All?gre Claude J. Isotope Geology
Подождите немного. Документ загружается.

With
T
¼4 10
9
years, the result for the mantle is: 8.68 10
16
þ10.56 10
16
þ6. 59 10
16
þ
18.4 10
16
¼44.23 10
16
cal g
1
s
1
¼1.84 10
14
Wg
1
s
1
.
For granite of the continental crust: 4.96 10
14
þ6.03 10
14
þ3.76 10
14
þ8.537 10
14
¼
23.28 10
14
cal g
1
s
1
or 97.3 10
14
Wg
1
s
1
.
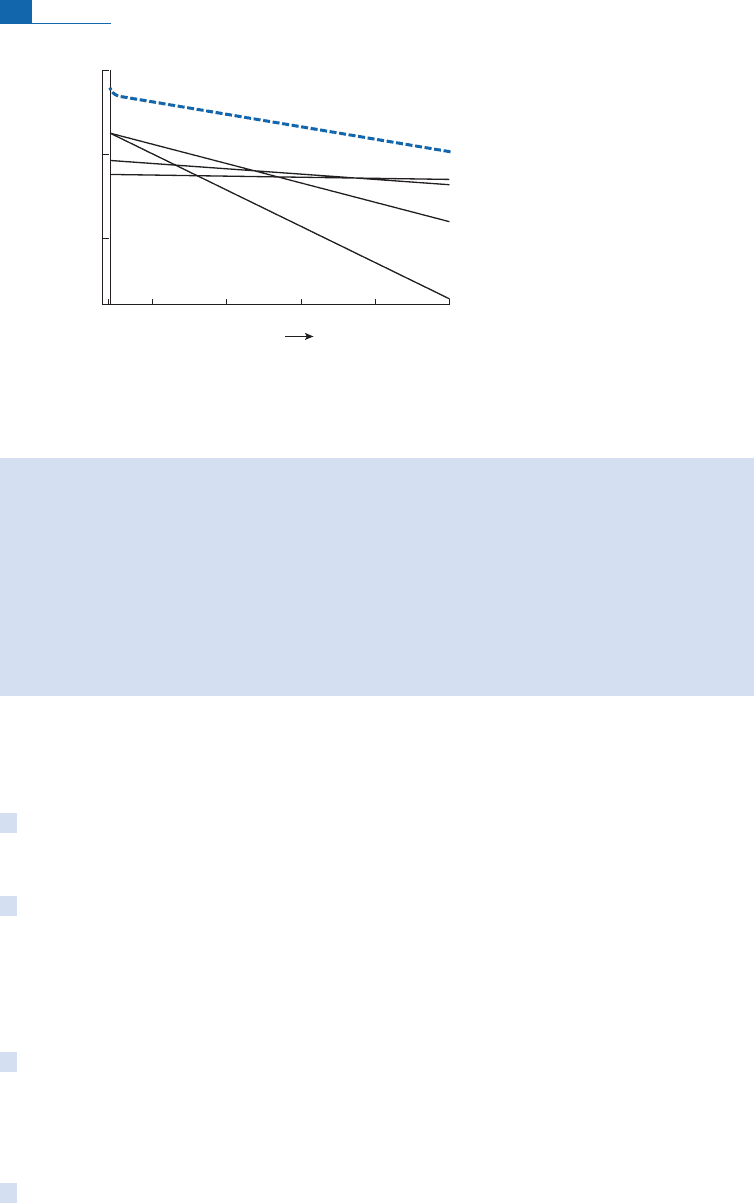
Notice that, at the present time, radioactive heat is supplied above all by the disintegration
of
238
U and to a lesser extent
40
K and
232
Th. Four billion years ago heat was supplied mainly by
40
K and
235
U (Figure 1.8). It will be observed, above all, that 4 billion years ago the mantle
produced 3.5 times as much heat as it does today. So it may be thought that the Earth was 3.5
times more ‘‘active’’ than today.
