ASM Metals HandBook Vol. 17 - Nondestructive Evaluation and Quality Control
Подождите немного. Документ загружается.


(a)
Approximately atmospheric pressure.
(b)
1 nm (nanometer) = 10
-9
m.
The level of vacuum is virtually always described in terms of absolute pressure. However, the mean free path or the
concentration of molecules controls such vacuum properties as viscosity, thermal conductance, and dielectric strength.
Furthermore, very few vacuum gages actually measure pressure, but instead measure the concentration of molecules.
Therefore, in the context of vacuum systems, the term pressure is largely inaccurate, although it remains in use.
Reference cited in this section
1.
Leak Testing, in Nondestructive Testing Handbook, R.C. McMaster, Ed.,
Vol 1, 2nd ed., American Society
for Nondestructive Testing, 1982
Leak Testing
Revised by Gerald L. Anderson, American Gas and Chemical Company
Leak Testing of Pressure Systems Without a Tracer Gas
Leak testing methods can be classified according to the pressure and fluid (gas or liquid) in the system. The following
sections describe the common fluid-system leak testing methods in the general order shown in Table 3, which also lists
methods used in the leak testing of vacuum systems (discussed in the section "Leak Testing of Vacuum Systems" in this
article). Table 4 compares leak testing method sensitivities.
Table 3 Method of leak testing systems at pressure or at vacuum
Gas systems at pressure
Direct sensing
Acoustic methods
Bubble testing
Flow detection
Gas detection
Smell
Chemical reaction
Halogen gas
Sulfur hexafluoride
Combustible gas
Thermal-conductivity gages
Infrared gas analyzers
Mass spectrometry
Radioisotope count
Ionization gages
Gas chromatography
Quantity-loss determination
Weighing
Gaging differential pressure
Liquid systems at pressure
Unaided visual methods
Aided visual methods
Surface wetting
Weight loss
Water-soluble paper with aluminum foil
Vacuum systems
Manometers
Halogen gas
Mass spectrometry
Ionization gages
Thermal-conductivity gages
Gas chromatography
Table 4 Sensitivity ranges of leak testing methods
Sensitivity range, cm
3
/s Method
Pressure Vacuum
Mass spectrometer 10
-3
to 10
-5
10
-3
to 10
-10
Electron capture 10
-6
to 10
-11
. . .
Colorimetric developer 1 to 10
-8
. . .
Bubble test--liquid film 10
-1
to 10
-5
10
-1
to 10
-5
Bubble test--immersion 1 to 10
-6
. . .
Hydrostatic test 1 to 10
-2
. . .
Pressure increase 1 to 10
-4
1 to 10
-4
Pressure decrease/flow 1 to 10
-3
. . .
Liquid tracer 1 to 10
-4
1 to 10
-4
High voltage . . . 1 to 10
-4
Halogen (heated anode) 10
-1
to 10
-6
10
-1
to 10
-5
Thermal conductivity (He)
1 to 10
-5
. . .
Gage . . . 10
-1
to 10
-7
Radioactive tracer 10
-13
. . .
Infrared 1 to 10
-5
. . .
Acoustic 1 to 10
-2
1 to 10
-2
Smoke tracer 1 to 10
-2
. . .
Source: Ref 2
Leak detection by monitoring changes in the pressure of the internal fluid is often used when leak detection equipment is
not immediately available. For the most part, detection can be accomplished with instruments that are already installed in
the system.
