All?gre Claude J. Isotope Geology
Подождите немного. Документ загружается.

Inth is case, wethereforehave:
l
1
N
1
¼ l
2
N
2
¼ l
3
N
3
¼
...
¼ l
n1
N
n1
;
therefore:
dN
n
dt
¼ l
1
N
1
:
It is asi fthere wasjust asingle direct decayreaction(1) !(n).
Consid ering the fact that in the course of g eological time natural radioactive chains
rapidly reach equilibrium, the initial products give the end products directly. Dating equa-
tions canthenbewritten:
206
Pb ¼
238
Uðe
l
8
t
1Þþ
206
Pb
0
207
Pb ¼
235
Uðe
l
5
t
1Þþ
207
Pb
0
208
Pb ¼
232
Thðe
l
2
t
1Þþ
208
Pb
0
where constantsl
8
, l
5
,andl
2
arethoseof
238
U,
235
U,and
232
Th.
92
92
90
88
90
88
86
84
82
90
88
86
84
86
84
82
124 126 128 130 132 134 136 138 140 142 144 146
82
206
Pb
208
Pb
208
TI
3.1 m
207
Pb
°
210
Po
138 d
207
Ti
4.8 m
211
Bi
2.2 m
211
Pb
36 m
215
Po
2x10
–3
s
219
Rn
3.9 s
223
Ra
11 d
227
Th
17 d
227
Ac
22 yr
224
Ra
3.6 d
220
Rn
55 s
216
Po
0.16 s
212
Po
3x10
–7
s
212
Bi
61 m
212
Pb
11 h
231
Th
26 h
210
Bi
50 d
214
Bi
20 m
214
Pb
27 m
218
Po
3.1 m
222
Rn
38 d
214
Po
2x10
-4
s
210
Pb
22 yr
226
Ra
1622 yr
235
U
713 Ma
232
Th
14 Ba
228
Ra
6.7 yr
230
Th
75 ka
234
Pa
1.2 m
234
Th
24 d
238
U
4.5 Ba
N
Z
228
Th
1.9 yr
231
Pa
34 ka
234
U
248 ka
228
Ac
6.1 h
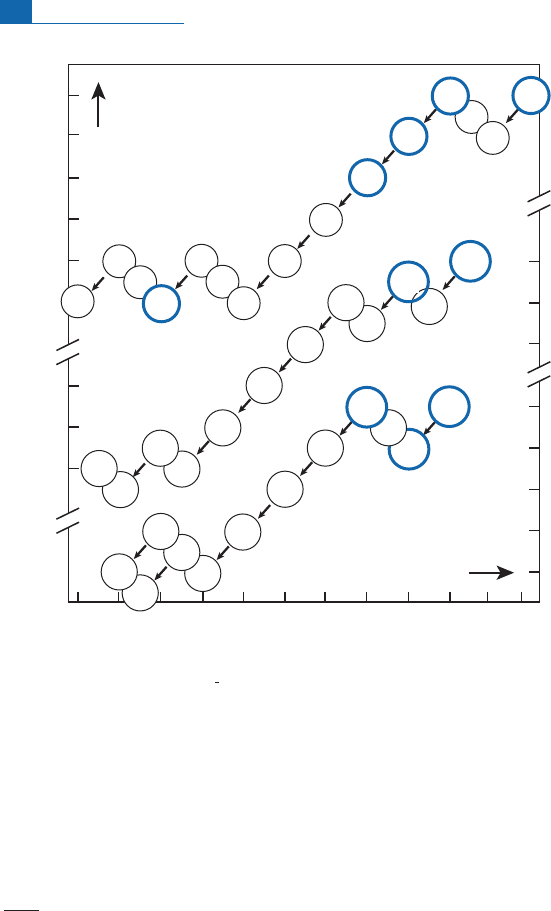
Figure 2.2 Radioactive chains represented on the proton–neutron graph. Each slot contains the symbol
of the isotope and its period
T
1
2
. Identify the various types of radioactivity of the three chains for yourself.
37 Radioactive chains

238
U !