Problems
1 Which molecules of simple hydrocarbons may interfere after ionization with the masses of
oxygen
16
O,
17
O, and
18
O when measured with a mass spectrometer? How can we make sure
they are absent?
2 The lithium content of a rock is to be measured. A sample of 0.1 g of rock is collected. It is
dissolved and 2 cm
3
of lithium spike added with a lithium concentration of 5 10
3
moles per
liter and whose isotope composition is
6
Li/
7
Li ¼100. The isotope composition of the mixture is
measured as
6
Li/
7
Li ¼10.
Given that the isotopic composition of natural lithium is
6
Li/
7
Li ¼0.081, what is the total
lithium content of the rock?
3 A sample contains 1 mg of strontium. What must be the maximum acceptable chemical blank,
that is, the quantity with which the sample is accidentally contaminated, if precision of
measurement with the mass spectrometer of
87
Sr/
86
Sr 0.7030 bears on 0.0001?
The
87
Sr/
86
Sr ratio of the blank is 0.7090. What must the blank be if precision is 10 times
better?
4 We are to construct a mass spectrometer for separating
87
Rb from
87
Sr. What should its
radius be?
1000 02000300040004600
0
1
10
100
total
Time (Ma)
Heat production (10
–12
W kg
–1
)
40
K
235
U
232
Th
238
U
Figure 1.8 Heat production by various forms of radioactivity in the Earth versus geological age.
27 Problems

5 Suppose that 1 mg of purified uranium has been isolated. It contains two (main) isotopes
238
U
and
235
U in the current proportions of
238
U/
235
U ¼137.8. What was the total activity of this
milligram of uranium 4.5 billion years ago and what is its activity today if
l
238
¼
0.155 125 10
9
yr
1
and
l
235
¼0.9875 10
9
yr
1
?
6 The Urey ratio is the ratio of heat from radioactivity to total heat which includes heat from the
accretion of the Earth and the formation of its core. The average heat flow measured at the
Earth’s surface is 4.2 10
13
W, which is 42 terawatts.
(i) In a first hypothesis the mantle composition is assumed to be uniform, with:
U ¼21 ppb (Th/U)
mass
¼4 and K ¼210 ppm.
Calculate the Urey ratio today.
(ii) In a second hypothesis, it is assumed that the entire mantle is similar to the upper mantle.
The upper mantle has a uranium content of 5 ppb, and the Th/U ratio is 2. Calculate the
Urey ratio.
28 Isotopes and radioactivity
CHAPTER TWO
The principles of radioactive dating
It can never be repeated enough that radioactive dating was th e greatest revolution in the
geological sciences. Geology is an historical science which cannot readily be practised
withoutaprecisewayofmeasuringtime.Itis safetosaythatno moderndiscover yingeology
could havebeen made without radioactive dating: reversalsofthe magnetic ¢eld, platetec -
tonics, the puzzle ofthe extinction ofthe dinosaurs, lunar exploration, the evolution oflife,
humanancestry, notto mentiontheageofthe Earthorofthe Universe!
The ages i nvolved in the earth sciences are very varied.Th ey are measured in years (yr),
thousands ofyears(ka), millionsofyears(Ma), andbillions ofyears(Ga). Geological clocks
mustthe reforebevariedtoo, with mean livesranging from ayear toabillionyears.
2.1 Dating by parent isotopes
Imagine we have a radioactive isotope R and N
R
is the number of atoms of this isotope.
Supposethatgeological circumstances (crystallization ofarockor mineral, say) enclos e an
initial quantity of R, i.e., the number ofatoms of R at time zero, written N
R
ð0Þ, in a‘‘box. ’’ If
thebox hasremained closedfromwhen it¢rstformeduntil today, the numberofatomsof R
remaining is N
R
ðtÞ¼N
R
ð0Þe
lt
,wheret is the time elapsed since the boxwas closed. Ifwe
know the quantity N
R
ð0Þand the decay constant l, by measuring N
R
ðtÞ we can calculate
th e age t at whichthebox closedby using the radioactivity formula‘‘upside down’’:
t ¼
1
l
ln
N
R
ð0Þ
N
R
ðtÞ
:
Methods where the initial quantities of radioactive isotopes are well enough known are
above all those where the radioactive isotope is produced by irradiation by cosmic rays.