Acoustic Methods
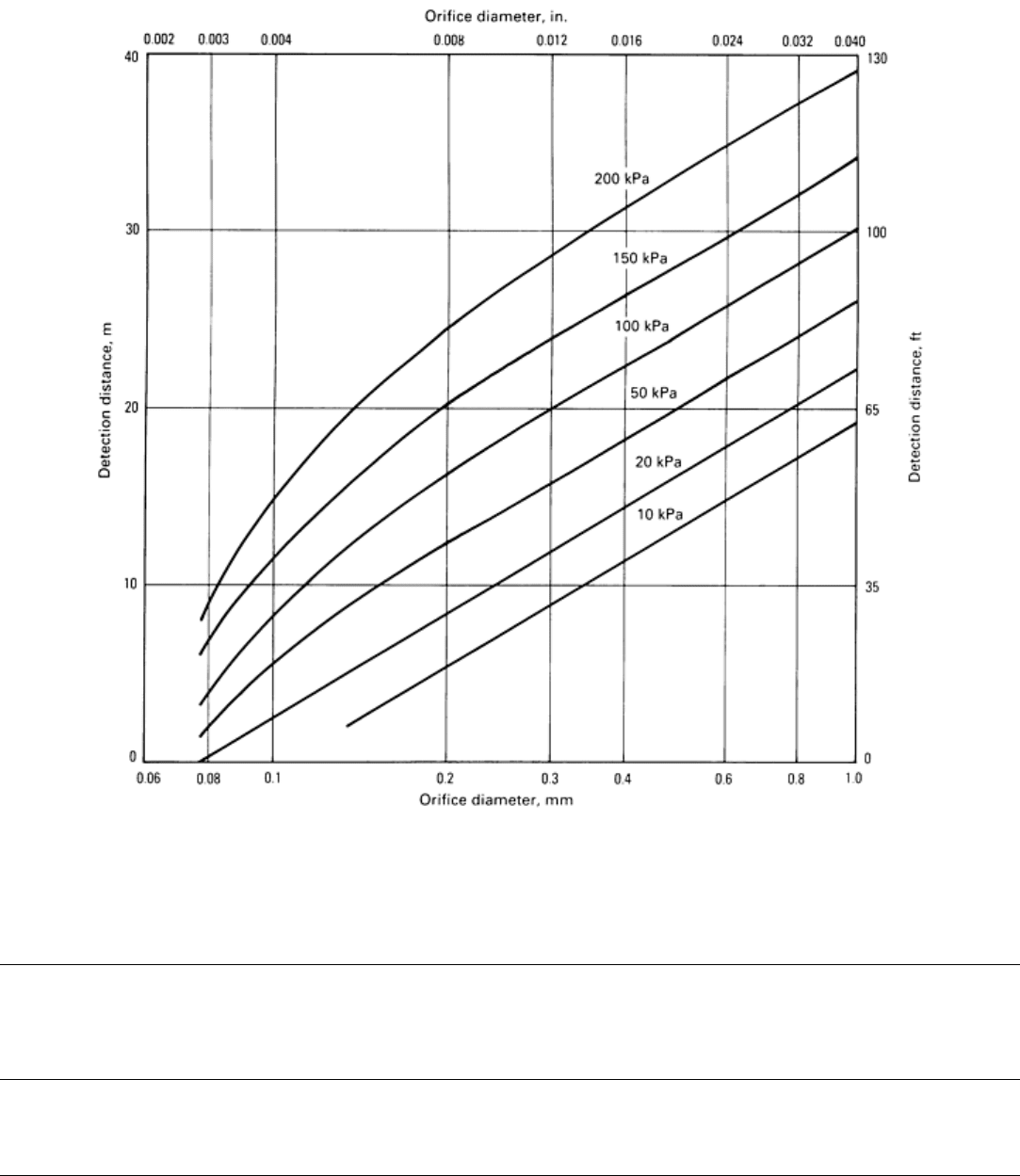
The turbulent flow of a pressurized gas through a leak produces sound of both sonic and ultrasonic frequencies (Fig. 1). If
the leak is large, it can probably be detected with the ear. This is an economical and fast method of finding gross leaks.
Sonic emissions are also detected with such instruments as stethoscopes or microphones, which have limited ability to
locate as well as estimate the approximate size of a leak. Electronic transducers enhance detection sensitivity.