206
Pb l
238
¼ l
8
¼ 1:551 25 10
10
yr
1
235
U !
207
Pb l
235
¼ l
5
¼ 9:8485 10
10
yr
1
232
Th !
208
Pb l
232
¼ l
2
¼ 4:9475 10
11
yr
1
:
This comes downto d i re ct parent^ daug hter dati ng.Warning! Linearapproximation can-
not be used with these chronometers.To be convinced of this, let us compare the values of
(e
lt
1) with (lt)for timeintervalsof 1 and 2 billion years for
235
Uand
238
U.
Letus consider the quantity ½ðe
lt
1Þlt=½e
lt
1
100, which is the relative e rror
thatcanbe expressed asapercentage.
With
235
U, for1billion the error is 41.2% and for 2 billion 68.2%! With
238
U, for1billion
the error is 7.5% and for 2 billion14.7%.These are highly non-linear systems, th en, as can
beseen from theshape ofthetypical curvesin Figure2.3.
2.3.3 The special case of lead–lead methods
We have seen that on the geological timescale radioactive chains attain equilibriu m and it
can be considered that
238
U decays directly to
206
Pb,
235
Uto
207
Pb, and
232
Th to
208
Pb.
For uranium-rich minerals li ke zi rcon, uraninite, or even monaz ite, the initial amount of
lead can be considered negligible. Assuming the system has remained closed, we can
write:
206
Pb ¼
238
Uðe
l
8
t
1Þ
207
Pb ¼
235
Uðe
l
5
t
1Þ:
Taking the ratio, remembering thatnowadays
1
the ratio
238
U/
235
U ¼137.8, we get:
207
Pb
206
Pb
¼
1
137:8
e
l
5
t
1
e
l
8
t
1
:
It can be seen that the
207
Pb/
206
Pb isotope ratio of lead alone gives a direct measurement
of time. This function is impli cit and calculating it requires prior numerical values
(Table 2.1 ).
Exercise
The
206
Pb/
207
Pb ratio of a uranium ore deposit is found to be 13.50. What is the age of the ore
supposing it has remained closed since it crystallized and that common lead can be ignored?
Answer
We shall invert the ratio so Table 2.1 can be used.
207
Pb/
206
Pb ¼0.074. The table shows that
the ratio measured lies between 1 and 1.2 10
9
years. The result can be improved either by
refining the table by calculating the ratio e
l
5
t
1=e
l
8
t
1