This isthe case ofcarbon-14 (
14
C) and beryllium-10 (
10
Be).
Exercise
The half-life of
14
C is 5730 years. The
14
C content of the atmosphere is 13.2 disintegrations per
minute and per gram (dpm g
1
) of carbon (initial activity
A
0
). We wish to date an Egyptian
artefact dating from approximately 2000 BC. What is the approximate activity (
A
) of this
artefact? If our method can measure 1 dpm, what mass of the (probably precious) sample will
have to be destroyed?

Answer
If the half-life
T
¼5730 years, the decay constant is l ¼ ln2=T ¼ 1:209 10
4
yr
1
. 2000 BC
corresponds to a time 4000 years before the present, therefore since
A
0
¼13.2 dpm g
1
and
A
¼
A
0
e
lt
,
A
¼7 dpm g
1
.
Making the measurement means using at least 1/7 g, or 142 mg of the sample.
As seen in the examples, the abundance of a radioactive isotope is estimated relative to a
reference. For
14
C we use total carbon. The dating formula is therefore:
t
¼
1
l
ln
ð
14
C=CÞ
0
ð
14
C=CÞ
t
where (
14
C) and (C) represent the concentrations of
14
C and total carbon, respectively.
In other cases, a neighboring stable isotope that is not subject to radioactive decay is taken
as the reference. So for
14
C, we use stable
13
C and we write:
t
¼
1
l
ln
ð
14
C=
13
CÞ
0
ð
14
C=
13
CÞ
t
"#
:
This formulation has the advantage of bringing out isotopic ratios, that is, the ratios mea-
sured directly by mass spectrometry.
2.2 Dating by parent–daughter isotopes
2.2.1 Principle and general equations
The di⁄culty with datingbytheparentisotopeisofcourseknowing N
R
ð0Þ,thatis,knowing
exactlyhowmanyradioactiveatomsweretrappedintheboxatthebeginning.This di⁄culty
is overcome by involving the stable daughter isotope produced by the disintegration noted
(D) (the asterisk being a reminder ofthe radioactive origin of the isotope R*).The parent^
daughter relation iswritten:
ðRÞ
!ðDÞ
l
:
Fromthe Curie^Rutherford^Soddylaw, we canwrite:
dN
R
ðtÞ
dt
¼l N
R
ðtÞ
dN
D
ðtÞ
dt
¼
dN
R
ðtÞ
dt
¼ l N
R
ðtÞ:
Integrating the ¢rst equ ation yields the decay l aw, N
R
ðtÞ¼N
R
ð0Þ e
lt
.Thesecondis
therefore written:
dN
D
ðtÞ
dt
¼ l N
R
ð0Þe
lt
;
30 The principles of radioactive dating
which integrates to:
N
D
ðtÞ¼N
R
ð0Þe
lt
þ C:
The integration constant C is determined by writing in t ¼0, N
D
¼N
D
(0), hence:
C ¼ N
D
ð0ÞþN
R
ð0Þ.Thisgives:
N
D
ðtÞ¼N
D
ð0ÞþN
R
ð0Þð1 e
lt
Þ:
But th is expression leaves the troublesome unknown N
R
ð0Þ. This is advantageously
replaced by:
N
R
ð0Þ¼N
R
ðtÞ e
lt
:
Thisgives:
N
D
ðtÞ¼N
D
ð0ÞþN
R
ðtÞðe
lt
1Þ:
If the box remains closed for both the radioactive isotope and the radiogenic isotope, by
measuring the present values N
D
(t)andN
R
(t), we can calculate t, providedwe know N
D
(0).
