the Competence of Calibration and Testing Laboratories,” ISO/IEC Guide
25, 1990
3. “Calibration and Testing Laboratory Accreditation Systems—General Requirements for Operation and
Recognition,” ISO/IEC Guide 58, 1993
4. “Guidelines for Auditing Quality Systems, Part 1: Auditing; Part 2: Qualification Criteria for Auditors,”
ISO 10011, 1992
5. ISO 9002, Quality Systems—Model for Quality Assurance in Production and Installation, 1994
6. “Good Laboratory Practice Principles,” Organization for Economic Cooperation and Development
(OECD), 1998
7. Fastener Quality Act, H. R. 3000 or PL 101–592, Federal Register, 26 Sept 1996

Mechanical Behavior Under Tensile and
Compressive Loads
*
George E. Dieter, University of Maryland
Introduction
THE MECHANICAL BEHAVIOR OF MATERIALS is described by their deformation and fracture
characteristics under applied tensile, compressive, or multiaxial stresses. Determination of this mechanical
behavior is influenced by several factors that include metallurgical/material variables, test methods, and the
nature of the applied stresses.
This article focuses on mechanical behavior under conditions of uniaxial tension and compression. The main
emphasis is on mechanical behavior during the engineering tension test, which is widely used to provide basic
design information on the strength of materials and as an acceptance test for the specification of materials. In
this test procedure, a specimen is subjected to a continually increasing uniaxial load (force), while simultaneous
observations are made of the elongation of the specimen. In this article, emphasis is placed on the interpretation
of these observations rather than on the procedures for conducting the tests. The article “Uniaxial Tensile
Testing” in this Volume discusses the influence of test procedure variables.
Footnote
*
Reprinted in part from Mechanical Metallurgy, 3rd ed., McGraw-Hill, New York, 1986, p 275–
295, with
permission
Mechanical Behavior Under Tensile and Compressive Loads*
George E. Dieter, University of Maryland
Engineering Stress-Strain Curve
In the conventional engineering tension test, an engineering stress-strain curve is constructed from the load-
elongation measurements made on the test specimen (Fig. 1). The engineering stress (s) used in this stress-strain
curve is the average longitudinal stress in the tensile specimen. It is obtained by dividing the load (P) by the
original area of the cross section of the specimen (A
0
):
(Eq 1)
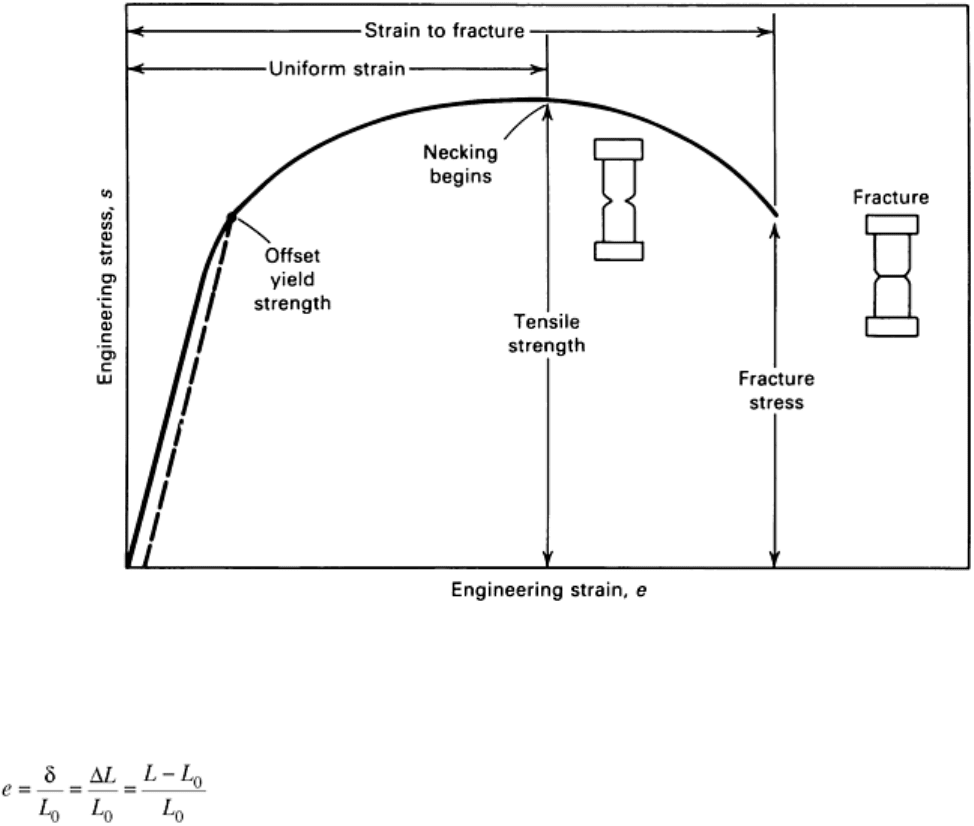
Fig. 1 Engineering stress-strain curve. Intersection of the dashed line with the curve
determines the offset yield strength. See also Fig. 2 and corresponding text.
