Adlard E.R. (ed.) Chromatography in the Petroleum Industry
Подождите немного. Документ загружается.


412
Chapter
14
are expensive and fragile, they are easily poisoned by the non-selective adsorp-
tion of analyte molecules to the gel material. Sieving buffers are less fiagile and
less expensive but have not been sufficiently investigated to determine their po-
tential for the analysis of industrial polymeric materials.
14.2.4
Capillary isoelectric focusing (CIEF)
This technique
is
also derived from classical electrophoresis in which sub-
stances are separated on the basis of their isoelectric point or PI values. The
capillary
is
filled with an ampholyte solution which forms a pH gradient in the
capillary when the voltage
is
applied. The analyte molecules (almost exclusively
proteins) migrate to the point where they have a net neutral charge, at which
point they stop migrating. The focused zones of the sample can be eluted
from
the capillary using pressure or the electro-osmotic flow of a different, non-
ampholytic buffer. This method provides very accurate determination of PI val-
ues and has a large sample-loading capacity; up to
1
pg of material can be loaded
onto the capillary, separated, and the purified fractions collected.
14.2.5
Instrumentation
As described briefly in the introduction to this chapter, CE systems have a
common architecture whatever their application. The different parts of the sys-
tem must be optimized to achieve reproducible analyses, ease of use, sensitive
detection and automatic operation. The main components of a
CE
system are
discussed
in
detail and the important parameters highlighted.
14.2.6
Capillary
Although
CE
is
possible using glass or polymer capillaries, silica capillaries
are the most widely used
as
they give the best combination of properties for
CE.
Alternatives to silica are usually explored in order to overcome difficulties with
the separation; in these circumstances it
is
usually more productive to investigate
an alternative technique. Alternatively, the chemistry of the electrolyte can be
adapted to dynamically coat the capillary surface.
A considerable amount of ef-
fort
is
being put into improving the stability and nature
of
coating materials; a
number of significant developments are expected in the near future.
The dimensions of the capillary are important parameters. The internal diame-
ter is usually between
25
and 150pm. Smaller diameters can improve separa-

Capillary electrophoresis
in
the petroleum industry
413
tions due to an increase in efficiency, but at the expense of detection limits
(injected volumes are smaller and the detector pathlength is shorter). The use of
larger capillary diameters can improve detection limits, but at the expense of the
separation. The recent development of Z-Flowcells for most commercial instru-
ments has improved detection limits
,
without the need to use large-bore capillar-
ies. The square of the diameter of the capillary is directly proportional to the cur-
rent generated in the capillary at a given potential. This limits the diameter of the
capillary since applying sufficient field strength to separate the analytes may
generate an excessive current if the capillary has
a
large internal diameter. This
can be manifest in
two
ways, the current generated exceeds the safety limits of
the system and it shuts down, or the heat generated by the current causes boiling
of the electrolyte and a break in the electrical continuity of the capillary. Before
either of these
two
points are reached, detector baseline instabilities will be ob-
served because of bubble formation.
The separation capacity of the capillary is proportional to its length. Keeping
other analytical parameters constant, increasing the length of the capillary de-
creases the electric field strength leading to longer run times.
14.2.7
High
voltage power supply
The most important feature of the power supply is stability; as the “pump”
of
the CE system, it is important to have a good-quality power supply. Most devices
will operate at up to
+30
kV
with a high degree of accuracy and precision. They
can run at constant voltage, constant current, or a mixture of both. Safety pre-
cautions are built into the CE system to prohibit inadvertent hazards. CE systems
cannot be considered spark-proof; this may limit the extension of their use in
hazardous areas such as oil platforms or refineries, unless they are operated in a
purged environment.
14.2.8
Temperature control
Applying a potential difference across the capillary and the resulting current
generates heat, this has to be dissipated to avoid boiling within the capillary. Dif-
ferent approaches are feasible, using either air flow across the capillary, or ex-
ternally cooled fluid circulation. With the latter approach, the capillary has to be
inserted into a cassette-type device to facilitate the coolant. Good temperature
control is necessary for reproducible migration times.