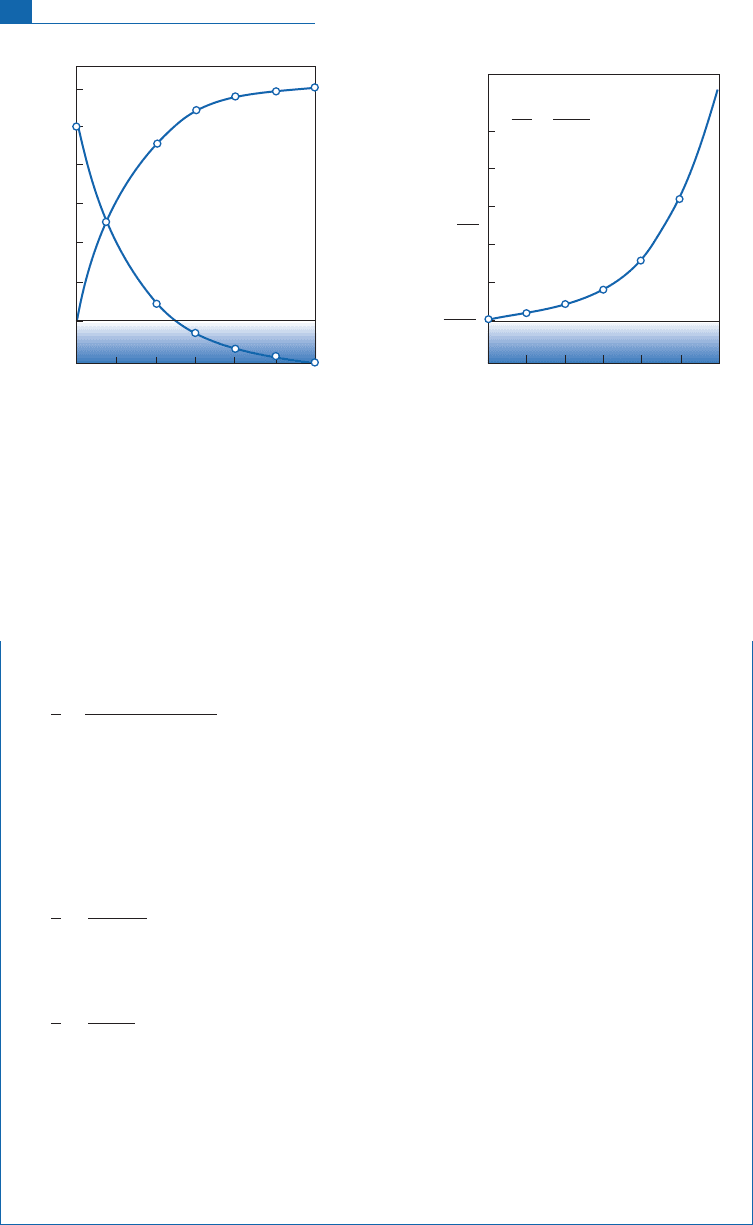
This canbe plotted as (N
D
(t), t).The slopeofthe curveateachpoint equals:
dN
D
ðtÞ
dt
¼
dN
R
ðtÞ
dt
¼ lN
R
ðtÞ¼lN
R
ð0Þe
lt
:
It therefore equals lN
R
(t), at l times the parentisotope content. Asthis contentdecays con-
stantly, theslope ofthetangentdoeslikewise, andthe curveis concavedownwards.To calcu-
latean age, wewritethe datingequation:
t ¼
1
l
ln
N
D
ðtÞN
D
ð0Þ
N
R
ðtÞ
þ 1
:
Thevalues of N
D
(t)andN
R
ðtÞcanbe measured, butt can onlybe calculated if N
D
(0) canbe
estimated or ignored and, of course, ifwe know the decay constant l. Figure 2. 1illustrates
all these points.
EXAMPLE
Rubidium–strontium dating
Let us take rubidium–strontium dating by way of an example. As seen,
87
Rb decays to
87
Sr with a decay constant
l
¼1.42 10
11
yr
1
. The parent–daughter dating equation is
written:
t
¼
1
l
Rb
ln
87
Srð
t
Þ
87
Srð0Þ
87
Rbð
t
Þ
þ 1
;
where
87
Sr(0) is the quantity of
87
Sr present at time
t
¼0, and
87
Rb(
t
) and
87
Sr(
t
) the quantities
of
87
Rb and
87
Sr present at time
t
. (The term
quantity
must be understood here as the number
of atoms or atom–grams.) Notice that time can be reversed and the present time considered
as the starting point, which is more practical. The initial time is then in the past, age (
t
) such
that
T
¼
t
.
