All?gre Claude J. Isotope Geology
Подождите немного. Документ загружается.


resolving power RP ¼
M
1
M
where M
1
isthe mass.M is de¢ned as M
2
¼M
1
þM,whereM
2
isthe closest mass to M
1
thatdoes notoverlapbymorethan 50% inthe collector.
We canalso de¢ne a resolving powerat1%.
The distance xbetween twobeams inthefocal plane is written:
x ¼ K
m
m
:
Dependingonthe angle oftheincidentbeam tothe m agnet, K ¼R foranangleof incidence
of908;K ¼2R for an angle of 278.
From the formula above:
RP ¼ C
R
x
;
R being the radius of curvature and x the distance between two beams of M and
M þM.
This is just to show that when one wants to separate two masses more e⁄ciently, the
radius has to be increased and then the voltage adjusted accordingly. Suppose we want to
separate
87
Rb and
87
Sr by the di¡erence in mass of neutrons and protons alone. A‘‘mon-
ster’’ mass spectrometer would be required. However, interfe rences between two masses
can be avoided when separating isotopes of an element from contaminating molecules.
(Methane
12
CH
4
has the same mass as
16
OandbenzeneC
6
H
6
interferes at mass 78 with
krypton.)
Abundance sensitivity
Another importantcharacteristicisthe xdistance(in millimeters)betweenthebeams.We
have to come back to the slits in the collectors.The problem is easy enough to understand.
At ¢rst, the bea m is rectangular. Collisions with residual air molecules means that, when it
reaches the collectorslit, thebeam is wider andtrapezoid-shaped with long tails. Collector
slits areopen so thatthey can receive one massbut no contribution from the adjacent mass.
When the abundances oftwoadjacent isotopes areverydi¡erent, the tail of the moreabun-
dant isotope forms background noise for th e less abundant one. Measuring the less abun-
dant isotope involves reconstructing the tail of the more abundant one mathematically.
This is possible only if the tail is not too big. Narrowing the collector slit brings about a
rapid decline in sensitivity.
Abundance sensitivity is the measurement ofthe contributi on of the tai l of o ne isotope
to the signal of the neighboring isotope. It is given as a signal/noise ratio multiplied by
the mass ratios. Special instruments have been developed for measuring abundance
sensitivity in extreme cases, such as measuring
14
C close to the massively more abundant
12
C. Abundanc e sensitivity is related to resolving power but also to the quality of the
ion opti c s.
7 The mass spectrometer
Exercise
The isotopic composition of the element rubidium (Rb) is measured, giving a current
i
¼10
11
A for the mass of
87
Rb. How many ions per second is that? If the measurement
lasts 1 hour how much Rb has been used if the ionization yield is 1%?
Answer
The intensity of an electrical current is defined by
i
¼d
q
/d
t
,whered
q
is the quantity of electrical
charge and d
t
the unit of time. Electrical current is therefore the quantity of electrical charge
flowing per unit time. The ampere, the unit of electrical current, is defined as being 1 coulomb
per second, the coulomb being the unit of electrical charge. The charge of an electron is
–1.6 10
19
coulombs. The positive charge is identical but with the opposite sign. An
intensity of 10
11
amps therefore corresponds to 10
11
coulombs per second / 1.6 10
19
coulombs ¼62.5 10
6
ions per second.
If this intensity is maintained for 1 hour: 6.25 10
7
3600 ¼2.2464 10
11
ions of
87
Rb
þ
.As
the ionization is 1%, this corresponds to 2.2464 10
13
atoms of 87
Rb
placed on the emitter
filament. As
85
Rb/
87
Rb ¼2.5933, Rb
total
(in atoms) ¼
87
Rb (in atoms) (1 þ2.5933).
So a total number of 8.0719 10
13
atoms of Rb is placed on the filament. As the atomic
mass of Rb is 85.468 g, the total weight of Rb is 11 ng.
Exercise
How much rock is needed to determine the isotopic composition of Rb by measuring a sample
for 20 minutes at 10
11
A if its concentration in Rb is 10 ppm (parts per million)?
Answer
If 11 ng of Rb are needed for 1 hour, for 20 minutes we need (11 20)/60 ¼3.66 ng, that is
3.66 10
9
/10
5
¼0.36 mg of rock or mineral.
It can be seen, then, that mass spectrometry is a very sensitive technique.
1.2.3 Various developments in mass spectrometers
Mass spectrometers have come a long way since the ¢rst instruments developed by J. J.
