Adlard E.R. (ed.) Chromatography in the Petroleum Industry
Подождите немного. Документ загружается.

402
Chapter
14
5
10
15
20
25
Minutes
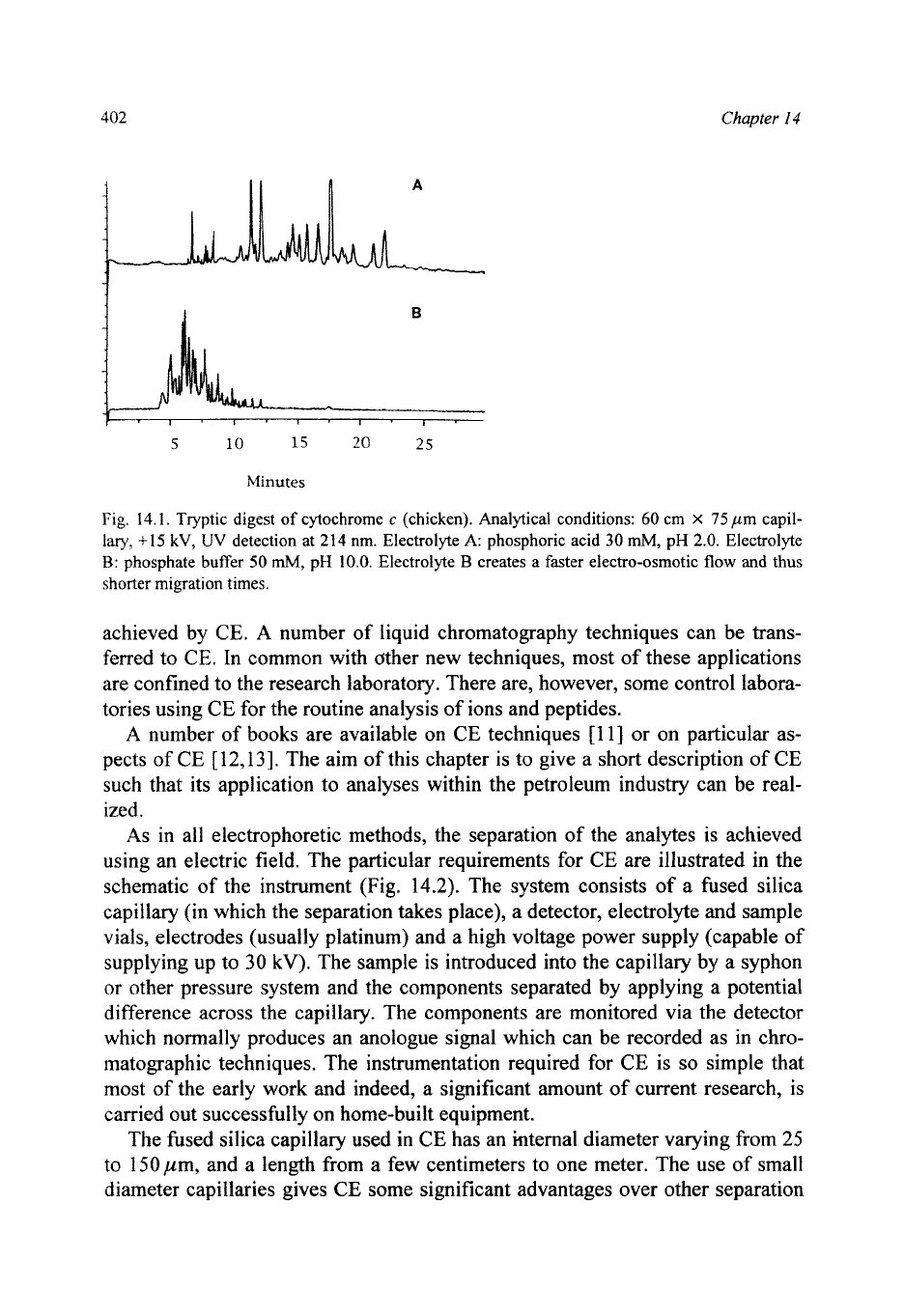
Fig.
14.1.
Tryptic digest of cytochrome
c
(chicken). Analytical conditions:
60
cm
X
75
pm capil-
lary,
+15
kV,
UV
detection at
214
nm. Electrolyte
A:
phosphoric acid
30
mM,
pH
2.0.