31 Dating by parent–daughter isotopes
The equation is then written:
T
¼
1
l
ln
87
SrðpÞ
87
Srð
T
Þ
87
RbðpÞ
þ 1
;
where
87
Sr(p) and
87
Rb(p) relate to the present-day values (p ¼present), and
87
Sr(
T
) relates to
the initial values at time (
T
). When dealing with minerals that are very rich in Rb such as
biotite and muscovite, the initial
87
Sr is negligible relative to the
87
Sr produced by decay of
87
Rb. For such systems, which are said to be radiogenically rich (or just rich for short), the
dating formula is written:
T
¼
1
l
ln
87
SrðpÞ
87
RbðpÞ
þ 1
:
This formula can be extended to rich systems in general:
T
¼
1
l
ln
N
D
ðpÞ
N
R
ðpÞ
þ 1
:
As seen, if
l
is known, the age can be calculated directly from measurements of the present-
day abundances of
N
D
(p) and
N
R
(p). The only assumption made, but which is crucial, is
that the box to be dated, that is, the mineral or rock, has remained closed ever since the
time it formed and that closure was short compared with the age to be measured. This
is indeed the case when a mineral crystallizes or a magma solidifies as with volcanic or
plutonic rock.
N
R(0)
N
D(0)
N*
R
Time graduated in periods
N*
R
N
D
N
D(0)
24 60
N*
R
N
D
=
0
Time graduated in periods
Number of atoms of R or D
24 6
N
R(0)
N
D
= N
D(0)
+ N
R(0)
(1–e
–
λt
)
N*
R
= N
R(0)
e
–
λt
N
D(
0)
+ (e
λ
t
–1)
Figure 2.1 Left: the decrease in the radioactive isotope and the increase in the radiogenic isotope. Right:
the increase in the radiogenic/radioactive isotope ratio.
N
*
R
number of atoms of the radioactive isotope (R)
N
D
number of atoms of the radiogenic isotope (D)
N
R
(0) number of atoms of R at time
t
¼0
N
D
(0) number of atoms of D at time
t
¼0
l
radioactive decay constant
32 The principles of radioactive dating

Remark
Where
l
is very small relative to the age, the exponential can be approximated by e
lt
1 þ
l
. This
is the case for the constant
l
of rubidium (
l
¼1.42 10
11
yr
1
) and for many others. The dating
formula is then:
T
1
l
87
SrðpÞ
87
RbðpÞ
:
Exercise
Suppose we have a specimen of biotite from Que
´
rigut granite (Pyre
´
ne
´
es Orientales, France)
whose age is to be determined from
87
Rb !
87
Sr decay. We measure the total content of
Rb ¼500 ppm and of Sr ¼0.6 ppm. Knowing that Rb is composed of
87
Rb and
85
Rb in the
proportions
85
Rb/
87
Rb ¼2.5, that the Sr is ‘‘pure’’ radiogenic
87
Sr, and that the decay constant
is
l
¼1.42 10
11
yr
1
, calculate the age of the biotite in Que
´
rigut granite.
Answer
The Rb content is written Rb
total
¼
85
Rb þ
87
Rb ¼(2.5 þ1)
87
Rb, therefore:
87
Rb ¼
Rb
total
3:5
142:8 ppm:
The
87
Sr content is 0.6 ppm. (There is no need to come back to atom–grams since
87
Rb and
87
Sr
have virtually the same atomic mass.) We can therefore write directly:
T
1
1:42 10
11
0:6
142:8
298 10
6
yr ¼ 298 million years:
If we had not adopted the linear approximation, we would have obtained:
T
1
1:42 10
11
ln
0:6
142:8
þ 1
297:36 10
6
yr:
As can be seen, the linear approximation is valid for the
87
Rb/
87
Sr system when the age is not
too great.