The strain, e, used for the engineering stress-strain curve is the average linear strain, which is obtained by
dividing the elongation of the gage length of the specimen (δ) by its original length (L
0
):
(Eq 2)
Because both the stress and the strain are obtained by dividing the load and elongation by constant factors, the
load-elongation curve has the same shape as the engineering stress-strain curve. The two curves frequently are
used interchangeably.
The shape and magnitude of the stress-strain curve of a metal depend on its composition, heat treatment, prior
history of plastic deformation, and the strain rate, temperature, and state of stress imposed during the testing.
The parameters that are used to describe the stress-strain curve of a metal are the tensile strength, yield strength
or yield point, percent elongation, and reduction in area. The first two are strength parameters; the last two
indicate ductility.
The general shape of the engineering stress-strain curve (Fig. 1) requires further explanation. In the elastic
region, stress is linearly proportional to strain. When the stress exceeds a value corresponding to the yield
strength, the specimen undergoes gross plastic deformation. If the load is subsequently reduced to zero, the
specimen will remain permanently deformed. The stress required to produce continued plastic deformation
increases with increasing plastic strain; that is, the metal strain hardens. The volume of the specimen (area ×
length) remains constant during plastic deformation, AL = A
0
L
0
, and as the specimen elongates, its cross-
sectional area decreases uniformly along the gage length.
Initially, the strain hardening more than compensates for this decrease in area, and the engineering stress
(proportional to load P) continues to rise with increasing strain. Eventually, a point is reached where the
decrease in specimen cross-sectional area is greater than the increase in deformation load arising from strain
hardening. This condition will be reached first at some point in the specimen that is slightly weaker than the
rest. All further plastic deformation is concentrated in this region, and the specimen begins to neck or thin down
locally. Because the cross-sectional area now is decreasing far more rapidly than the deformation load is
increased by strain hardening, the actual load required to deform the specimen falls off, and the engineering
stress defined in Eq 1 continues to decrease until fracture occurs.
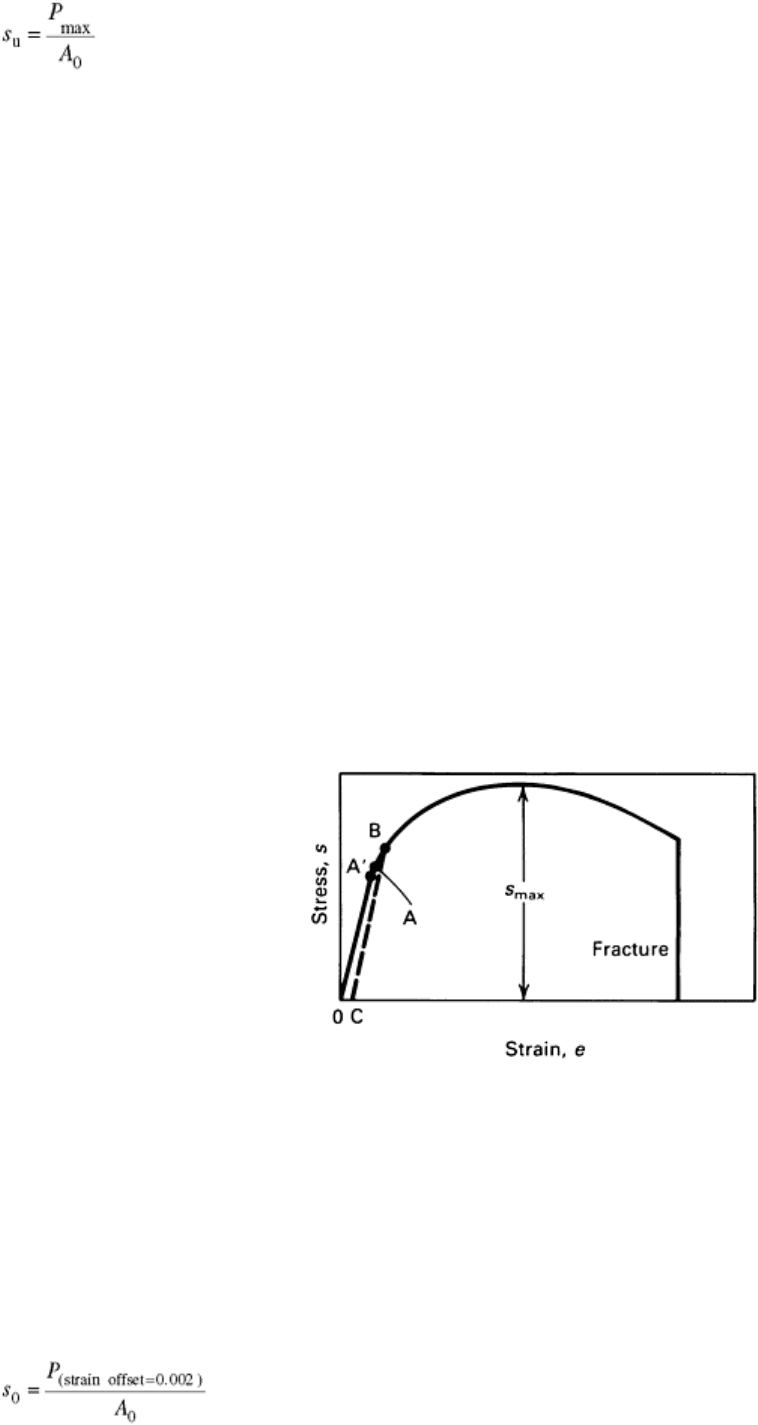
The tensile strength, or ultimate tensile strength (s
u
) is the maximum load divided by the original cross-