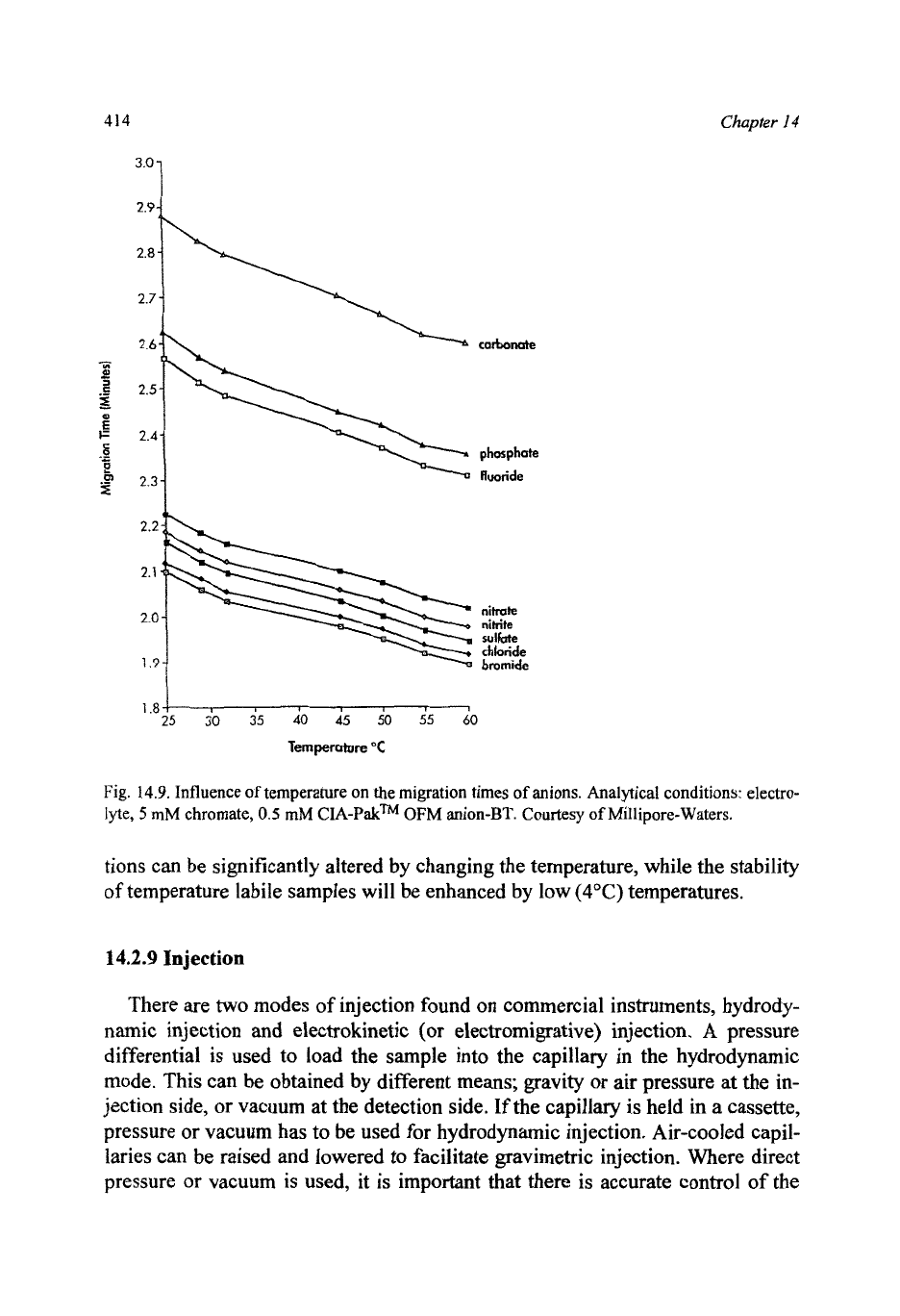
A
change of 1°C causes a
2%
change in ionic mobility (Fig. 14.9). Temperature control of the electrolyte
and sample compartments is also important. The selectivity of MEKC separa-
References pp.
425-426
414
3.0
1
I
2.9
J
Chapter
14
carbonate
phosphote
fluoride
nitrate
nihite
sulfate
chloride
bromide
Fig.
14.9.
Influence
of
temperature on the migration times
of
anions. Analytical conditions: electro-
lyte,
5
mM chromate,
0.5
mM
CIA-Pakm
OFM
anion-BT. Courtesy
of
Millipore-Waters.
tions can be significantly altered by changing the temperature, while the stability
of temperature labile samples will be enhanced by low
(4°C)
temperatures.
14.2.9
Injection
There are
two
modes of injection found on commercial instruments, hydrody-
namic injection and electrokinetic (or electromigrative) injection. A pressure
differential is used to load the sample into the capillary in the hydrodynamic
mode. This can be obtained by different means; gravity or air pressure
at
the in-
jection side,
or
vacuum at the detection side.
If
the capillary is held in
a
cassette,
pressure or vacuum has to be used for hydrodynamic injection. Air-cooled capil-
laries can be raised and lowered to facilitate gravimetric injection. Where direct
pressure or vacuum is used, it is important that there
is
accurate control of the

Capillary electrophoresis in the petroleum industry
415
pressure and the sample vials are well sealed, so that reproducible injections can
be made. When using gravimetric techniques for injection, the capillary is placed
in the sample vial and elevated to a given height above the detection end of the
capillary. An aliquot of the sample will syphon into the capillary. The injected
volume is proportional to the pressure differential, sample viscosity, capillary
diameter and time. If any of these parameters cannot be controlled, the injections
will be irreproducible. Normally, the capillary diameter will stay the same, as
will the pressure differential if all seals are in good condition. One can manipu-
late the time of injection to facilitate loading; viscosity is more difficult to con-
trol, particularly if the sample matrix is subject to change. Temperature control
is important here because of its effect on the viscosity of solutions.