Thomson and F. Aston.To givesome ideaofthe advances made, when Al Nier was measur-
ing lead isotopes as apostdoctoral fellow at Harvardin1939 (moreaboutthis later), heused
agalvano meter projectingabeam oflightontothewallandmeasured thepeakwith aruler!
Nowadayseverything takestheform ofa computeroutput.
Ionizat ion
The ¢rst technique was to use the element to be measured as a gaseous compound.When
b ombarded byelectrons, atoms ofthe gas lose electrons and so become ioniz ed (Nier,1935,
1938,1939). Later came the thermal-ionization technique (TIMS) (Inghram an d Chupka,
1953). In the so-called solid-source mass spectrometer, a saltofthe element is depositedon
ametal ¢lament (Ta,W, Pt).The¢lamentisheatedbytheJoule e¡ectofanincreasingelectric
cur rent. At a certain temperature, the element ionizes (generally as positive ions [Sr, Rb,
Sm, Nd, U, Pb ] but alsoas negativeion s [ Os]). Ionizationbecame afundamental character-
istic ofmass spectrometry.
8 Isotopes and radioactivity

Nowadays, asanalternative,plasmaisused foroptim al ionization in instruments named
ICP-MS.
Ion optics
Substantial e¡ort has been put into optics combin ing various geometries and assemblies.
Bai nb ri dge and Jordan (1936) used a magnetic ¢eld to turn the beam through 1808.
Mattauch and Herzo g (1934) combined electric an d magnetic ¢elds to separate ions and
focusbeams. Magnetshapesweremodi¢edtoimprovetransmission e⁄ciency.
Computerized numerical simulation has allowed tremendous advances in ion optics
des ign. All of the techniques u sed tended to maximize ionization and transmission , to
increase resolution power and abun dance sensitivity, and to minimize the high voltage
requirement and the si ze of the magnet, which areboth big factors in c ost. However, when
the ionization process created a wide dispersion in ion energies, more sophisticated ion
optics were require d to refocus the ion beam in a narrow band on the collectors. So ICP-
MS, ionprobe, and AMSinstrumentshavebecomelarge and more expensive.
Collectors areanother importantissue.Early massspectrometershadasinglecollector. By
scanningthemagnetic¢eld,theionbeampassedinsequencethroughthecollectorandaspec-
trum of ion abundance was recorded (Figure 1.1). Nowadays most mass spe ctrometers use
simultaneous ion collectionwithan arrayofcollectorssidebyside, each collectorcorrespond-
ing to a distinct mass. This se ems an obvious technique to use as it eliminates £uctuations
betweentherecordingsofonemass(peak)toanother.However,itistechnicallyextremelydi⁄-
culttoachieve,both mechanically,accuratelyinstalling severalcollectors in asmallspace,and
electronically, controlling drifting of the electronic circuits with time. These problems have
now been virtually eradicated. It is worth noting that, un like in most areas of science, all
advancessince 1980havebeenmadebyindustr ialengineersratherthanbyacademicscientists.
However, because ofelectronic‘‘noise’’and electrical instabilities, all isotopic measurements
are statistical. On each run, thousands of spectra are recorded and statistically processed.
Onlysince microcomputershavebeenavailablehave suchtechniquesbecomefeasible.
1.2.4 Preparatory chemistry and final accuracy
In most mass spectrometry techniques (except for ion probes) chemic al separation is used
before m easurement to puri fy the element whose isotopic composition is under study.
Since the elements tobe measured are present as traces, th ey have to be separated from the
majorelements wh ichwould otherwiseprevent any ionization asthe majorelementsrather
than the trace elements would give out their electrons. For example, an excess of K inhibits
any Rb ionization. Chemical separation also prevents isobaric interference between, say,
87
Rb and
87
Sror
187
Re and
187
Os.
Chemicalseparationcanbedoneingaseousform inpuri¢c ationlines,asforraregasesor
for oxygen or hydrogen measurement, or in liquid solution for most elements. The basic
technique in the latter case is the ion-exchange column as introduced by Aldrich et al.
(1953).Alltheseoperationshavetobeperformedinverycleanconditions,otherwisesample
contaminationwill ruin measurement.The greater the accuracyofmass spectrometry, the
cleaner the chemistry required. The chemistry is carried out in a clean room with special
9 The mass spectrometer

equipmentusing specially preparedultra-clean reagents,thatarefarcleaner than anycom-
mercialversions.