sectional area of the specimen:
(Eq 3)
The tensile strength is the value most frequently quoted from the results of a tension test. Actually, however, it
is a value of little fundamental significance with regard to the strength of a metal. For ductile metals, the tensile
strength should be regarded as a measure of the maximum load that a metal can withstand under the very
restrictive conditions of uniaxial loading. This value bears little relation to the useful strength of the metal under
the more complex conditions of stress that usually are encountered.
For many years, it was customary to base the strength of members on the tensile strength, suitably reduced by a
factor of safety. The current trend is to use the more rational approach of basing the static design of ductile
metals on the yield strength. However, due to the long practice of using the tensile strength to describe the
strength of materials, it has become a familiar property, and as such, it is a useful identification of a material in
the same sense that the chemical composition serves to identify a metal or alloy. Furthermore, because the
tensile strength is easy to determine and is a reproducible property, it is useful for the purposes of specification
and for quality control of a product. Extensive empirical correlations between tensile strength and properties
such as hardness and fatigue strength are often useful. For brittle materials, the tensile strength is a valid design
criterion.
Measures of Yielding. The stress at which plastic deformation or yielding is observed to begin depends on the
sensitivity of the strain measurements. With most materials, there is a gradual transition from elastic to plastic
behavior, and the point at which plastic deformation begins is difficult to define with precision. In tests of
materials under uniaxial loading, three criteria for the initiation of yielding have been used: the elastic limit, the
proportional limit, and the yield strength.
Elastic limit, shown at point A in Fig. 2, is the greatest stress the material can withstand without any measurable
permanent strain remaining after the complete release of load. With increasing sensitivity of strain
measurement, the value of the elastic limit is decreased until it equals the true elastic limit determined from
microstrain measurements. With the sensitivity of strain typically used in engineering studies (10
-4
in./in.), the
elastic limit is greater than the proportional limit. Determination of the elastic limit requires a tedious
incremental loading-unloading test procedure. For this reason, it is often replaced by the proportional limit.