2.2.2 Special case of multiple decay
Letus considerthe caseofthelong-period
40
K radioactiveisotope which decays in two dif-
ferent ways, either by electron capture giving
40
Arorby
decaygiving
40
Ca, eachwith its
own decayconstantl
e
and l
, respectively:
e
–
cap
40
Ar
40
K
β
–
40
Ca
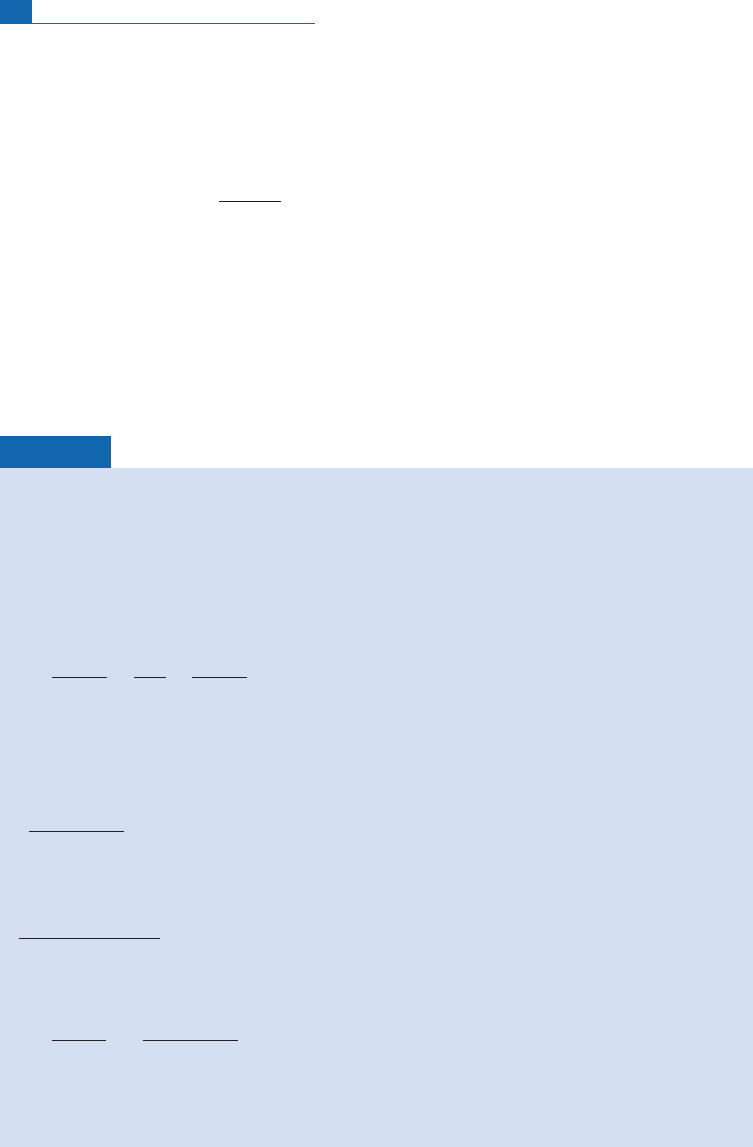
Whatis the dating formula? Letusgetbacktobasics:
d=dt½N
K
ðtÞ ¼ ðl
e
þ l
ÞN
K
ðtÞ
33 Dating by parent–daughter isotopes
where N
K
isthe numbe rof
40
K nuclides andN
Ar
the numberof
40
Ar nuclides.
d=dt½N
Ar
ðtÞ ¼ l
e
N
K
ð0Þe
ðl
e
þl
Þt
:
Integrating using theusual notationwith
40
Kand
40
Ar
0
gives:
40
ArðtÞ¼
40
Ar
0
þ
40
KðtÞ
l
e
l
e
þ l
e
ðl
e
þl
Þt
1
withl
e
¼0.581 10
10
yr
1
and l
¼4.962 10
10
yr
1
.
l ¼ l
e
þ l
¼ 5:543 10
10
yr
1
:
The initial
40
Ar is usually negligible.We are therefore generally dealing with r ich systems
but not withvery young systems where whatis known as excess argon raises di⁄culties for
accurateage calculations.