Electrolyte
D:
phosphate
buffer
50
mM,
pH
10.0.
Electrolyte
B
creates a faster electro-osmotic
flow
and
thus
shorter migration times.
achieved by CE.
A
number of liquid chromatography techniques can be trans-
ferred to CE. In common with sther new techniques, most
of
these applications
are confined to the research laboratory. There are, however, some control labora-
tories using CE for the routine analysis
of
ions and peptides.
A
number of books are available on CE techniques
[l
13
or on particular as-
pects of CE
[
12,131.
The aim of this chapter is to give a short description
of
CE
such that its application to analyses within the petroleum industry can be real-
ized.
As
in all electrophoretic methods, the separation of the analytes is achieved
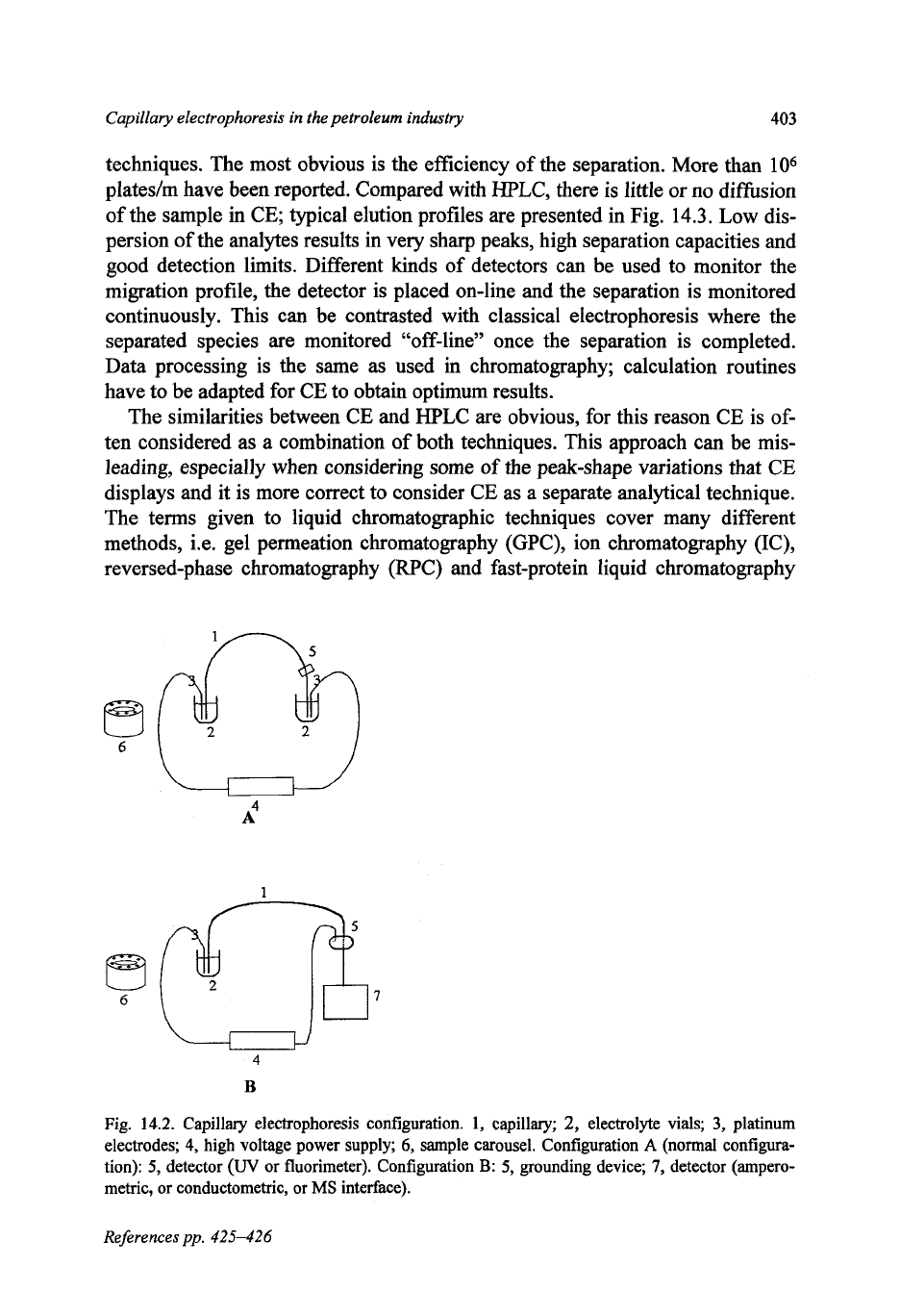
using an electric field. The particular requirements for CE are illustrated in the
schematic of the instrument (Fig. 14.2). The system consists
of
a
fused silica
capillary (in which the separation takes place), a detector, electrolyte and sample
vials, electrodes (usually platinum) and a high voltage power supply (capable
of
supplying up to
30
kV).