Fig. 2 Typical tension stress-strain curve for ductile metal indicating yielding criteria.
Point A, elastic limit; point A′, proportional limits; point B, yield strength or offset (0 to
C) yield strength; 0, intersection of the stress-strain curve with the strain axis
Proportional limit, shown at point A′ in Fig. 2, is the highest stress at which stress is directly proportional to
strain. It is obtained by observing the deviation from the straight-line portion of the stress-strain curve.
The yield strength, shown at point B in Fig. 2, is the stress required to produce a small specified amount of
plastic deformation. The usual definition of this property is the offset yield strength determined by the stress
corresponding to the intersection of the stress-strain curve offset by a specified strain (see Fig. 1 and 2). In the
United States, the offset is usually specified as a strain of 0.2 or 0.1% (e = 0.002 or 0.001):
(Eq 4)
Offset yield strength determination requires a specimen that has been loaded to its 0.2% offset yield strength
and unloaded so that it is 0.2% longer than before the test. The offset yield strength is often referred to in Great
Britain as the proof stress, where offset values are either 0.1 or 0.5%. The yield strength obtained by an offset
method is commonly used for design and specification purposes, because it avoids the practical difficulties of
measuring the elastic limit or proportional limit.
Some materials have essentially no linear portion to their stress-strain curve, for example, soft copper, gray cast
iron, and many polymers. For these materials, the offset method cannot be used, and the usual practice is to
define the yield strength as the stress to produce some total strain, for example, e = 0.005.
Some metals, particularly annealed low-carbon steel, show a localized, heterogeneous type of transition from
elastic to plastic deformation that produces a yield point in the stress-strain curve. Rather than having a flow
curve with a gradual transition from elastic to plastic behavior, such as Fig. 1 and 2, metals with a yield point
produce a flow curve or a load-elongation diagram similar to Fig. 3. The load increases steadily with elastic
strain, drops suddenly, fluctuates about some approximately constant value of load, and then rises with further
strain.
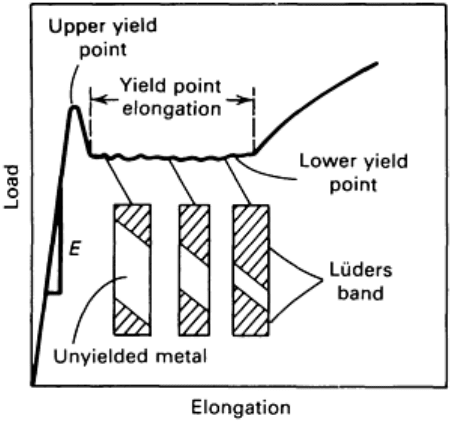
Fig. 3 Typical yield-point behavior of low-carbon steel. The slope of the initial linear
portion of the stress-strain curve, designated by E, is the modulus of elasticity.
The load at which the sudden drop occurs is called the upper yield point. The constant load is called the lower
yield point, and the elongation that occurs at constant load is called the yield-point elongation. The deformation
occurring throughout the yield-point elongation is heterogeneous. At the upper yield point, a discrete band of
deformed metal, often readily visible, appears at a stress concentration, such as a fillet. Coincident with the
formation of the band, the load drops to the lower yield point. The band then propagates along the length of the
specimen, causing the yield-point elongation. A similar behavior occurs with some polymers and superplastic
metal alloys, where a neck forms but grows in a stable manner, with material being fed into the necked region
from the thicker adjacent regions. This type of deformation in polymers is called “drawing”.
In typical cases, several bands form at several points of stress concentration. These bands are generally at
approximately 45° to the tensile axis. They are usually called Lüders bands or stretcher strains, and this type of
deformation is sometimes referred to as the Piobert effect. When several Lüders bands are formed, the flow
curve during the yield-point elongation is irregular, each jog corresponding to the formation of a new Lüders
band. After the Lüders bands have propagated to cover the entire length of the specimen test section, the flow
will increase with strain in the typical manner. This marks the end of the yield-point elongation. Lüders bands
formed on a rimmed 1008 steel are shown in Fig. 4.