Fig. 1 Turbulence caused by fluid flow through an orifice
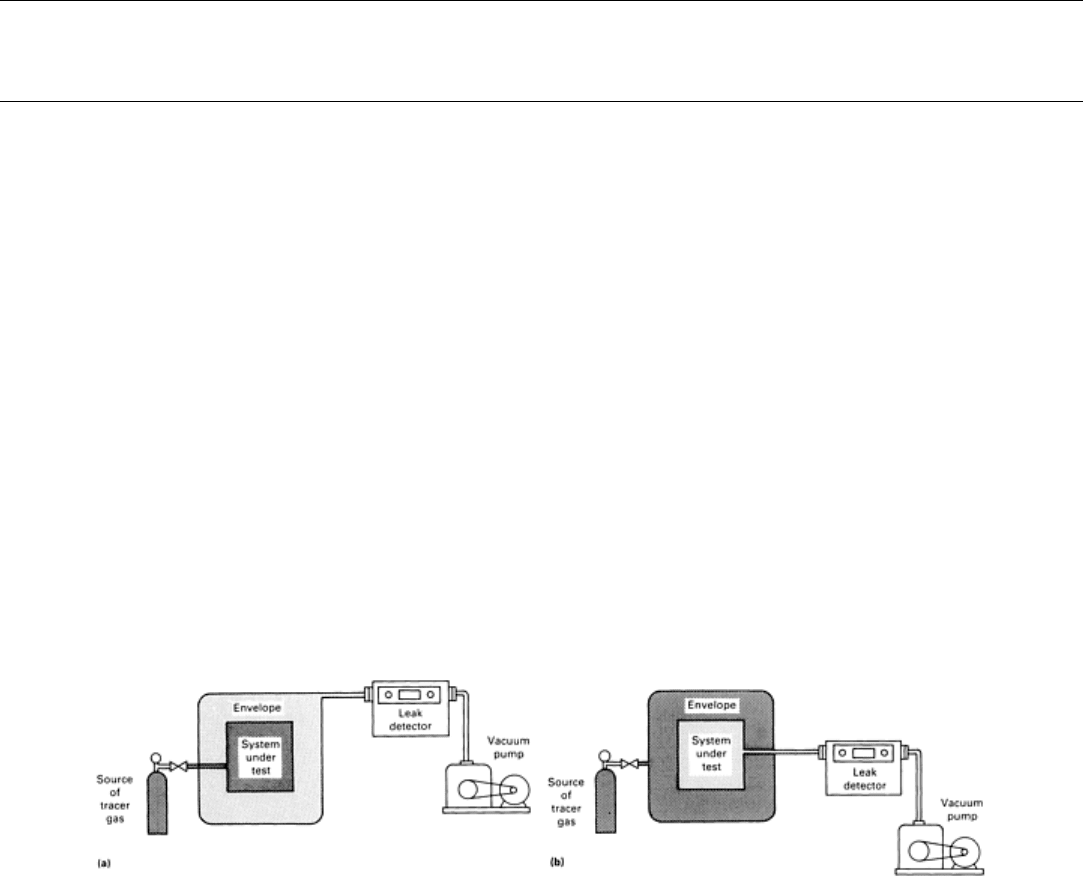
Smaller leaks can be found with ultrasonic probes operating in the range of 35 to 40 kHz, although actual emissions from
leaks range to over 100 kHz. Ultrasonic detectors are considerably more sensitive than sonic detectors for detecting gas
leaks and from distances of 15 m (50 ft) are capable of detecting air leaking through a 0.25 mm (0.010 in.) diam hole at
35 kPa (5 psi) of pressure. The performance of an ultrasonic leak detector as a function of detection distance, orifice
diameter, and internal air pressure is shown in Fig. 2. The sound level produced is an inverse function of the molecular
weight of the leaking gas. Therefore, a given flow rate of a gas such as helium will produce more sound energy than the
same flow rate of a heavier gas such as nitrogen, air, or carbon dioxide. If background noise is low, ultrasonic detectors
can detect turbulent gas leakage of the order of 10
-2
atm cm
3
/s. Ultrasonic leak detectors have also been successfully used
with ultrasonic sound generators when the system to be tested could not be pressurized.
Fig. 2 Relation
of orifice diameter to detection distance with an ultrasonic leak detector for various internal air
pressures
Reference cited in this section
2.