Whenjudging measurementreliability, investigatorshavetostatetheleveloftheirblan ks.
Theblankistheamountofthetargetelements measuredin achemi calprocess donewithout
anysample.Theblankhastobeneglig ibleor verysmallcomparedwiththeamountofmater-
ial tobe measured. Soincreases in accuracyarelinked notonly withthe imp rovementofthe
mas s spe ctrometerbut also oftheblanks.
Althoughthis isnotthe placetogive full technical details about conditions for preparing
and measuring samples, as these can only be learned in the laboratory and not from text-
b ooks,afewgeneral remarks maybemade.
Moderntechniquesallow isotope ratiostobe measuredwithadegree ofprecision of10
5
or10
6
(a few ppm!) on samples weighing justa few nanograms (10
9
g) or even a few pic o-
grams (10
12
g). For example, if a rock contains10 ppm of strontium, its isotope composi-
tion can be measured on10
9
g with a degree of pre cision of 30 ppm.Therefore just10
4
g,
that is, 0.1mg would be needed to m ake the measurement. This method can be used for
studying precious rocks, such as s amples of moon rock or meteorites, or minor or rare
minerals,that is, minerals that are di⁄culttoseparate and concentrate.What do such levels
ofprecision mean? They meanwe can readily tell apart twoisotope ratios ofstrontium, say
0.702 21and0.702 23, thatis,towithin 0.000 03, evenwherelowconcentrations areinvolved.
To achieve such precision the measurement mustbe‘‘internally calibrated.’’ When measur-
ing the abundance ratio (A
1
/A
2
) of two isotopes, the electrical current ratio (I
1
/I
2
) detected
is slightlydi¡erentfrom (A
1
/A
2
).The di¡erenceis engendered by the measurementits elf.This
istermed mass discrim ination.
6
Eitheroftwo methods isusedforcalibrating measurements.
The ¢rst is the internal standard method. If the element has three or more isotopes one
particular ratiois chosen asthe reference ratioandc orrection is made for mass discrimina-
tion. So if the abundances are A
1
, A
2
, A
3
,wetake(A
1
/A
3
) ¼R.The measurement (I
1
/I
2
)is
written R(1 þm), where m is the di¡erence in massbetween A
1
and A
3
.The fractiona-
tion coe⁄ci ent is calculatedand then appliedtothe measurementofthe ratio (A
1
/A
2
).
7
The second method is to measure a standard sample periodically and to express the
values measuredin termsofthatstandard.
The extraordinary prec ision the mass spectro meter can achieve must notbe jeopardized
byaccidental contamination when preparing samples.To this end ultra-clean preparatory
chemistry isdevelopedusing ultra-pure chemical reag ents in cleanrooms(Plate 3bottom).
1.2.5 Ionization techniques and the corresponding spectrometers
Four major ionization techniques are used depending on the characteristics of the various
chemicalelements(ioni zationpotential).
Th e r mal-io nization ma ss spe ctromet r y (T I M S)
The element to be analyzed is ¢rst puri¢ed chemically (especially to separate any isobars)
and depositedon arefractory ¢lament. Heating the ¢lamentin avacuumby the Joule e¡ect
6
Such discrimination depends on the type of mass spectrometer used. It decreases with mass for any given
type.
7
In high-precision mass spectrometry an exponential law rather than a linear one is used to correct mass
fractionation.
10 Isotopes and radioactivity

ion izes the elements, whi ch eitherlose an electronbecoming positive ions, as in the cases of
Rb
þ
,Sr
þ
,andPb
þ
, or gain an electron b ecoming negative ions as with OsO
3
and WO
3
.
Instrumental mass fractionation is of the order of1% by mass deviation for light elements
(Li)and 0.1%bymass deviation forheavyele ments(Pb, U).
Electronicbombardment
Lightelementssuchashydrogen (H), carbon (C), nitrogen (N),andoxygen (O) or raregases
are analyzed as gases (H
2
,CO
2
,N
2
,O
2
, or ato ms of He, Ne, At, or Xe) bombarded in a
vacuum by an electron beam. Positive ions are thus formed by stripping an electron from
such molecules or atoms. The ions are then accelerated and sorted magnetically as in
TIMS. Substances are prepared for analysis in gaseous form by extracting the gas from the
sample under vacuum either by fusion or by chemical reaction.The gas is then puri¢ed in
vacuum lines where other gases are captured either byadsorption orby manipulating their
liquefactiontemperatures.