The sample is introduced into the capillary by a syphon
or other pressure system and the components separated by applying a potential
difference across the capillary. The components are monitored via the detector
which normally produces an anologue signal which can be recorded as in chro-
matographic techniques. The instrumentation required for CE
is
so simple that
most of the early work and indeed, a significant amount
of
current research,
is
carried out successfully on home-built equipment.
The fused silica capillary used
in
CE has an internal diameter varying from
25
to ISOpm, and a length from a few centimeters to one meter. The use
of
small
diameter capillaries gives
CE
some significant advantages over other separation
Capillary electrophoresis in the petroleum industry
403
techniques. The most obvious is the efficiency of the separation. More than
106
plateslm have been reported. Compared with HPLC, there is little or
no
diasion
of the sample in CE; typical elution profiles are presented in Fig.
14.3.
Low dis-
persion of the analytes results in very sharp peaks, high separation capacities and
good detection limits. Different kinds of detectors can be used to monitor the
migration profile, the detector is placed on-line and the separation is monitored
continuously. This can be contrasted with classical electrophoresis where the
separated species are monitored “off-line” once the separation is completed.
Data processing is the same as used in chromatography; calculation routines
have to be adapted for CE to obtain optimum results.
The similarities between CE and HPLC are obvious, for this reason CE is of-
ten considered as
a
combination of both techniques. This approach can be mis-
leading, especially when considering some of the peak-shape variations that CE
displays and it is more correct to consider CE as a separate analytical technique.
The terms given to liquid chromatographic techniques cover many different
methods, i.e. gel permeation chromatography (GPC), ion chromatography
(IC),
reversed-phase chromatography
(RPC)
and fast-protein liquid chromatography
Fig.
14.2.
Capillary electrophoresis configuration.
1,
capillary;
2,
electrolyte vials;
3,
platinum
electrodes;
4,
high voltage power supply;
6,
sample carousel. Configuration A (normal configura-
tion):
5,
detector
(W
or fluorimeter). Configuration
B:
5,
grounding device;
7,
detector (ampero-
metric, or conductometric, or
MS
interface).
References pp.
425426

404
Chapter
14
A
Fig.
14.3.
Elution profiles in CE and HPLC.
(A)
Capillary electrophoresis;
(B)
HPLC. The electro-
osmotic flow generates a flat profile, compared to the profile
of
pressure driven
flow.
As
a result
analyte
zones in
CE
are very sharply defined compared with HPLC.
(FPLC). Different chemical approaches are used to obtain the separation in these
methods, but the instrumentation remains the same. Similarly, the term
“CE”
refers to a group of different chemical approaches. The various separation meth-
ods,
instrumentation and applications are described in the following pages.
14.2
SEPARATION TECHNIQUES
There are four main separation techniques used in CE. Other modes of sepa-
ration could be considered, but they generally belong
to
at least one of the four
main modes. These are:
-
free-zone capillary electrophoresis (FZCE)
-
micellar electrokinetic chromatography (MEKC)
-
gel-filled capillary electrophoresis (GFCE)
-
capillary isoelectrofocussing (cIEF)
Each separation technique is considered in turn and where appropriate is ref-
erenced to applications within the petroleum industry.