Injection volumes are very small, less than
10
nl
is
common, and should be
considered if sample homogeneity is not reliable. The resolution of the separa-
tion can be compromised if the injection volume is too large. It is difficult to de-
fine exactly the volume of sample that will disrupt a CE separation.
Electromigrative injection is used to selectively introduce sample components
into the capillary by generating a potential difference between the capillary and
the ions in the sample. The injection of negatively charged species is obtained by
making the end of the capillary positive with respect to the sample. The negative
ions migrate to the capillary where they can accumulate. Positively charged spe-
cies are injected by making the end of the capillary negative with respect to the
sample. Differing amounts of sample are injected by varying either the injection
voltage or the length of time the voltage is applied. Unlike hydrodynamic injec-
tion, electromigration is a selective injection technique, only the positive or
negative ions in the sample will be injected, neutral species will not. This can be
a
significant benefit if the sample matrix is complex and the operator knows
which ionic species are of interest.
A
disadvantage of this technique is the non-
linear relationship between the field-strength used and the mobility of the ions in
the sample. The most mobile ions will migrate through the sample and into the
capillary faster than slower ions. There will therefore be an enhanced accumula-
tion of the most mobile ions that can lead to inaccuracies in quantitation unless
the sample matrix is well defined and stable. A good use of electromigration in-
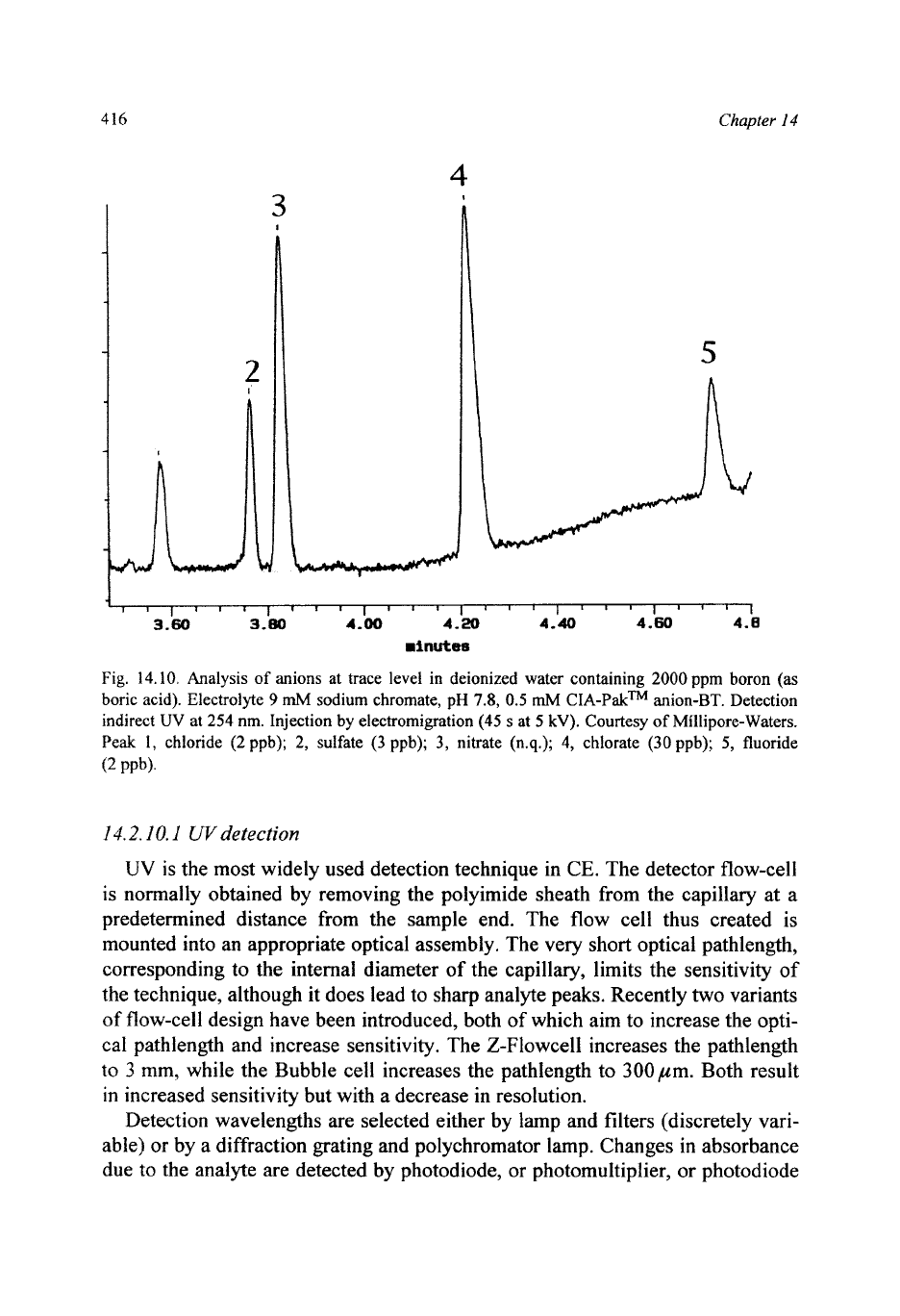
jection is in the analysis of very low levels of anions and cations in ultra-pure
water (Fig.