Encyclopedia of Materials Science and Engineering, Vol 4, MIT Press, 1986
Leak Testing
Revised by Gerald L. Anderson, American Gas and Chemical Company
Bubble Testing

A simple method for leak testing small vessels pressurized with any gas is to submerge them in a liquid and observed
bubbles. If the test vessels is sealed at atmospheric pressure, a pressure differential can be obtained by pumping a partial
vacuum over the liquid or by heating the liquid. The sensitivity of this test is increased by reducing the pressure above the
liquid, the liquid, density, the depth of immersion in the liquid, and the surface tension of the liquid.
Immersion Testing. Oils are a more sensitivity medium than ordinary water. Therefore, it is common practice to test
electric components in a bath of hot perfluorocarbon. When testing by reducing the pressure above the liquid, several
precautions must be observed, particularly if the reduced pressure brings the liquid close to its boiling point. If the liquid
begins to boil, a false leak indication will be given. The test vessel must be thoroughly cleaned to increase surface
wetting, to prevent bubbles from clinging to its surface, and to prevent contamination of the fluid. If water is used, it must
be distilled or deionized and should be handled with minimal sloshing to reduce the absorbed-gas content. A small
amount of wetting agent is normally added to water to reduce surface tension. With the addition of the proper wetting
agent, water can be even more sensitive than oils. Water-base surfactant solutions can be used successfully to detect leaks
as small as 10
-6
atm cm
3
/s.
Immersion testing can be used on any internally pressurized item that would not be damaged by the test liquid. Although
this test can be relatively sensitive, as previously stated, it is more commonly used as a preliminary test to detect gross
leaks. This method is inexpensive, requires little operator skill for low-sensitivity testing, and enables an operator to
locate a leak accurately.
Bubble-forming solutions can be applied to the surface of a pressurized vessels if it is too large or unwieldy to
submerge. However, care must be taken to ensure that no bubbles are formed by the process itself. Spraying the bubble
solution is not recommended; it should be flowed onto the surface. A sensitivity of about 10
-5
atm cm
3
/s is possible with
this method, if care is taken. Sensitivity may drop to about 10
-3
atm cm
3
/s with an untrained worker or to 10
-2
atm cm
3
/s if
soap and water is used.
Like immersion testing, the use of bubble-forming solutions is inexpensive and does not require extensive training of the
inspector. One disadvantage is that the test does not normally enable the operator to determine the size of a leak accuracy.
Leak Testing
Revised by Gerald L. Anderson, American Gas and Chemical Company
Flow Detection
Three separate methods are frequently grouped under the heading of flow detection:
• Pressure increase
• Pressure decrease
• Flow
The sensitivity for each of these method is not the same, nor can any one method always be substituted for the other. If an
internally pressurized vessel or system is enclosed within a larger vessel that is then sealed except for a small duct,
leakage from the vessel being tested will result in an increase in pressure within the larger enclosing vessel, and gas will
flow through the attached duct. If a device sensitive to the movement or flow of gas is installed in the attached duct, it can
serve as a leak-rate indicator. Extremely sensitive positive-displacement flowmeters have been developed for this
purpose.
Alternatively, a volumetric-displacement meter, which consists essentially of a cylinder with a movable piston, can be
used. When attached to the duct from the enclosing vessel, the piston moves as the pressure of the enclosing vessel rises,
effectively increasing the volume of the vessel and returning the internal pressure to the ambient atmospheric pressure.
The piston must be very nearly free of frictional drag, and its motion must be accurate horizontally. Sensitive volumetric-
displacement meters are equipped with micrometric cathetometers, which can accurately measure extremely small
displacements of the piston. Some volumetric-displacement meters will detect leaks of about 10
-4
atm cm
3
/s. This is a
very simple method to use when it is inconvenient to measure directly the pressure change of the container being tested.
The simplest method of leak detection and measurement is the bubble tube. If the end of the duct from the outer enclosing
vessel empties into a liquid bath, appreciable leakage will generate bubbles. Even very small leak rates can be detected
simply by the movement of the liquid meniscus in the tube.