In duct ivelycoupled plasma mas s spec trometry (ICPMS) inanargon plasma
The sample is ionized in an argon plasma induced by a high-frequency electrical ¢eld
(plasma torch).The high temperature of the plasma, about 10 000 K, means elements like
hafnium or thorium, which are di⁄cult to ionize by thermal emission, can be completely
ion ized. The ele ment to be analyzed is atomized and th en ionized. It is sprayed into the
plasma from a solution as a liquid aerosol. Or it may be released from a solid sample by
laserablation.Thesolid aerosolsoformed isinjected into theplasmatorch.Mass fractiona-
tion is between a twentieth of1% for a light element like boron and 1% for heavy elements.
Fractionation is corrected for by using the isotope ratios ofother similar elements as inter-
nal standards,because,atthe te mperature ofthe plasma, fractionation is duetomass alone
and is nota¡ectedbythe element’s chemical characteristics.
Ionic bombardment in secondary-ion mass spectrometry (SIMS)
The solid sample (rock, mineral) containing the chemic al element for analysis is cut,
polishe d, and put into the vacuum chamber where itis bombarded by a‘‘primary’’ beam of
ions (argon, oxygen, or c esium). This bombardment creates a very-high- te mperature
plasma at about 40 000 K in whi ch the element is atomized and ionized.The development
ofhigh resolution secondary-ion mass spectrometers (ion m icroprobe s) means in-situ iso-
tope measurements can be made on very small samples and, above all, on tinygrains.T his
is essential for studying, say, thefewgrainsof interstellar materialcontainedin meteorites.
Remark
All of the big fundamental advances in isotope geology have been the result of improved
sensitivity or precision in mass spectrometry or of improved chemical separation reducing con-
tamination (chromatographic separation using highly selective resins, use of high-purity materials
such as teflon). These techniques have recently become automated and automation will be more
systematic in the future.
1.3 Isotopy
Assaid,eachchemicalelementis de¢nedbythenumberofprotonsZ in itsatomicstructure.
It is the numberofprotons Z thatde¢nes the element’s position in the periodic table. But in
11 Isotopy
each position there are several isotopes which di¡er by the numberof neutrons N they con-
tain, that is, by their mass.These isotopes are created during nuclear processes which are
collectively referred to as nucle osy nthe sis and which have been tak ing place in the stars
throughoutthehistoryofthe Universe (see Chapter 4).
The isotopic composition of a chemical element is expressed either as a percentage or
more convenientlyas aratio. A referenceisotope is chosen relativeto whichthequantitiesof
other isotopes are expressed. Isotop e rati o s are expressed in terms of numbers of atoms
and notofmass. Forexample,tostudy variations intheisotopic composition ofthe element
strontium brought about by the radioactive decay of the isotope
87
Rb, we choose the
87
Sr/
86
Sr isotope ratio. To study the isotopic variations of lead, we consid er the
206
Pb/
204
Pb,
207
Pb/
204
Pb,and
208
Pb/
204
Pb ratios.
1.3.1 The chart of the nuclides
The isotopic composition of all the naturallyoccurring chemical elements has been deter-
mined, that is, the numberof isotopes and their proportions havebeen identi¢ed.The ¢nd-
ings havebeen plotted as a (Z, N) graph, that is, the number ofprotons against the number
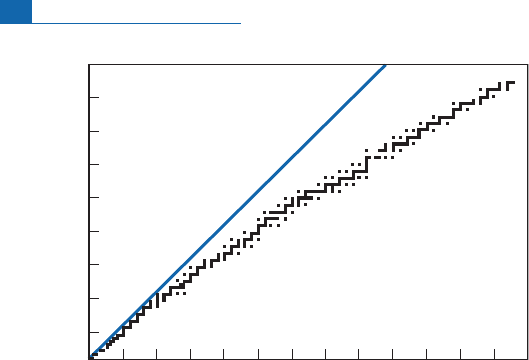
ofneutrons. Figure1.3, details ofwhich aregiven inthe Appendix, prompts a few remarks.