14.10).
14.2.10
Detection
There are a number of detection techniques available for
CE
systems; all are
taken from HPLC, although a number of modifications are necessary for opti-
mum
CE
performance. The detector types and their uses are presented here.
References
pp.
425-426
416
Chapter
14
4
3
2
i
5
i,
,
,,,~IIl~I~l~~~~~~~~~"'
3.60 3.80
4.00
4.20
4.40
4.60
4.8
minutes
Fig.
14.10.
Analysis
of
anions at trace level in deionized water containing
2000
ppm boron (as
boric acid). Electrolyte
9
mh4
sodium chromate, pH
7.8,
0.5
mh4
CIA-PakTM anion-BT. Detection
indirect
UV
at
254
nm. Injection by electromigration
(45
s
at
5
kV).
Courtesy
of
Millipore-Waters.
Peak
I,
chloride
(2
ppb);
2,
sulfate
(3
ppb);
3,
nitrate (n.q.);
4,
chlorate
(30
ppb);
5,
fluoride
(2
PPb).
I4.2.10.1
W
detection
UV
is the most widely used detection technique in CE. The detector flow-cell
is
normally obtained by removing the polyimide sheath from the capillary at a
predetermined distance from the sample end. The flow cell thus created
is
mounted into an appropriate optical assembly. The very short optical pathlength,
corresponding to the internal diameter
of
the capillary, limits the sensitivity
of
the technique, although it does lead to sharp analyte peaks. Recently
two
variants
of
flow-cell design have been introduced, both of which aim to increase the opti-
cal
pathlength and increase sensitivity. The Z-Flowcell increases the pathlength
to
3