Leak Testing
Revised by Gerald L. Anderson, American Gas and Chemical Company
Leak Testing of Pressure Systems Using Specific-Gas Detectors
Many available types of leak detectors will react to either a specific gas or a group of gases that have some specific
physical or chemical property in common. Leak-rate measurement techniques involving the use of tracer gases fall into
two classifications:
• Static leak testing
• Dynamic leak testing
In static leak testing, the chamber into which tracer gas leaks and accumulates is sealed and is not subjected to pumping to
remove the accumulated gases. In dynamic leak testing, the chamber into which tracer gas leaks is pumped continuously
or intermittently to draw the leaking tracer gas through the leak detector. The leak-rate measurement procedure consists of
first placing tracer gas within (Fig. 3a) or around the whole system being tested (Fig. 3b). A pressure differential across
the system boundary is established either by pressurizing one side of the pressure boundary with tracer gas or by
evacuating the other side. The concentration of tracer gas on the lower-pressure side of the pressure boundary is measured
to determine the leak rate.
Fig. 3
Modes of leakage measurement used in dynamic leak testing techniques utilizing vacuum pumping. (a)
Pressurized system mode for the leak testing
of smaller components. (b) Pressurized envelope mode for the
leak testing of larger-volume systems
Specific-Gas Detection Devices
Some of the more commonly used gas detectors are described below. These range from the simple utilization of the
senses of sight and smell, when possible, to complex instrumentation such as mass spectrometers and gas
chromatographs.
Odor Detection Via Olfaction. The human sense of smell can and should be used to detect odorous gross leaks. The
olfactory nerves are quite sensitive to certain substances, and although not especially useful for leak locations, they can
determine the presence of strong odors. However, the olfactory nerves fatigue quite rapidly, and if the leakage is not
noted immediately, it will probably not be detected.
Color Change. Chemical reaction testing is based on the detection of gas seepage from inside a vessel by means of
sensitive solutions or gas.
The ammonia color change method is probably the best known. In this method, the vessel surface is cleaned and
then a calorimetric developer is applied to the surface of the vessel, where it forms an elastic film that is easily removed
after the test. The developer is fairly fluid, and when applied with a spray gun it sets rapidly and adheres well to metal
surfaces, forming a continuous coating. An air-ammonia mixture (usually varying from 1 to 5% NH
3
) is then introduced
into the dry vessel. Leakage of gas through the discontinuity causes the indicator to change color. The sensitivity of this
method can be controlled by varying the concentration of ammonia, the pressure applied to the air-ammonia mixture, and
the time allowed for development.
Indicator tapes are useful for the leak testing of welded joints; the method used for this application is as follows. After
the surface of the joint to be tested has been cleaned with a solvent, the indicator tape is fixed on the weld either by a
rubber solution applied on the edge of the tape or by a plastic film. Then the inspection gas, which consists of an
ammonia-air mixture with 1 to 10% NH
3
, is fed into the vessel at excess pressure. If microdiscontinuities exist, the gas
leaks out the reacts chemically with the indicator, forming colored spots that are clearly visible on the background of the
tape.
The indicator tape method has the following advantages:
• Remote control is possible, ensuring safety for the operators
• Leaks of approximately 10
-7
atm cm
3
/s can be detected
• The color of the tape is not affected by contact with the hands, high humidity, or the passage of time
•
Tapes can sometimes be used more than once; if there are spots caused by the action of ammonia, they
can be removed by blowing the tape with dry compressed air
Ammonia and Hydrochloric Acid Reaction. Another method involves pressurizing the vessel with ammonia gas,
then searching for the leak with an open bottle of hydrochloric acid. A leak will produce a white mist of ammonium
chloride precipitate when the ammonia comes into contact with the hydrogen chloride vapor. Good ventilation is
necessary because of the noxious characteristics of both hydrogen chloride vapor and ammonia gas.