Firstofall,thestableisotopesfallintoaclearlyde¢ned zone,knownasthevalleyofstability
b ecause it corresponds to the minimum energy levels of nuclides. Initially this energy valley
followsthe diagonal Z ¼N.Then,afterN ¼20, the valley fallsaway from the diagonal onthe
side of a surplus of neutrons. It is as if, as Z increases, an even g reater number of neutrons is
neededtopreventthe electrical lychargedprotonsfrom repelling eachotherandbreaking the
nucleusapart.(Things areactually morecomplicatedthanthissimplisticimagesuggests!)
80
60
40
20
20 40 60 80
Neutron number (N)
Proton number (Z)
100 120
Z
=
N
Natural stable isotopes
Figure 1.3 The distribution of natural stable isotopes in the neutron–proton diagram. The diagram is
stippled because natural or artificial radioactive isotopes lie between the stable isotopes. After
N
¼20,
the zone of stable nuclei moves away from the diagonal for which the number of neutrons equals the
number of protons. For
N
> 20, the number of neutrons then exceeds the number of protons. This zone
is called the valley of stability as it corresponds to a minimum energy level of the nuclides.
12 Isotopes and radioactivity

A second remark relates to parity. Elementsfor which Z is an even numb erhave far more
isotopes than elements for which Z is an odd number. Fluorine (Z ¼9), sodium (Z ¼11),
phosphorus (Z ¼15), andscandium (Z ¼21)havejusta single isotope.
Lastly, and notl east importantly, theheaviest ele mentwithstable isotopes islead.
8
1.3.2 Isotopic homogenization and isotopic exchange
As the isotopes ofanygiven chemical element all have the same electron suite, theyall have
pretty much the same chemical properties. But in all chemical, physical, or biological pro-
cesses, isotopes ofanygiven elementbehaveslightlydi¡erently from each oth er, thus giving
rise to isotopic f ract ionatio n. Such fractionation is very weak and is apparent above all in
lightelements. Itis also exploited in isotopegeologyas shallbe seen in Chapter7.
Initially we shall ignore such fractionation, except where allowance has tobe made for it
as with
14
C or when making measurements with a mass spectrometer where, as has been
seen, correction mustbe madefor mass discrimination.This virtuallyidenticalbehaviorof
chemical isotopes entails afundamental consequenceinthetendencyfor isotopic homogen-
ization to occur.Where two or more geochemical objects (minerals within the same rock,
ro cks in solution, etc.) are in thermodynamic equilibrium, the isotope ratios ofthe chemical
elements present are generally equal. If theyare unequal initially, they exchange some atoms
until they equalize them. It is important to understand that is otopic homogen ization occurs
through isotopic exchange without chem ical homogeni zat ion. Each chemi cal component
retains its chemicalidentity, ofcourse.Thispropertyof isotopichomogenization‘‘across’’che-
mical diversityis one ofthefundamentalsof isotope geochemistry. A simplewayofobserving
this phenomenon is to put calcium carbonate powder in the presence of a solution ofcarbo-
nate in water in proportions corresponding to thermodynamic equ ilibrium. Therefore no
chemical reaction occurs. Repeat the experiment but with radioactive
14
C in solution in the
form ofcarbonate. Ifafter10 daysorsothesolidcalcium carbonateis isolated, it willbefound
to have become radioactive. It will have exchanged some of its carbon-14 with the carbonate
ofmass12and13whichwereinthesolution.
Exercise
A liter of water saturated in CaCO
3
whose Ca
2 þ
content is 1 10
2
moles per liter is put in the
presence of 1 g of CaCO
3
in solid form. The isotopic ratio of the solid CaCO
3
is
40
Ca/
42
Ca ¼151.
The isotopic ratio of the dissolved Ca
2þ
has been artificially enriched in
42
Ca such that
40
Ca/
42
Ca ¼50. What is the common isotopic composition of the calcium when isotopic
equilibrium is achieved?
Answer
40
Ca/
42
Ca ¼121.2.
As said, when two or more g eoche mical objects with di¡erent isotopic ratios are in
each other’s presence, atom exchange (whi ch occurs in all chemical reactions, including at
8
Until recently it was believed to be bismuth (Z ¼83), whose only isotope is
209
Bi. In 2003 it was
discovered to be radioactive with a half-life of 1.9 10
19
years!