14.2.1
Free-zone
capillary electrophoresis
(FZCE)
The term FZCE is used to describe the separation that takes place in a capil-
Capillary electrophoresis
in
the petroleum industry
Injector Detector
405
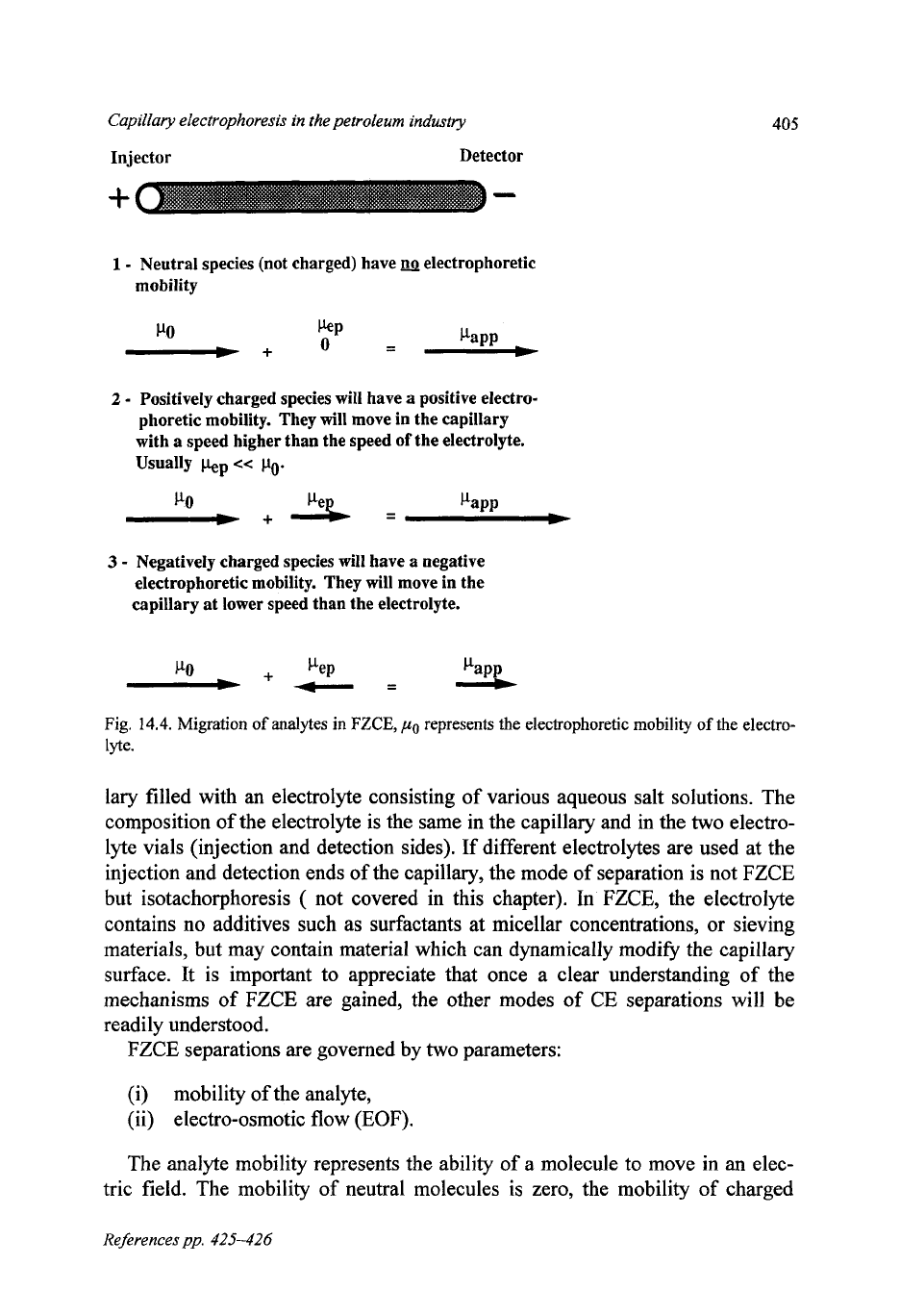
1
-
Neutral species (not charged) have
electrophoretic
mobility
2
-
Positively charged species will have
a
positive electro-
phoretic mobility. They will move in the capillary
with
a
speed higher than the speed
of
the electrolyte.
Usually
kP
<<
po.
CI.0
3
-
Negatively charged species will have
a
negative
electrophoretic mobility. They will move in the
capillary
at
lower speed than the electrolyte.
PaPP
L
PO
pep
L+
-=
Fig.
14.4.