mm, while the Bubble cell increases the pathlength to 300pm. Both result
in
increased sensitivity but with a decrease in resolution.
Detection wavelengths are selected either by lamp and filters (discretely vari-
able) or by
a
diffraction grating and polychromator lamp. Changes
in
absorbance
due to the analyte are detected by photodiode, or photomultiplier,
or
photodiode
Capillary electrophoresis
in
the petroleum industry
417
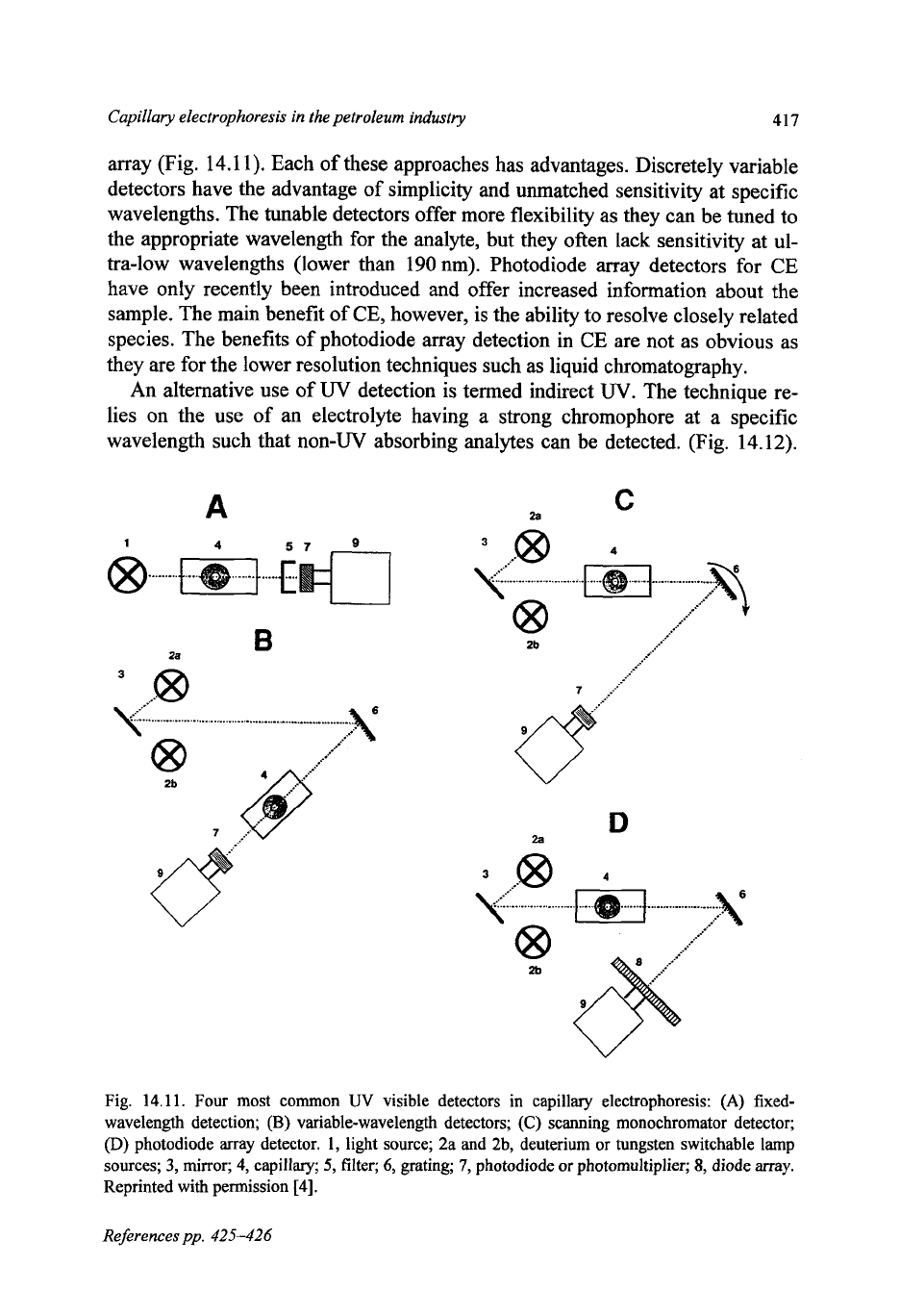
array (Fig. 14.1 1). Each of these approaches has advantages. Discretely variable
detectors have the advantage of simplicity and unmatched sensitivity at specific
wavelengths. The tunable detectors offer more flexibility as they can be tuned to
the appropriate wavelength for the analyte, but they often lack sensitivity at ul-
tra-low wavelengths (lower than 190 nm). Photodiode array detectors for CE
have only recently been introduced and offer increased information about the
sample. The main benefit of
CE,
however, is the ability to resolve closely related
species. The benefits of photodiode array detection in
CE
are not as obvious as
they are for the lower resolution techniques such as liquid chromatography.
An
alternative use of
UV
detection is termed indirect
UV.
The technique re-
lies on the use of an electrolyte having a strong chromophore at a specific
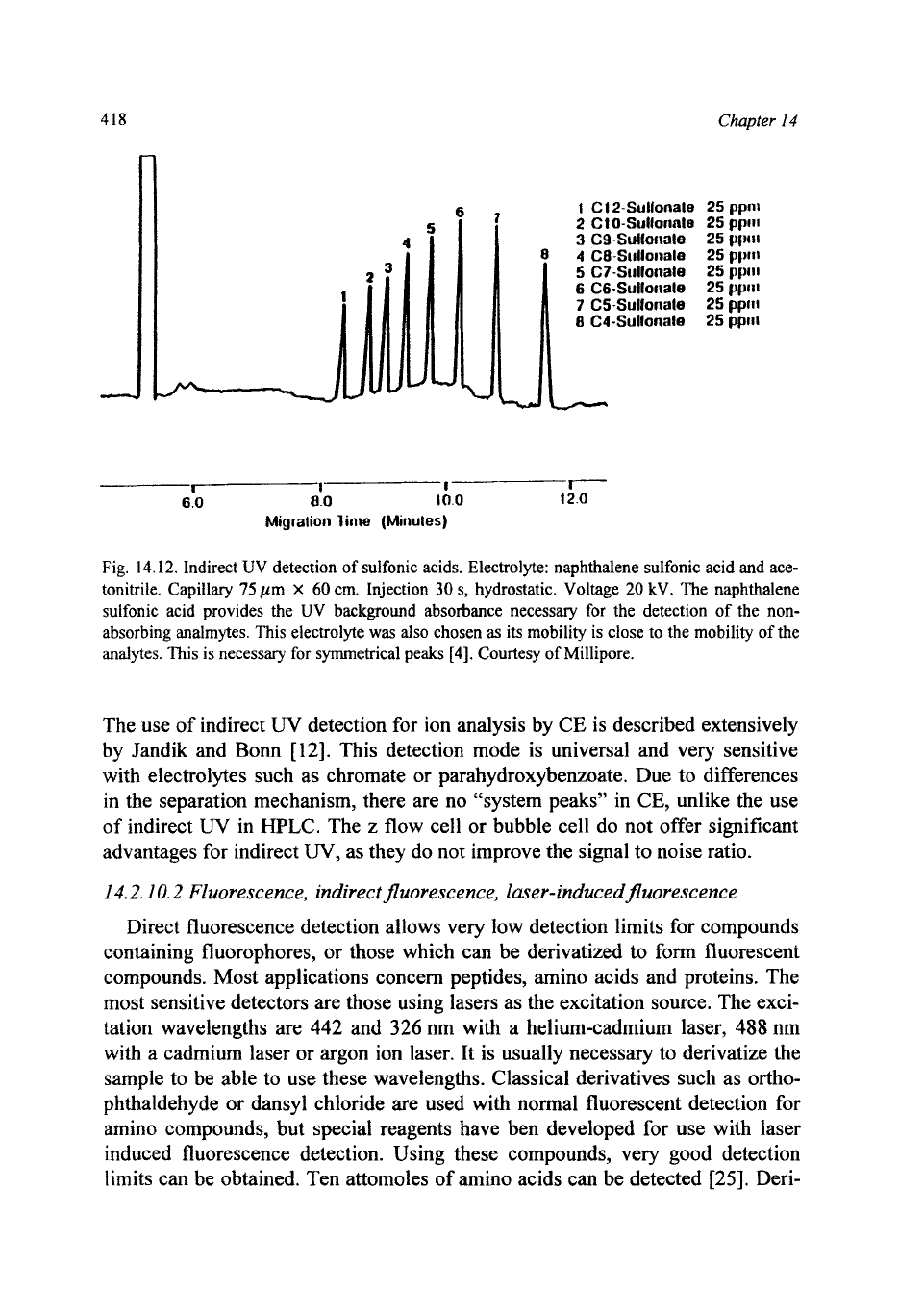
wavelength such that non-UV absorbing analytes can be detected. (Fig. 14.12).