in the interval 1 and 1.2, or by
1
The two uranium isotopes decay each at their own rate from the time that they are first formed. Their
ratio is constant as there is no isotopic fractionation between them. However, this ratio has varied over
geological time.
38 The principles of radioactive dating
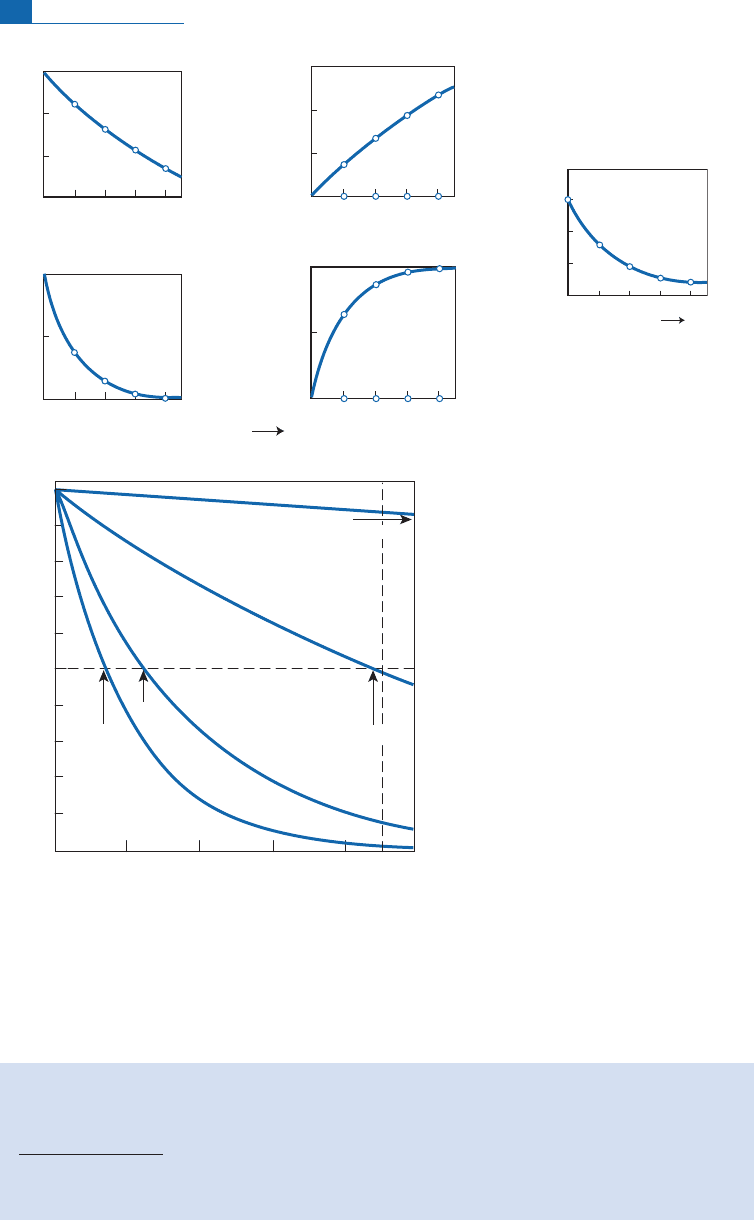
using a linear approximation between 1 and 1.2 10
9
years. The value
207
Pb/
206
Pb for 1.2
billion is 0.080 12 and for 1 billion is 0.0725. The variation is therefore:
0:080 12 0:0725
200
¼ 3:8 10
5
per million years:
The difference is between 0.0740 and 0.0725, that is, 1.5 10
3
million years.
1
0.4
0.2
207
Pb*/
206
Pb*
0
0.6
0.8
234
Time (Ga)
Time (Ga)
1.0
0.5
0.0
01 2 3
Age (Ga)
P/P
0
235
U-
207
Pb
40
K-
40
Ar
238
U-
206
Pb
T
= 0.70
Age of the Earth
4.56 Ga
87
Rb-
87
Sr
45
T = 48.8
T
= 4.47
T = 1.25
235
U
0
0.5
1
2134
207
Pb*
0
1
0.5
1234
0.2
206
Pb*
0
0.4
0.6
1234
0.6
0.4
0.8
1
238
U
1234
Figure 2.3 Non-linear decay of
235
U and
238
U and their end products
207
Pb and
206
Pb. (a) The radioactive
constants of these reactions are very different. The curve on the right shows the change in radiogenic
ratio
207
Pb*/
206
Pb* versus time. (b) Comparison in the same figure of the principal radioactive clocks
used in geology, emphasizing the behavior of U–Pb systems compared to others.
39 Radioactive chains

The approximate age is therefore 1040 Ma. Direct calculation from the
207
Pb/
206
Pb ratio for
1040 Ma gives 0.073 98. The age is therefore 1042 Ma, but such precision is illusory because
error is far greater (see Chapter 5) than the precision displayed.
2.3.4 The helium method
Natural chains feature many instances of decay, that is, expulsion of
4
He nuclei. Thus
238
Udecay ultimately produces eight
4
He ,
235
Ude cay produces seven
4
He, and
232
Th decay
p roduces six
4
He.We cantherefore write:
d
4
He
dt
¼ 8l
8
238
U þ 7l
5
235
U þ 6l
2
232
Th:
Remark
This equation underlies the first helium dating method thought up by Rutherford (1906).