Migration of analytes
in
FZCE,
po
represents the electrophoretic mobility
of
the electro-
lyte.
lary filled with an electrolyte consisting of various aqueous salt solutions. The
composition of the electrolyte is the same in the capillary and in the
two
electro-
lyte vials (injection and detection sides). If different electrolytes are used at the
injection and detection ends of the capillary, the mode of separation is not FZCE
but isotachorphoresis
(
not covered in this chapter). In
FZCE,
the electrolyte
contains no additives such as surfactants at micellar concentrations, or sieving
materials, but may contain material which can dynamically modifL the capillary
surface. It is important to appreciate that once
a
clear understanding
of
the
mechanisms of FZCE are gained, the other modes of
CE
separations will be
readily understood.
FZCE separations are governed by
two
parameters:
(i) mobility of the analyte,
(ii) electro-osmotic
flow
(EOF).
The analyte mobility represents the ability of a molecule to move in an elec-
tric field. The mobility of neutral molecules is zero, the mobility
of
charged
References pp.
425426

406
Chapter
14
molecules is proportional to their charge and inversely proportional to their
mass. The charge to mass ratio of an ion
is
therefore an important parameter in
CE separations.
Both
anions and cations can be separated in the same run.
Cations are attracted towards the cathode and under “normal” conditions, their
speed is additive to the electro-osmotic flow. Anions are attracted towards the
anode, but are “normally” carried to the cathode by the faster EOF. Neutral
molecules are carried towards the cathode by the EOF. The order of migration
is
cations with the highest charge/mass ratio followed by cations with smaller ra-
tios. The neutral species migrate as one, their charge/mass ratio is zero. Finally
the anions with the lower charge/mass migrate earlier than those with greater
ratios.
The electro-osmotic flow within the capillary is a product of the electrical
field strength, the charge
on
the
capillary surface, the ionic strength,
pH
and vis-
cosity of the electrolyte. In most cases, the electro-osmotic flow and the mobility
of
the analyte ion are additive, although a number of well established applica-
tions
of
CE rely on differences between the electro-osmotic flow and analyte
mobility.
The mechanics
of
osmotic flow has been described in a number of publica-
tions
[
141
and is presented schematically in Fig.
14.5.
Osmotic flow is created by
the presence
of
positive or negative charges on the interior walls of the capillary.
When using bare silica capillaries, the ionization of the silanol (SiOH) groups
occurs at
pH
34.
The negative charges induce
an
accumulation of positive
charges
(H,O+)
near the walls. These hydroxonium ions may also be involved in
the solvation
of
analyte cations. When the voltage is applied across the capillary,
the positive charges are attracted by the cathode and they move (migrate) to-
wards it. The net effect
is
a movement of the electrolyte in the capillary in the
direction
of
the cathode. It is important to note that the EOF exists only if the
walls
of
the capillary are charged (e.g. if the electrolyte pH exceeds 3) and if the
internal diameter is small enough (less than
200
pm).
The strength of the EOF increases if the electrolyte pH is raised as a result of
the increased ionization (i.e. charge) of the silica surface of the capillary. Silica
Fig.
14.5.
Electro-osmotic
flow.

Capillary electrophoresis
in
the petroleum industry
407
is fully ionized at pH 6.8, therefore increasing the pH of the electrolyte above
6.8 does not lead to an increase in EOF. It can, however, influence CE separa-
tions due to the effect on the ionic state of the analyte. The EOF can be affected
by modifying the state of the capillary walls. The addition of cationic surfactants
at millimolar concentrations can reverse the EOF. The positively charged surfac-
tant molecules form a layer at the surface of the capillary and effectively create a
positively-charged surface instead of a negative silica surface. Hydroxide ions in
the electrolyte will therefore associate with the coated surface. When the voltage
is applied, the hydroxide ions migrate towards the anode, i.e. the “normal” elec-
tro-osmotic flow is reversed. Similar results can be obtained by coating the capil-
lary with positively charged polymers. The stability of the coating, however, can
be adversely affected by the applied electric field, leading to poor reproducibil-
ity. Coating of the capillary with a neutral polymer can almost eliminate the
EOF, alternatively the injected samples may react with the walls and modify the
EOF. It is also possible to alter the ionized state of the capillary wall by coating
it with a weak acid. Positive or negatively-charged walls can be generated de-
pending on the pH
of
the electrolyte used. Further control of the EOF can be
gained by modifying the viscosity of the electrolyte; as the viscosity increases,
the EOF velocity decreases and this has been used to successfully separate sur-
factant species (Fig. 14.6).