13 Isotopy

equilibrium) tends to homogenize the whole in terms of isotopes.This is known as isotopic
exchan ge. Itis akineticphenomenon,depending thereforeonthetemperatureandphysical
state of the phases present. Simplifying, isotope exchange is fast at high temperatures and
slow at low temperatures like all chemical reactions which are accelerated by temperature
increase. In liqu ids an d gases, di¡us ion is fast and so isotope exchange is fast too. In solids,
di¡usionis slowandsoisotopeexchangeisslowtoo.In magmas (high-temperatureliquids),
then, both trends are compounded and isotope homogenization occurs quickly.The same
istrueofsupercritical£uids,thatis,£uidsdeepwithintheEarth’scrust.Conversely,insolids
at ordinary temperatures, exchange occurs very slowlyan d isotope heterogeneities persist.
Two important consequences follow from these two properties. The ¢rst is that a mag ma
has the sam e isotope composition asthe solid sourcefromwhich ithas issuedbyfusion, but
notthe same chemical composition.The second is that, conversely, a solid atordinary tem-
peratures retains its isotopic composition over time without becomi ng homogeneous with
itssurroundings.Thisiswhyro cks are reli ab l e isotopere c ords.Thispropertyisadi rectcon-
sequenceofthe di¡usionproperties ofnaturalisotopes in liquids andsolids.
The theory of di¡usion, that is, the spontaneous motion of atoms in£uenced by di¡er-
encesi n con centration, provides anapproximatebut adequateformula:
x
ffiffiffiffiffiffi
Dt
p
wherex isthe distancetraveledbythe element,t istime inseconds, andD the di¡usion coef-
¢cient(cm
2
s
1
).
Exercise
In a liquid silicate at 1200 K the diffusion coefficient for elements like Rb, Sr, or K is
D
¼10
6
cm
2
s
1
. In solid silicates heated to 1200 K,
D
¼10
11
cm
2
s
1
.
How long does it take for two adjacent domains of 1 cm diameter to become homogeneous:
(1) within a silicate magma?
(2) between a silicate magma and a solid, which occurs during partial melting when 10% of
the magma coexists with the residual solid?
Answer
(1) In a silicate magma if
D
¼10
6
cm
2
s
1
,
t
x
2
/
D
¼10
6
s, or about 11 days.
If it takes 11 days for the magma to homogenize on a scale of 1 cm, on a 1-km scale
(¼10
5
cm), it will take
t
x
2
/
D
¼10
10
¼10
16
s, or close to 3 10
8
years, given that
1 year 3 10
7
s.
In fact, homogenization at this scale would not occur by diffusion but by advection or
convection, that is, a general motion of matter, and so would be much faster.
(2) In the case of a magma impregnating a residual solid with crystals of the same dimen-
sions (1 cm),
t
x
2
/
D
¼10
11
s or about 3 10
5
years, or 300 000 years, which is rather fast
in geological terms. For 1-mm crystals, which is more realistic,
t
10
2
/
D
¼3 10
3
years,
or 3000 years. So isotope equilibrium is established quite quickly where a magma is in the
presence of mineral phases.
A second important question is whether rocks at ordinary temperatures can retain their
isotope compositions without being modi¢ed and without being re-homogenized. To
14 Isotopes and radioactivity

answer this, it must be remembered that the di¡usion coe⁄cient varies with temperature
bythe Arrheniuslaw:
D ¼ D
0
exp
DH
RT
where H is the activation energy, which is about 40 kcal per mole, R is the ideal gas con-
stant(1.987 calper K per mole), and T theabsolutetemperature(K). If D ¼10
11
cm
2
s
1
in
solids at1300 8C, what is the di¡usion coe⁄cientD
or
atordinarytempe ratures?
D
or
D
1300
¼ exp
DH
R
I
T
or
I
T
1300
¼hBi
from which D
or
¼ B
hi
D
1300
.
Calculate Bhito¢ndthat itgives1.86 10
25
, thereforeD
or
¼2 10
36
cm
2
s
1
.
Tohomogenizea1-mmgrainatordinary temperaturestakes
t ¼
10
2
2 10
36
¼ 0:5 10
34
s 1:5 10
26
years;
whi ch is in¢nitelylongto allintentsandpurposes.
Important remark
Rocks therefore retain the memory of their history acquired at high temperatures. This is the prime
reason isotope geology is so incredibly successful and is the physical and chemical basis of isotope
memory. The phenomenon might be termed isotopic quenching, by analogy with metal which, if it is
immersed when hot in cold water, permanently retains the properties it acquired at high temperature.