Integrating the equation, assuming
4
He(0) ¼0, gives:
4
He ¼ 8
238
Uðe
l
8
t
1Þþ7
235
Uðe
l
5
t
1Þþ6
232
Thðe
l
2
t
1Þ:
Table 2.1 Numerical values of the radiogenic
207
Pb/
206
Pb isotope ratio
Time (Ga) e
l
1
t
1e
l
2
t
1
207
Pb
206
Pb
radiogenic
0 0.0000 0.0000
0.2 0.03 15 0.2 177 0.0501 2
0.4 0.0640 0.4828 0.05471
0.6 0. 097 5 0.8056 0.05992
0.8 0.1321 1.1987 0.06581
1.0 0.1678 1.6774 0.07250
1.2 0.2046 2.260 3 0.08012
1.4 0.2426 2.9701 0.08879
1.6 0.2817 3.834 4 0.0 9872
1 .8 0.322 1 4.8869 0. 1 1 000
2. 0 0. 3638 6.1685 0.12298
2.2 0.4 0 67 7.7292 0.13783
2.4 0 . 4511 9.6296 0. 15482
2.6 0.4968 11.9437 0.17436
2.8 0 . 5440 14.76 17 0.1 9680
3.0 0.5926 18.1931 0.22266
3.2 0.6428 22.3716 0.25241
3.4 0.6946 27.4597 0.28672
3.6 0.74 80 33.6556 0.32634
3.8 0.8030 41.20 04 0.37212
4.0 0.8599 50.3878 0.42498
4.2 0.9185 61.57 52 0.48623
4.4 0.9789 75.1984 0.55714
4.6 1 . 04 13 91 .7873 0.63930
40 The principles of radioactive dating
Byadmittinglinearapproximations, which isvalidonly fordurations thatarenotexcessive,
e
lt
1 lt andobservingthat
235
U/
238
U ¼1/137.8 andthat
232
Th/
238
U 4 weobtain:
4
He ¼
238
U8l
8
þ
7
137:8
l
5
þ6 4l
2
t:
By noting L ¼ 8l
8
þ
7l
5
137:8
þ 24l
2
¼ 14:889 10
10
yr
1
, we obtain
1
L
4
He
238
U
:
This formulaisvalidfor you ng ages.
Exercise
Take 1 kg of rock containing 2 ppm of uranium. How many cubic centimeters of
4
He will it
have produced in 1 billion years?
Answer
At 2 ppm 1 kg of rock represents 10
3
g 2 10
6
¼2 10
3
g of uranium, and 2 10
3
gof
uranium corresponds to
2 10
3
238
¼ 8:4 10
6
moles. Using the approximate formula:
4
He ¼ L
t
U ¼ 14:89 10
10
10
9
8:4 10
6
4
He 125 10
7
moles:
If 1 mole of
4
He corresponds to 22.4 liters at standard temperature and pressure, the amount
of
4
He produced in 1 billion years is 0.28 cm
3
.
A LITTLE HISTORY
The beginnings of radioactive dating
Rutherford performed the first radioactive age determination in 1906. He calculated the
amount of helium produced by uranium and radium per year and per gram of uranium and
found 5.2 10
8
cm
3
yr
1
g
1
of uranium.
Ramsay and Soddy had measured the helium content (long confused with nitrogen
because it is inert like the noble gases) in a uranium ore known as fergusonite. The
fergusonite contained 7% uranium and 1.81 cm
3
of helium per gram. Rutherford calculated
an age of 500 million years. The following year he found uranium ores more than 1 billion
years old! At that time Lord Kelvin was claiming the Earth was less than 100 million years old!
(See previous chapter.)
2.3.5 The fission track method
Fission reactions emit heavy atoms. The atoms ejected by ¢ssion create £aws in crystals.
Such defects ^ knownastracks ^ canbeshownup on mineralsurfaces throughacid etchi ng
41 Radioactive chains

which preferentiallyattacks damaged areas, those where atoms havebeen displaced (Price
and Walker,1962) (Figure2.4).