Application work using FZCE must be defined by reference to the actual EOF
observed. The EOF can be determined using a neutral-charged marker molecule
(such as a UV-absorbing organic compound, e.g. formamide). Migration of a
neutral marker in a
75
pm
X
60 cm capillary, at pH
7.0
and at an applied voltage
of
20
kV,
will take approximately 10 min, giving a calculated value of the EOF
of 0.26pVmin. This value will vary, depending on the pH, nature and concen-
tration of the electrolyte and the applied voltage. The EOF can be compared to
the flow
of
solvent from
an
HPLC pump. It
is
necessary to maintain a constant
EOF to obtain stable migration times, just
as
it is important in HPLC for the
pump to give a stable flow for reproducible retention times. FZCE is a popular
mode of CE as it is relatively easy to predict the effect that modifications to the
analytical conditions will have
on
the subsequent separation. For this reason,
there are a large number of published papers and referenced applications.
Manipulation of the EOF and the factors that affect it are both the benefit and
the problem with FZCE. For the technique to be successful in a modern analyti-
cal laboratory, it is necessary to ensure that the EOF
is
correctly controlled. This
is especially true when analysing unknown samples; the operator must be wary
of the effect the sample analytes can have on the EOF. For this reason, there are
a
number of capillary conditioning steps that can be used to minimize these ef-
fects. Rinsing the capillary with potassium hydroxide (normally at
0.5
M
strength) between samples is often used to remove sample components which
References pp.
425426
408
Chapter
I4
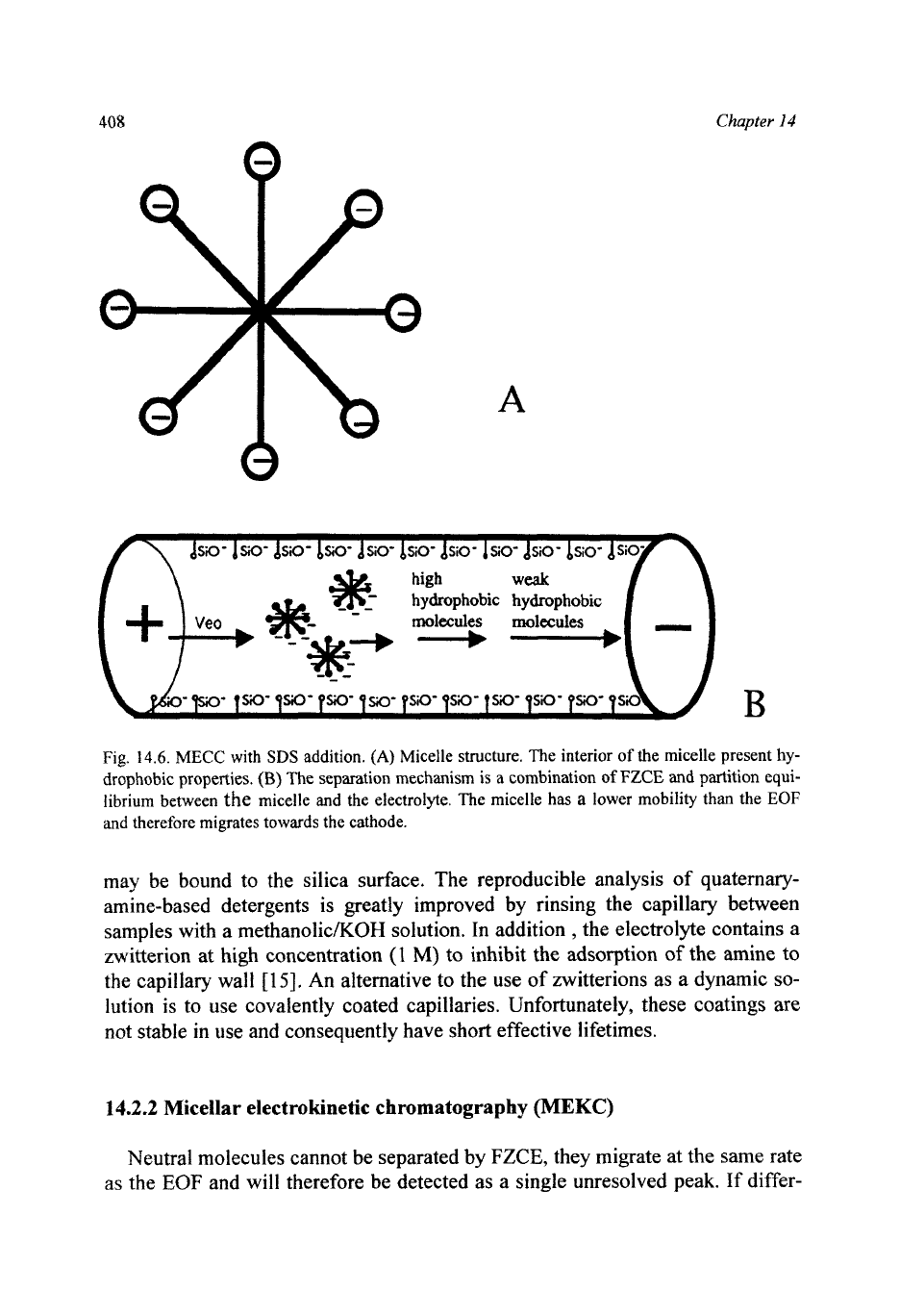
A
molecules molecules
__+____,
B
Fig.