Ammonia and Sulfur Dioxide Reaction. Another modification of the ammonia chemical indicator test involves the
use of ammonia and sulfur dioxide gas to produce a white mist of ammonium sulfide. Sulfur dioxide is not as irritating or
corrosive as hydrogen chloride, but it is still a noxious gas and should be used only in well-ventilated areas.
Halide Torch. Still another type of chemical reaction test uses the commercial halide torch, which consists of a gas tank
and a brass plate (Fig. 4). Burning gas heats the brass plate; in the presence of halogen gas, the color of the flame changes
because of the formation of copper halide. (The flame is also used to inspirate gas through the probe, which is a length of
laboratory tubing.) The halide torch locates leaks as small as 1 × 10
-3
atm cm
3
/s.
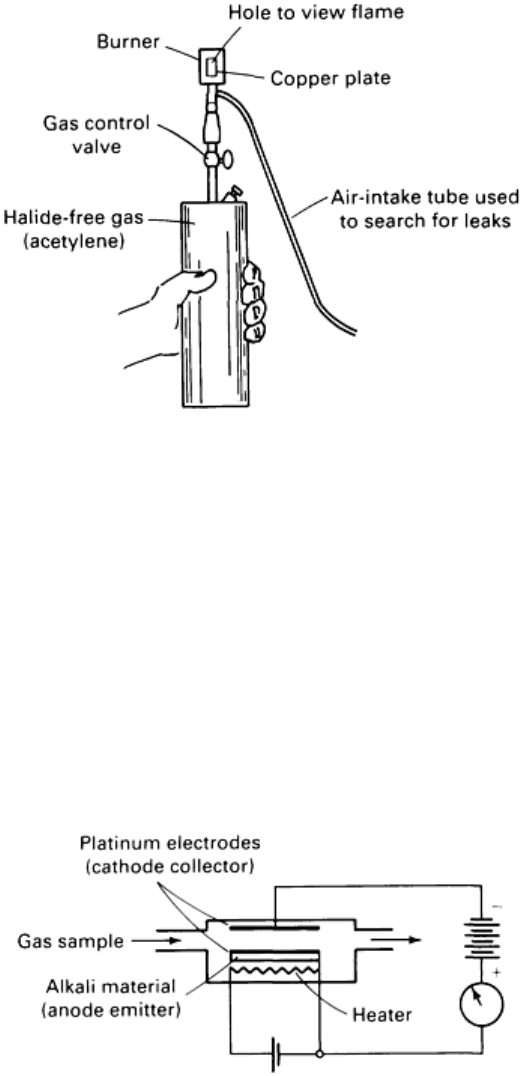
Fig. 4 Halide torch used for leak location
Halogen-Diode Testing. In halogen-diode testing, a leak detector is used that responds to most gases containing
chlorine, fluorine, bromine, or iodine. Therefore, one of these halogen-compound gases is used as a tracer gas. When a
vessel is pressurized with such a tracer gas, or a mixture of a halogen compound and air or nitrogen, the sniffing probe of
the leak detector is used to locate the leak.
The leak detector sensing element operates on the principle of ion emission from a hot plate to a collector (Fig. 5).
Positive-ion emission increases with an increase in the amount of halogen-compound gases present. This ion current is
amplified to give an electrical leak signal. The sensitivity of halogen detectors operating at atmospheric pressure is about
10
-6
atm cm
3
/s, but this will vary depending on the specific gas that is being used.
Fig. 5
Schematic of sensing element of a heated anode halogen leak detector used at atmospheric pressure for
leak location with detector probe system. Positive-
ion current from heated alkali electrode responds to
refrigerant gases and other halogenated hydrocarbon tracer gases.
Several different types of halogen leak detectors are available. Each includes a control unit and a probe through which air
is drawn at about 4.9 × 10
5
mm
3
/min (30 in.
3
/min). When searching for leaks from an enclosure pressurized with a tracer
gas, the probe tip is moved over joints and seams suspected of leaking. The probe tip should lightly touch the surface of
the metal as it is moved. Where forced ventilation is required to keep the air free of halogen vapors, it must be stopped
during actual testing, or care must be taken to ensure that drafts do not blow the leaking gas away from the test probe.