Thenumberof¢ssiontracks iswritten:
F
s
¼
l
fission
l
238
Uðe
l
t
1Þ
l
fission
¼ 7 10
17
yr
1
t
1=2
¼ 9:9 10
15
yr
1
:
In practice, a thin section is cut and the number of surface tracks (and not in a volume) is
measured.Thevisibleproportion
s
is calculated:
s
¼ q
l
fission
l
238
Uðe
l
t
1Þ
where qis ageometric factorforswitching from a surface toavolume.
To measure
238
U, we take advantage of knowledge that
238
U/
235
U ¼137.8 and measure
235
Ubycausinginduced ¢ssion (by placing the ore under study in a reactor), which pro-
duces tracks that are revealed and counted.We then have the number of tracks before (
s
)
and after (
i
) irradiation.Thegeometric factordisappears:
s
i
¼
l
fission
l
137:8
G
ðe
l
t
1Þ:
We calibratethe £uxofneutrons inducing
235
U¢ssionandthe e¡ectivecross-section.
2
By using a standard sample whose uranium content is kn own an d which is irradiated atthe
same time as the study sample and by counting the ¢ssion tracks p roduced, we can then
calculatet.
Ionization Electrostatic
displacement
Relaxation and
elastic deformation
Figure 2.4 Creation of fission tracks in an insulating material.
2
The effective cross-section is the probability of a reaction occurring, that is, here, the probability of
producing a fission with a given flux of neutrons. The characteristics of this will be seen in Chapter 4.
42 The principles of radioactive dating
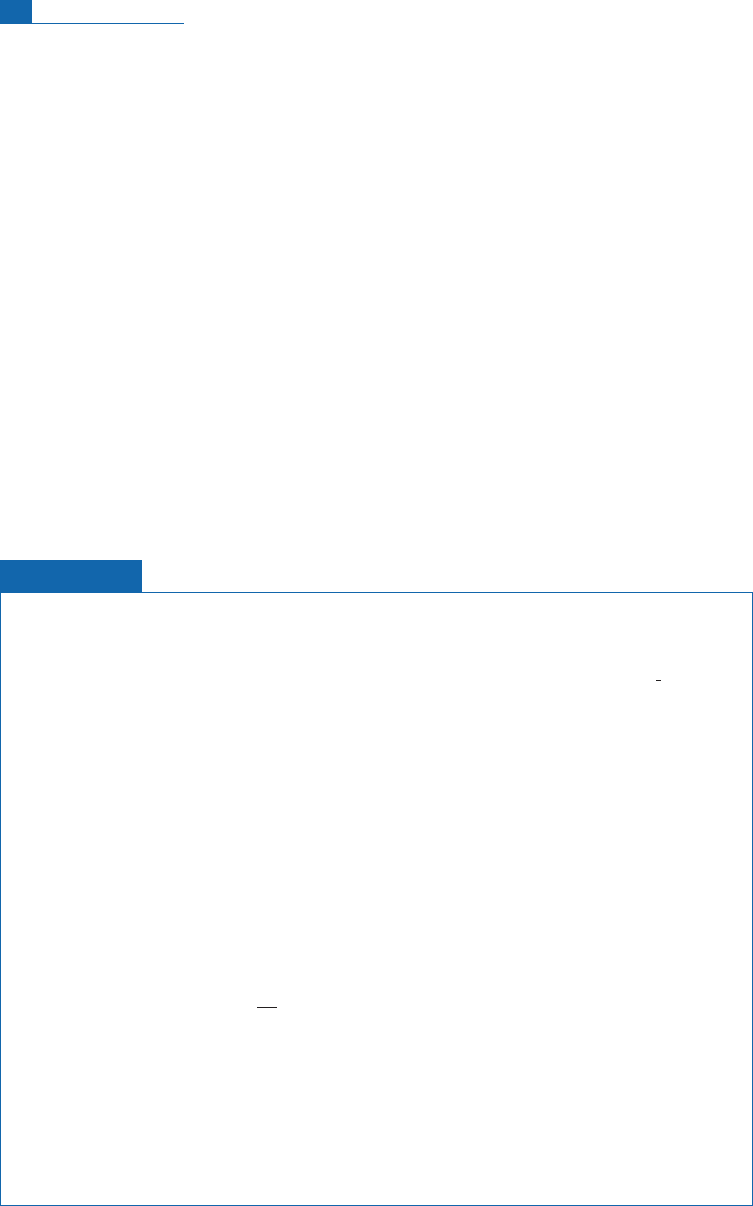
2.3.6 Isolation of a part of the chain and dating young
geological periods
The various radioactive isotopes of the radioactive chain belong to di¡erent chemical ele-
mentssothat,undercertaingeologicalconditions,oneortwoisotopesinthechainfraction-
ate chemically and become isolate d, thereby breaking the secular equilibrium. Once
isolated they create a new partial chain in turn. Two straightforward speci¢c cases are of
practicalimportance.