C
2a
A
1
4
57
9
.........
...
..................
....
/'
....
Zb
,..'
Za
B
*.."
3@
.....
7
.:-.
\
-9"
.......................................................
0
o'..
2a
........................
.....
.y
.....
0
f
2b
Fig.
14.11.
Four most common
UV
visible detectors in capillary electrophoresis:
(A)
fixed-
wavelength detection;
(B)
variable-wavelength detectors;
(C)
scanning monochromator detector;
(D)
photodiode array detector.
1,
light source; 2a and 2b, deuterium
or
tungsten switchable lamp
sources;
3,
mirror;
4,
capillary;
5,
filter;
6,
grating;
7,
photodiode
or
photomultiplier;
8,
diode array.
Reprinted with permission
[4].
References pp.
425-426
Chapter
14
418
-c
1
;I
1
1
CIP-Sullonate
25
ppnl
2
CtO-Sullonnte 25
ppiii
3
C9-Sulloriale 25
ppiii
4
CB-Stillonale 25
ppi~
5
C7-Sullonate 25 ppit~
6
C6-Sulloiiale 25
ppiil
7 CS-Sulfonate 25
ppiit
8
C4-Sullonale
25
ppri~
I
JL
I1
I
I
6.0
8.0
10.0
12.0
Migralion
lime
(Mnules)
Fig.
14.12.
Indirect UV detection of sulfonic acids. Electrolyte: naphthalene sulfonic acid and ace-
tonitrile. Capillary
75
pm
X
60
em. Injection
30
s,
hydrostatic. Voltage
20
kV. The naphthalene
sulfonic acid provides
the
UV
background absorbance necessary for the detection of the non-
absorbing analmytes. This electrolyte
was
also
chosen
as
its
mobility
is
close to the mobility of the
analytes.
This
is
necessary
for
symmetrical peaks
[4].
Courtesy of Millipore.
The use of indirect
UV
detection for ion analysis by CE is described extensively
by Jandik and Bonn
[
121. This detection mode
is
universal and very sensitive
with electrolytes such as chromate
or
parahydroxybenzoate. Due to differences
in the separation mechanism, there are no “system peaks” in CE, unlike the use
of
indirect
W
in HPLC. The
z
flow cell or bubble cell do not offer significant
advantages for indirect
W,
as they do not improve the signal to noise ratio.
14.2.10.2
Fluorescence, indirect fluorescence,
laser-inducedfluorescence
Direct fluorescence detection allows very low detection limits for compounds
containing fluorophores,
or
those which can be derivatized to form fluorescent
compounds. Most applications concern peptides, amino acids and proteins. The
most
sensitive detectors are those using lasers as the excitation source. The exci-
tation wavelengths are 442 and
326
nm with a helium-cadmium laser, 488 nm
with a cadmium laser
or
argon ion laser. It is usually necessary to derivatize the
sample to be able to use these wavelengths. Classical derivatives such as ortho-
phthaldehyde or dansyi chloride are used with normal fluorescent detection for
amino compounds, but special reagents have ben developed for use with laser
induced fluorescence detection. Using these compounds, very good detection
limits can be obtained. Ten attomoles
of
amino acids can be detected [25]. Deri-

Capillary electrophoresis
in
the petroleum industry
419
vatization at such low levels is very difficult and these results were obtained by
dilution of the sample from higher (nanomole) concentrations.
Indirect laser induced fluorescence detection permits the detection
of
non-
fluorescent analytes by using an electrolyte containing a fluorescent compound.
Results have been published for the determination
of
both cations and anions
using indirect laser fluorescence. The detection limits, however, are in the same
order of magnitude as the detection limits by indirect
UV
with a less expensive
instrument.