When the probe passes over the close to a leak, the tracer gas is drawn into the probe with the air and through a sensitive
element, where it is detected. The leak signal is either audible or visual.

Certain precautions are necessary in this probe exploration. Probing too rapidly may result in missing small leaks. To
avoid this risk, the speed at which the probe is moved must be in proportion to the minimum leak tolerance. When testing
a vessel for allowable leaks of the order of 0.001 kg (0.04 oz) per year, the probe travel can be 25 to 50 mm/s (1 to 2
in./s), but for smaller leaks the probe speed should be reduced to 13 mm/s ( in./s).
Sulfur hexafluoride detectors operate on the principle of electron-capture detectors, which are used widely in the
field of gas chromatography. The sensing chamber of a sulfur hexafluoride detector consists of a cylindrical cell that has a
centrally mounted insulated probe. The inner wall of the cell is coated with a radioactive element (10 gigabecquerel
(GBq), or 300 millicuries (mCi), of tritium). Low-energy electrons emitted by the tritium are collected on the central
probe by means of a polarizing voltage maintained between the probe and the cell wall. The resulting electric current is
amplified and displayed on a conventional meter. Leak-rate sensitivity is 10
-8
mL/s (3 × 10
-10
oz/s), and concentration
sensitivity is 1 part sulfur hexafluoride in 10
10
parts air. The equipment is fully portable.
Pure nitrogen (oxygen free) is passed through the detector and the standing current is set by a zero control to a given
position on the meter scale. When an electron-capturing compound such as sulfur hexafluoride enters the cell, the
electrons forming the current are captured by the sulfur hexafluoride molecules, resulting in a reduction of the standing
current, which is indicated by a meter deflection. This meter reading is proportional to the amount of sulfur hexafluoride
entering the cell and is therefore an approximate indication of the size of the leak. The oxygen molecule also possesses
the electron-capture characteristic, although to a lesser extent than sulfur hexafluoride; hence the requirement for purging
the instrument with oxygen-free nitrogen during testing.
Combustible-gas detectors are often used as monitors or as leak locators where combustible fumes are likely to
accumulate, as in basements. These instruments warn of potentially hazardous conditions by their ability to measure
combustible-gas mixtures well below the dangerous concentration level. Catalytic combustible gas instruments measure
the gas concentration as a percentage of its lower explosive limit. The temperature of a heated catalytic element will rise
in the presence of a combustible gas. The minimum sensitivity of a catalytic bead is about 500 ppm, which is a leak rate
of approximately 10
-3
atm cm
3
/s. As leak locators, therefore, catalytic elements are not sufficiently sensitive. To locate a
combustible gas, a solid-state sensor or flame ionization detector should be used. These sensors can detect a leak of
approximately 10
-5
atm cm
3
/s.
Thermal-Conductivity Gages. The thermal conductivity of a gas can be measured by a hot-wire bridge method (Fig.
6). A resistance element, usually a thin wire or filament, is heated electrically and exposed to the gas. The temperature,
and therefore the resistance of the wire, depends on the thermal conductivity of the surrounding gas, provided the power
input is held constant. Alternatively, the temperature (and resistance) of the wire can be maintained at a constant value,
and the required power input measured either directly or indirectly in terms of applied voltage or current. Many types of
thermal-conductivity detectors are commercially available, including differential detectors, simple Pirani gages, hydrogen
Pirani gages, differential or trapped Pirani gages, charcoal Pirani gages, and thermocouple gages.
Fig. 6 Schematic of a thermal-conductivity gage using a Pirani-
type detector. Thermal losses from the
electrically heated resistance wire vary with heat conduction by gas molecules. He
at losses are reduced as gas
pressure is lowered.
Pirani-Type Detectors. When Pirani-type detectors are used, the search gas should have low density, low viscosity,
and low molecular weight. Most important, the gas should have a thermal conductivity markedly different from that of
air.