Exercise
We analyze 1 g of biotite extracted from Que
´
rigut granite by the
40
K–
40
Ar method. The biotite
contains 4% K and
40
K ¼1.16 10
4
of K
total
.
The quantity of argon measured at standard temperature and pressure is 4.598 10
5
cm
3
of
40
Ar. What is the radiometric age of this biotite?
Answer
The dating formula to calculate the age is written:
T
¼
1
l
e
þ l
b
ln
40
Ar
40
K
l
e
þ l
b
l
e
þ 1
:
We must therefore calculate the
40
Ar/
40
K ratio in atoms.
As there are 22 400 cm
3
in a mole at standard temperature and pressure, the value of
40
Ar
in number of moles is:
4:598 10
5
22 400
¼ 2:053 10
9
moles of
40
Ar:
The value of
40
K is:
0:04 1:16 10
4
40
¼ 1:16 10
7
moles of
40
K:
Therefore:
T
¼
10
9
0:5543
ln
2:053 10
9
1:16 10
7
0:1048
þ 1
¼ 280 million years:
Comparing this with the result obtained previously using the
87
Rb–
87
Sr method, we find
about the same age but slightly younger.
There is another branched decay used in geology, that of
138
La which decays into
138
Ba
and
138
Ce(Naka
¨
ı etal.,1986).
34 The principles of radioactive dating
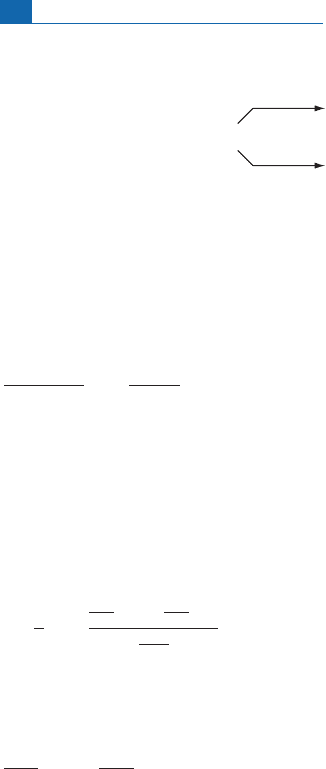
e
–
cap
138
Ba
λ
Cap e
– = 4.44
.
10
–12
yr
–1
λ
β
= 2.29
.
10
–12
yr
–1
138
La
β
–
138
Ce
There is also a case where even more intense multiple decay occurs, in the spontaneous
¢ssion of
238
U, which yields a whole series of isotopes. Luckily, ¢ssion decay is negligible
comparedwith decay. In the dating equationwe can consider the constant l
alone asthe
decay constant, but allowance mustbe made, of course, for the ¢ssion products. For exam-
ple, for
136
Xewewritethe dating equation:
136
Xe
radio
238
U
¼ Y
l
fission
l
ðe
l
t
1Þ
whereY is th e yieldof
136
Xeproducedduring ¢ssion 0.067 3, with l
¢ssion
¼8.47 10
17
yr
1
and l
¼1.55 10
10
yr
1
.
Wesawwhen lookingatdatingbyparentisotopesthatitwasconvenienttoexpressthe dat-
ing equation by introducing isotope ratios rather than moles of radioactive and radiogenic
isotopes.Thisis callednormalization.Thusfor
87
Rb^
87
Sr weuseastablestrontium isotope,
86
Sr.The dating equation isthenwritten:
t ¼
1
l
ln
87
Sr
86
Sr
ðtÞ
87
Sr
86
Sr
ð0Þ
87
Rb
86
Sr
!