14.2.10.3
Amperometric detection, conductometric detection,
MS
detection
All the detection modes used in WLC will be available for CE in the near
future. The following three modes
of
detection are still in the development stage
but will certainly soon be in routine use.
Amperometric detection.
This technique is often termed electrochemical detec-
tion, it requires that the analyte molecules have a redox potential, i.e. that they
can be either oxidized or reduced by applying a low voltage. Classical examples
are catecholamines, carbohydrates and some inorganic compounds such as sul-
phite, sulphide, cyanide and iodide. Detection of metal ions has also been de-
scribed. The design of a cell is shown Fig.
14.13.
Working electrodes can be
made
of
carbon fibre (catecholamine analysis), copper (carbohydrates), silver
(sulphur species), mercury, gold or platinum. Interest in this technique lies in the
selectivity and sensitivity it can offer, for example attomoles
of
catecholamines
can be detected
[26].
Compared to HPLC, amperometric detection in
CE
gives
better coulombic efficiency (percentage
of
the electroactive analyte that is oxi-
dized or reduced in the cell).The main difficulty to overcome in CE is that rou-
tinely the detector is set to approximately
1.0
V
(in order to oxidizeheduce the
analyte) which generates a current
of
nanoamps, in the presence
of
the high volt-
age and microamp current generated by
CE.
It is therefore necessary to ground
Fig.
14.13.
Amperometric detection cell design. Reprinted with the permission of ref.
[25].
A,
fused silica capillary;
B,
drop of carrier electrolyte;
C,
stainless steel plate;
RE,
reference electrode;
WE,
working electrode;
AE,
auxiliary electrode.
References pp.
425-426

420
Chapter
I4
the high voltage at the detection end of the capillary before the amperometric
detector flow cell
[27].
A similar approach
is
required for conductometric detec-
tion.
Conductometric detection.
This mode of detection works in exactly the same
way as the HPLC detector and it
is
an
attractive detector to use for measuring
ions. The detection principle is to measure the change in conductance of a solu-
tion due to the presence of an ion. The conductance can either be enhanced or
reduced by the presence of the ion. It is difficult to measure small changes in
conductance
in
the presence of the highly conducting CE electrolyte and high
voltage. An interesting experimental approach
is
the use
of
chemical suppression
1281.
Using this method, good detection limits were presented for anion analysis,
but a number of problems still need to be resolved. It is difficult to assemble the
suppressor and conductivity cell on the capillary and the lifetime of the suppres-
sor is compromised by the cationic surfactants used to control the EOF. If the
EOF
is
not controlled, the separation becomes very unstable. There are difficul-
ties therefore in the selection of electrolytes which are compatible with both
separation and suppressioddetection.
Once these problems have been overcome, conductometric detection of ions
separated by CE should offer advantages against present methods
of detection.
Mass
spectroscopy detection.
The mass spectrometer is an attractive detection
technique as it can give absolute identification of the separated analytes based on
their molecular mass and, if the appropriate technique is used, mass spectrum. It
is
for this reason that
LC-MS
is
a fast growing technique. One of the difficulties
with LC-MS is the incompatibility between the optimum flow rates for LC
analysis versus the optimum flow rates for most MS interfaces. One possible
solution attempted by MS manufacturers is to modify the interface to accommo-
date high flow rates. This can, however, compromise the performance of the MS.
An
alternative solution is to use capillary LC with microbore columns and flow
rates in the microliter per minute range.
In
CE, the EOF usually ranges from
0.1
to
1
,uI/min, which makes it more suitable for
MS
interfaces than HPLC.
A review of CE-MS published recently
[28]
comments that the electrospray
ionization interface (ESI) is more frequently used than fast atom bombardment
(FAB). This is probably due to the extensive use of ESI for protein and peptide
analysis, for which it
is
well suited, as is CE.
There are compatibility problems with CE electrolytes and
MS,
this can be
reduced by the use of a sheathing liquid which effectively dilutes the CE electro-
lyte to ionic levels that the
MS
can tolerate (Fig.
14.14).