T he radioactive isotope becomes isolated on its own
First, when a radioactive isotope in the chain (but with a long enough period) becomes iso-
lated on its own, it gives rise to a partial chain, but being isolated fro m the parent it dec ays
accordingtothe law:
N
i
ðtÞ¼N
i
ð0Þe
lt
where N
i
(0) is the number of nuclides ofth e intermediate element at time t ¼0. Ifthis num-
ber can be estimated, the decay scheme can be used as a chronometer. This is equivalent,
then, to datingby theparentisotope.
EXAMPLE
The ionium method and the rate of sedimentation
Thorium is virtually insoluble in sea water. Thus
230
Th (still known as ionium from the
terminology of the pioneers of radioactivity), a decay product of
234
U with
T
1
2
¼ 75 ka,
precipitates on the sea floor, is incorporated in the sediment and so gradually buried. There,
now isolated, it decays.
At any depth
x
of sediment from the surface (Figure 2.5) we can write:
230
Thð
x
Þ¼
230
Thð0Þe
l
230
t
where
230
Th(0) is the surface content which is assumed constant over time. If the sedimenta-
tion rate is constant, time can be replaced by the ratio
t
¼
x
/
V
s
where
V
s
is the sedimentation
rate and
x
the length (depth):
230
Thð
x
Þ¼
230
Thð0Þexp
x
=
V
s
½
l
ðÞ
or in logarithms:
lnð
230
ThðxÞÞ ¼ ln
230
Thð0Þ
x
V
s
l:
The slope of the curve (ln
230
Th,
x
) gives a direct measure of sedimentation rate and the
ordinate at the origin gives
230
Th(0). (Note its order of magnitude of a millimeter per
thousand years.)
This method only works, of course, if it is assumed that
230
Th(0), that is the thorium content
at the sediment surface, is constant and if the sediment has not been disturbed by chemical,
physical, or biological phenomena.
43 Radioactive chains
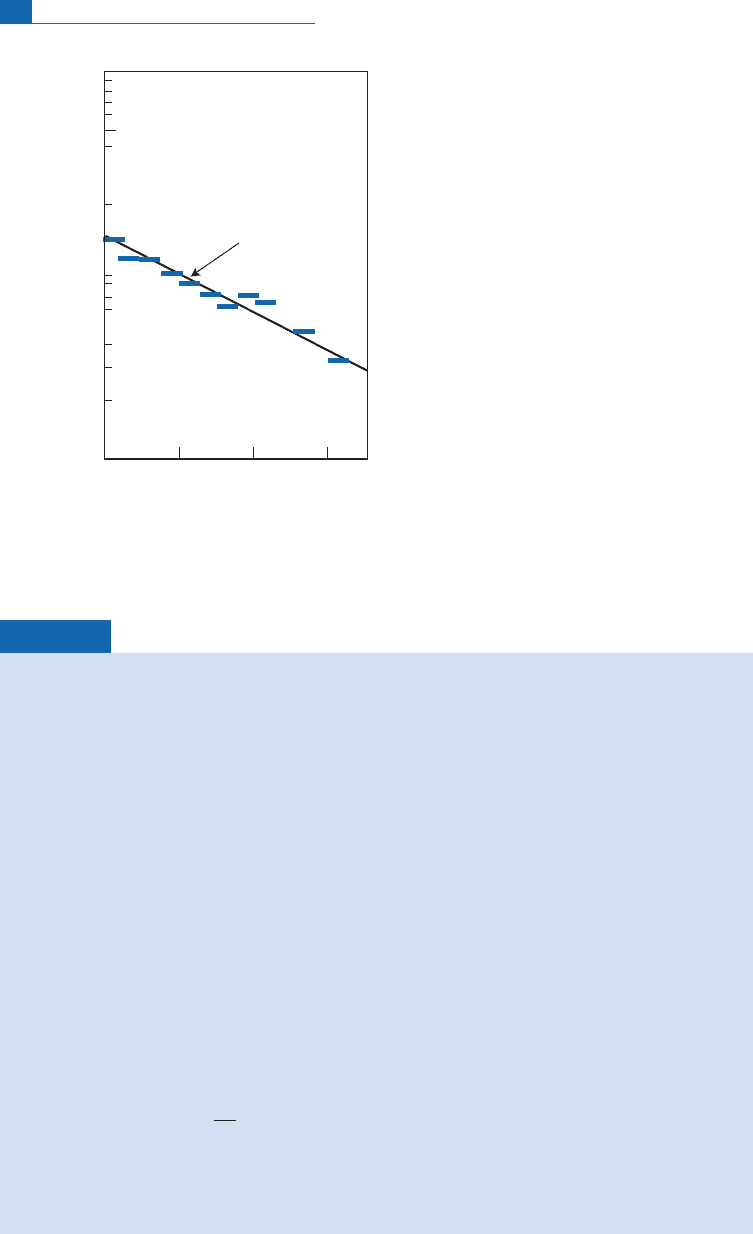
Exercise
The lead isotope
210
Pb (as a member of the radioactive chain) is radioactive with a decay