The component or structure to be tested is filled with the tracer gas under positive pressure. This gas will consequently
escape through even the most minute leak; hence the need for the desirable physical properties mentioned above. The
escaping gas is drawn into the narrow-bore probe of the leak detector by a small suction pump. The sample is then
allowed to expand into the sensing head, which contains an electrically heated filament. Simultaneously, a sample of pure
air is drawn by the same pump into a second, identical chamber that also contains a heated filament. The two filaments
form two arms of a conventional Wheatstone bridge circuit, which is initially balanced by an external variable resistance
while both arms are simultaneously exposed to air. As soon as one arm receives a sample containing a trace of the tracer
gas, such as helium, heat is extracted at a greater rate because of the substantially greater thermal conductivity of helium
than air. This will cause a corresponding change in the resistance of the filament, thus unbalancing the Wheatstone bridge
network. This is shown by an appropriate deflection on a center-zero milliammeter; simultaneously, an audio alarm
circuit is triggered.
Infrared gas analyzers can detect a gas mixture that has a clear absorption band in the infrared spectrum by
comparing it to the absorption characteristics of a pure standard sample of the same gas. The tracer gas used must possess
a strong absorption in the infrared region. Nitrous oxide possesses this property markedly. The known characteristic is
converted into a measurable response by allowing a heat source to radiate through two absorption tubes that contain the
gases under comparison. These tubes are separated by a thin metal diaphragm that, in combination with an adjacent
insulated metal plate, forms an electrical condenser. If the system is in balance (that is, if the same gas is in each tube), the
heating effect will be equal and no pressure differential on the diaphragm will occur. However, if one tube contains
nitrous oxide admixed with air, absorption of heat will occur to a greater extent that in the tube containing pure air. This
will cause a higher pressure on one side of the diaphragm, as a result of the increase in temperature, and will cause it to
move slightly in relation to the insulated plate. The resulting change in capacity of the condenser is amplified
electronically and rendered visible on an output meter.
Infrared absorption is also a very sensitive way to measure small concentrations of hydrocarbons, such as methane.
Infrared lasers are becoming more common as monitors for a variety of toxic and combustible gases. For some
compounds, infrared laser spectroscopy can detect gas at parts per trillion levels.
Mass Spectrometer Testing. A mass spectrometer is basically a device for sorting charged particles. The sample gas
enters the analyzer, where its molecules are bombarded by a stream of electrons emitted by a filament. The bombarded
molecules lose an electron and become positively charged ions, which are electrostatically accelerated to a high velocity.
Because the analyzer lies in a magnetic field perpendicular to the ion path, the ions travel in distinct, curved paths
according to their mass. The radii of these paths are determined by ion mass, the magnitude of initial acceleration, and the
strength of the magnetic field. With a constant magnetic field, any group of ions having the same mass can be made to
travel the specific radius necessary to strike the ion collector. The positive charge of the ions is imparted to the target, or
collector, and the resulting current flow is proportional to the quantity of the ions of that particular mass.
Specialized mass spectrometers are available, such as residual-gas analyzers, partial pressure analyzers, and helium mass
spectrometers, which have been tuned to respond only to certain ranges of atomic mass units. In particular, the helium
mass spectrometer is constructed so that it does not scan but is tuned to the helium peak. It will detect only helium; all
other molecules passing through the detector tube will miss the target or collector because of their differences in mass or
momentum from helium.
The theoretical sensitivity of the helium mass spectrometer is about 10
-12
atm cm
3
/s; the sensitivity of the residual-gas
analyzer is about one order of magnitude less. General-purpose mass spectrometers have a sensitivity even less than this,
depending on the range of atomic mass units that the instrument is designed to measure, Helium mass spectrometers,
however, may not detect leaks smaller than approximately 10
-8
atm cm
3
/s in large systems. Because of background,
outgassing of sorbed gases, noise, permeation, and other such factors, 10
-8
to 10
-9
atm cm
3
/s is often the minimum
detectable vacuum leak rate for helium mass spectrometers.