14.6.
MECC
with
SDS
addition.
(A)
Micelle structure. The interior
of
the micelle present
hy-
drophobic properties.
(B)
The separation mechanism is
a
combination
of
FZCE
and partition equi-
librium between
the
micelle and the electrolyte. The micelle
has
a
lower mobility than the
EOF
and therefore migrates towards the cathode.
may be bound to the silica surface. The reproducible analysis of quaternary-
amine-based detergents
is
greatly improved by rinsing the capillary between
samples with a rnethanolicKOH solution. In addition
,
the electrolyte contains a
zwitterion at high concentration
(1
M)
to inhibit the adsorption of the amine to
the capillary wall
El.51.
An alternative to the use
of
zwitterions as a dynamic
so-
lution is to use covalently coated capillaries. Unfortunately, these coatings are
not stable in use and consequently have short effective lifetimes.
14.2.2 Micellar electrokinetic chromatography
(MEKC)
Neutral molecules cannot be separated by
FZCE,
they migrate at the same rate
as
the
EOF
and will therefore be detected as
a
single unresolved peak. If differ-

Capillary
electrophoresis
in
the petroleum
industry
409
ences in hydrophobicity exist between the various neutral molecules in the sam-
ple, it is possible to separate them using MEKC.
The addition of an anionic surfactant to the electrolyte at an appropriate con-
centration can result in the formation of micelles (Fig. 14.6a). The interior
of
the
micelle has a hydrophobic character while the exterior is hydrophilic. The neu-
tral analytes can be separated according to their affinity with the hydrophobic
part of the micelle. The technique has been extensively studied by Terabe and
co-workers
[S,
16-20]
who developed the technique working with phenolic com-
pounds as a model system, but they have looked at a broad spectrum of analytes
including chiral drug formulations
[21].
MEKC uses an ionic micelle which will
normally have an electrophoretic mobility opposite to that of the
EOF.
The
mobility
of
the micelle is lower than that of the EOF such that adsorbed analytes
in the micelle will be detected, as they migrate to the detector at the cathodic end
of the capillary.
Separation is based on the analytes partitioning between the micellar phase
and the solution phase. Micelles form in solution when a surfactant
is
added to
water in a concentration above its critical micelle concentration. The most com-
monly used surfactant in MEKC is sodium dodecyl sulphate
(SDS),
which is an
anionic surfactant.
A
typical electrolyte contains
SDS
at
50
mM
concentration
and a buffer such as sodium phosphate at pH
8.0.
The hydrophobic analytes in the sample are the most retained. This is similar
to what is observed in reversed-phase HPLC. In MEKC, the anticipated migra-
tion order is the same as the elution order in reversed-phase HPLC. One of the
limiting factors of HPLC is the minimum particle size of the column packing; the
smallest particles in common use today have a diameter of
3pm.
The dimen-
sions of the micelles, however, are much smaller and as a result, much better
efficiency can be obtained with MEKC.