constant
l
¼3.11 10
2
yr
1
. This natural radioactive lead is incorporated into ice deposited
in Greenland by forming successive layers of ice which can be studied like sedimentary strata.
The activity of
210
Pb is measured at four levels in disintegrations per hour per kilogram of ice
(dph kg
1
). Table 2.2 shows the results.
Calculate the sedimentation rate of the ice. Assuming a constant rate and a compaction
factor of 5, how thick will the glacier be in 5000 years? Calculate the
210
Pb content of fresh ice.
Answer
The dating equation is written noting activity by square brackets:
210
Pb
¼
210
Pb
e
l
t
:
If the rate of deposition is
V
and height
h
, we have
t
¼
h
/
V
.
The equation becomes:
210
Pb
¼
210
Pb
exp l
h
=
V
ðÞ
or
ln
210
Pb
¼ ln
210
Pb
0
l
h
V
:
If the
210
Pb content has remained constant over time, the data points must be aligned in a (ln
[activity,
h
]) plot. The slope is therefore l=
V
. The data points are plotted on the graph and
the slope determined. This gives
V
¼45 cm yr
1
.
Depth (cm)
50
10
5
1
02040
230
Th
ex
(dpm g
–1
)
60
100
4.3
mm
ka
–1
Figure 2.5 Decreasing
230
Th content in a core from the sea floor and determination of the
sedimentation rate. Th
ex
is the excess thorium compared with the equilibrium value counted in
disintegrations per minute per gram (dpm g
1
).
44 The principles of radioactive dating

In 5000 years’ time, allowing for compaction, there will be 5000 45=5 ¼ 450 m of ice.
The
210
Pb content is calculated: surface activity is 75 dph kg
1
ice.
l
210
N
210
¼ 75 dph kg
1
hence
N
210
¼
75
3:1110
2
8760ðÞ
1
¼
75
3:5 10
7
¼ 21:4 10
7
atoms of
210
Pb nuclides
per kilogram of ice;
8760 being the number of hours in a year. As a mass that gives:
21:4 10
7
210
6:023 10
23
¼ 7:5 10
14
kg of ice;
where Avogadro’s number is in the denominator and the previous atomic mass of
210
Pb in the
numerator.