þ1
"#
:
This is the form in which dating equations will be expressed fro m now on. A system will be
richwhen:
87
Sr
86
Sr
ð0Þ
87
Sr
86
Sr
ðtÞ:
2.2.3 Main geochronometers based on simple
parent–daughter ratios
Rubidium ^ Strontiu m
87
Rb
87
Sr ; l ¼1.42 10
11
yr
1
.The normalization isotope is
86
Sr. Developed in its modern formbyAldrichet al.(1953).
Potassium ^Argon
40
K^
40
Ar, with the constants alreadygiven.The reference isotope is
36
Ar.DevelopedinitsmodernformbyAldrichandNier(1948a).
Rhenium^ Osmium
187
Re
187
Os with l ¼1.5 10
11
yr
1
. The reference isotope is
186
Os and more recently
188
Os. Developed by Luck, Bi rck,andAlle
'
gre in 1980 after a
¢rstattemptbyHirtetal.in1963.
These are the three simple clocks that are commonly found as rich systems in nature.We
shallseethatother formsofdecay c anbeusedbutunder moredi⁄cultcircumstances.
Sam a ri um ^Neodymi um
147
Sm
143
Nd ; l ¼6.54 10
12
yr
1
. Normalization by
14 4
Nd.
Develop edbyLug mai rand Mart i in1977 after an at temptbyNotsu etal.in1973.
35 Dating by parent–daughter isotopes
Lutetium ^ Hafnium
176
Lu
176
Hf ; l ¼2 10
11
yr
1
. Norm alization with
177
Hf.
DevelopedbyPatchett andTa t s u m ot o (1980a,1980b).
These methods are supplemented by others related to radioactive chains which are to be
examined next, by the extinct radioactive methods covered in Section 2.4,andbythe
inducedradioactivemethods examined inChapter 4.
2.3 Radioactive chains
2.3.1 Principle and general equations
When
238
U,
235
U, a n d
232
Th decay, theygive risetothreeother radioactiveisotopeswhich,in
turn, decay into new radioactive elements, and so on.The process stops when the last iso-
topes produced are the three lead isotopes
206
Pb,
207
Pb, and
208
Pb, which are stable. It was
radioactive chains which allowed both Pierre and Mar i e Cu r ie and Rutherford and
Soddy to discover the mechanisms ofradioactivity. A radioactive chain can be represented
by writing:
ð1Þ
!ð2Þ
!ð3Þ
!ð4Þ
! ...!ðnÞ stable:
Decayinvolves and radioactivity. Alpharadioactivitygives o¡helium nuclei.Their path,
inthe (Z, N)plot,bringsthe end product into thevalleyofstability.
Figure 2.2 shows the prec ise structure ofthe three chains. Mathematically, as studied by
Bateman (1910), radioactive chains can be described by the Curie^Ruth erford^Soddy
lawswritten one after the other:
dN
1
ðtÞ
dt
¼l
1
N
1
ðtÞ
dN
4
ðtÞ
dt
¼ l
3
N
3
ðtÞl
4
N
4
ðtÞ
dN
2
ðtÞ
dt
¼ l
1
N
1
ðtÞl
2
N
2
ðtÞ
.
.
.
dN
3
ðtÞ
dt
¼ l
2
N
2
ðtÞl
3
N
3
ðtÞ
dN
n
dt
¼ lN
n1
ðtÞ
where N
i
is the number of nuclides of elements i and N
n
the number of nuclides of the ¢nal
stable isotope. Successive integration of these equation s presents no real di⁄culty and is
even agood revision exercise for integrating ¢rst-order di¡e rential equations with constant
coe⁄cients. Letus leave thatfor nowand concentrate on a fewsimple and importantlimit-
ing cases.
2.3.2 Secular equilibrium: uranium–lead methods
We suppose that, in view of the length of geological time, the radioactive chain reaches a
stationarystatewhere the contentofall the intermediate radioactive isotopes remains con-
stant(this is known assecularequilibrium):
dN
2
dt
¼
dN
3
dt
¼ ¼
dN
n1
dt
¼ 0:
36 The principles of radioactive dating