1.3.3 A practical application of isotopic exchange:
isotopic dilution
Supposewe wishto measurethe rubidium contentofa rock. Rubidium has two isotopes, of
mas s 85 and 87, in the proportion
85
Rb/
87
Rb ¼2.5933. (This is the value found when mea-
suring the Rb isotope composition ofnatural rocks.) The rock is dissolvedwitha mixtureof
aci ds.To the solution obtained, we add a solution witha known Rb content which has been
arti¢cially enriched in
85
Rb (spike), whose
85
Rb/
87
Rb ratio in the spike is known.The two
solutionsmixandbecomeisotopicallybalanced.Onceequilibriumisreached, theabsol ute
Rb content ofthe rock canbe determined bysimply m easur ing the isotope composition of
afraction ofthemixture.
Writing
85
Rb
87
Rb
¼
85
87
gives:
85
87
mixture
¼
ð
85
RbÞ
rock
þð
85
RbÞ
spike
ð
87
RbÞ
rock
þð
87
RbÞ
spike
¼
85
87
spike
þ
85
87
rock
87
Rb
rock
87
Rb
spike
hi
1 þ
87
Rb
rock
87
Rb
spike
hi
:
To p a n d b o tt o m a r e d i v i d e d b y
87
Rb, bringingout
85
87
.
A little manipulation gives:
87
Rb
rock
¼
87
Rb
spike
85
87
spike
85
87
spike
85
87
mixture
85
87
mixture
85
87
rock
"#
:
15 Isotopy

Because we knowthe
87
Rb contentofth espike,theRb contentoftherockcanbeobtainedby
simply measur ing the isotope ratio of the mixture, without having to recover all the Rb by
chemi calseparation.
Suppose, say, the isotopic composition of the spike of Rb is
85
Rb/
87
Rb ¼0. 12.The natu-
rally occurri ng
85
Rb/
87
Rb ratio is 2.5933.We dissolve1g of rock an d add to the solution1g
ofspi ke containing 3 ngof
87
Rb percubic centimeter. After thoroughlymixing thesolution
containing the dissolved rockand the spike solution, measurement of a fraction of the mix-
tureyields aratio of
85
Rb/
87
Rb ¼1.5.Th e Rb contentofthe rockcanbe calculated.We sim-
plyapply theformula:
87
Rb
rock
¼
87
Rb
spike
0:12 1:5
1:5 2:59
¼ 1:266;
or
87
Rb
rock
¼ 1:266
87
Rb
spike
¼ 1:266 3 10
9
g ¼ 3:798 10
9
g:
As we took a sample of 1g of rock, C
87
R
b
¼ 3:798 ng g
1
¼ 3:798 ppb. Therefore
C
87
total
¼ C
87
R
b
ð1 þ 2:5933Þ¼13:42 ppb.
This method can be used for all chemical elements with several stable isotopes for which
spikeshavebeenpreparedthatarearti¢ciallyenrichedinoneormoreisotopesandforelements
with a single isotope, provided it is acceptable to use a radioactive isotope (and so potentially
dangerousfor whoeverconductstheexperiment)asaspike.Themethod hasthreeadvantages.
First,aftermixingwiththespike,chemicalseparation methodsneednotbe entirelyquanti-
tative. (The yields of the various chemical operations during analysis do not count.) Isotope
ratios alonematter, aswell as anycontamination,whichdistortsthemeasurement,ofcourse.
Then, as th e mass spectrometer makes very sensitive and very precise measurements of
isotope ratios, isotopic dilution may be used to measure the amounts of trace elements,
eventhetiniesttraces, withgreatprecision.
Isotope dilutionwas invented for the needs ofl aboratoryanalysisbut maybe extended to
natural processes. As shall be seen, variable isotope ratios occur in nature. Mixes of them
can be used to calculate proportions by mass of the geochemical elements involved just by
simple measurements of isotope ratios.
As can be seen, isotope dilution is an essential method in isotope geochemistry. But just
howpreciseisit?This exercisewillallowustospecifythe error(uncertainty)inisotopedilu-
tion measurement.
Exercise
The isotope ratios of the spike, sample, and mixture are denoted
R
T
,
R
S
, and
R
M
, respectively.
We wish to find out the quantity
X
of the reference isotope
C
j
in the sample. To do this,
quantity
Y
of spike has been mixed and the isotope ratios (
C
i
/
C
j
) ¼
R
of the spike, sample, and
mix have been measured. What is the uncertainty of the measurement?
Answer
Let us begin with the formula
X
¼
Y
R
T
R
M
R
M
R
S
16 Isotopes and radioactivity