The combination of the
resolving capacities of
MS
and CE are synergistic. The only issue
is
sensitivity
since the injected amounts
in
CE are so small, and the need to dilute the CE
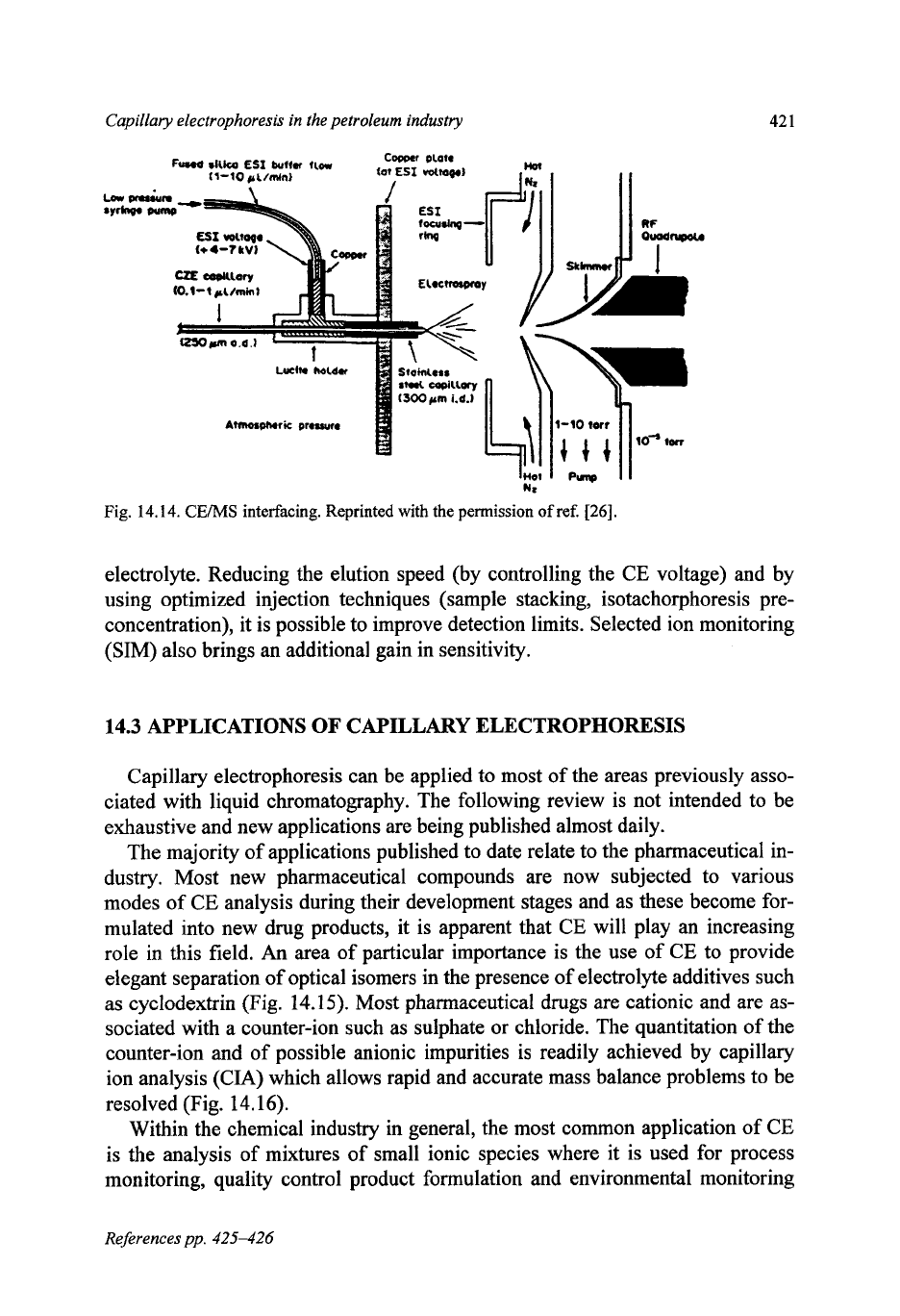
Capillary electrophoresis in the petroleum industry
lo,
Coowr
E5~
rotcogr,
elate
Hoc
Fund
sllka
ESI
buff-
flow
t+lOpl/mln)
-r
'Y
42
1
Inol
I
Purp
I
I
Nz
Fig.
14.14.
CEMS
interfacing. Reprinted with the permission
of
ref.
[26].
electrolyte. Reducing the elution speed (by controlling the CE voltage) and by
using optimized injection techniques (sample stacking, isotachorphoresis pre-
concentration), it is possible to improve detection limits. Selected ion monitoring
(SIM) also brings an additional gain in sensitivity.
14.3
APPLICATIONS
OF
CAPILLARY ELECTROPHORESIS
Capillary electrophoresis can be applied to most of the areas previously asso-
ciated with liquid chromatography. The following review is not intended to be
exhaustive and new applications are being published almost daily.
The majority of applications published to date relate to the pharmaceutical in-
dustry. Most new pharmaceutical compounds are now subjected to various
modes of CE analysis during their development stages and as these become for-
mulated into new drug products, it is apparent that CE will play an increasing
role in this field.
An
area of particular importance is the use of CE to provide
elegant separation of optical isomers in the presence of electrolyte additives such
as cyclodextrin (Fig.
14.15).
Most pharmaceutical drugs are cationic and are as-
sociated with a counter-ion such as sulphate or chloride. The quantitation of the
counter-ion and of possible anionic impurities is readily achieved by capillary
ion analysis
(CIA)
which allows rapid and accurate mass balance problems to be
resolved (Fig.
14.16).
Within the chemical industry in general, the most common application of CE
is the analysis of mixtures of small ionic species where it is used for process
monitoring, quality control product formulation and environmental monitoring
References pp.
425426