The
210
Pb content is 7.5 10
17
, which is very little! This shows the incredible sensitivity of
radioactive methods because
210
Pb in Greenland’s glaciers really can be measured and used
for estimating the rate of sedimentation of ice.
Th e parent isotope is isolated an d enge nder s it s daughte r s
Thisiswhathappenswithuranium;forexample, when certain solidphasesl ike calciumcar-
bonate are precipitated uranium is entrained with calcium and then isolated. For the ¢rst
radioactiveproductofanynotablehalf-life, wethen have:
l
23
N
23
ðtÞ¼l
1
UðtÞ e
l
1
t
e
l
23
t
where N
2^3
is the number of nuclides in the th ird intermediate product in the chain.Why?
Because the chain includes very short-lived radioactive products such as thorium-234
(
234
Th) or protactinium-234 (
234
Pa) which reach equilibrium very quickly. Thus,
238
U
decays to
234
Th, an element whose lifespan is 24 days, then
234
Pa, whose lifespan is 1.18
minutes: it canbe considered, then, that
238
Udirectlygives
234
Uwhose half-life is 2.48 10
5
yearsforal l typesofusualsamples (corals,speleothems, travertines, etc.).
Such a method is applied, for example, to se condary mineralizations of uranium. The
soluble uranium migrates and is deposited further away, leavi ng the insoluble thorium
whereitis. Itthen‘‘resumes’’its decaygiving
230
Th andwe canwrite:
l
230
230
Th ¼ l
230
238
Ue
l
238
t
e
l
230
t
:
Thereforetheageofmigration canbe determinedbymeasur ing
230
Thand
238
U.
Table 2.2 Activity of
210
Pb with depth
Depth
0m 1m20 cm 1.50 m 40 cm 2.50m 50 cm
dph k g
1
75 32 5245103
45 Radioactive chains
Exercise
Uranium-238 (
238
U) decays to
234
U which is itself radioactive with
l
¼2.794 10
6
yr
1
.
Sea water is not in secular equilibrium relative to uranium isotopes as
234
U weathers better
than
238
U from rocks of the continental crust and is enriched in the rivers flowing into the
ocean. In activity, noted in square brackets [ ],
234
U=
238
U½
seawater
¼ 1:15. When limestone
forms from sea water, it is isotopically balanced with the sea water and so takes the value 1.15.
A fossil mollusk has been found in a Quaternary beach formation and its activity ratio measured
as
234
U=
238
U
½
¼ 1:05. Work out the dating equation. What is the age of the mollusk?
Answer
The dating equation is:
234
U
238
U
¼
234
U
238
U
0
e
l
t
þ 1 e
l
234
t
because l
234
l
238
and l
238
t
0if
t
> 10
6
years.
This gives:
T
¼
1
l
ln
234
U
238
U
hi
0
1
234
U
238
U
1
0
@
1
A
and
T
¼393 000 years.
2.3.7 General equation and equilibration time
Weshall doan exercisetohelp underst andequilibrationtimeandbythe sametokengivethe
theoretical an swer to theprevious exercise.
Exercise
Establish the general equation for evolution of an isotope in a radioactive chain and where the
parent has a longer half-life and the daughter a markedly shorter half-life. We shall take the
example of
234
U !
230
Th decay to get our ideas straight.
Answer
This is a review exercise for mathematics on integrating a linear differential equation with
constant coefficients:
d
234
U
d
t
¼ l
238
238
U l
234
234
U
d
230
Th
d
t
¼ l
234
234
U l
230
230
Th
with
238
U being considered constant.
Integrating the two previous equations in succession for the example in question gives:
l
234
234
U ¼ l
234
234
U
0
e
l
234
t
þ l
238
238
U1 e
l
234
t
l
230
230
Th ¼ l
230
230
Th
0
e
l
230
t
þ l
234
234
Ue
l
234
t
e
l
230
t
:
These two equations can be written with the activity notation in square brackets:
lN
¼[
N
],
which simplifies notation and means the
l
constants can be dispensed with. This gives:
46 The principles of radioactive dating