As
in HPLC, the addition of organic modifiers influences the separation by
altering the degree of adsorption of the analytes, depending on the amount of
modifier present. Increasing the amount of organic modifier in the electrolyte
will cause a decrease in the adsorption of hydrophobic analytes into the micelle
and a reduction in their migration times. It may also increase the viscosity
of
the
electrolyte, thereby reducing the EOF leading to an enhanced separation.
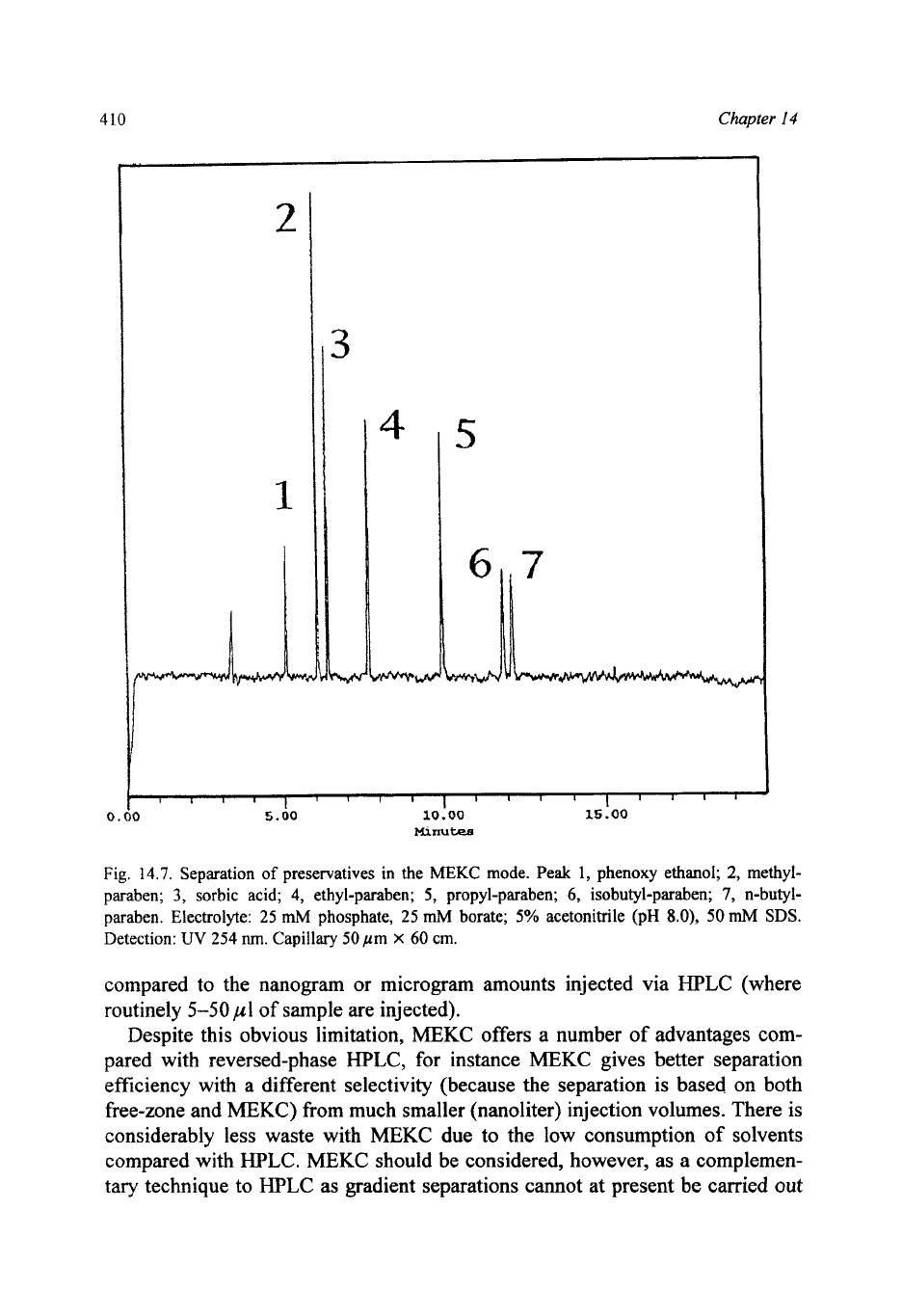
MEKC has been used for a wide variety of applications from the separation of
amino acid derivatives to the separation of preservatives (Fig. 14.7) or metal
complexes. The potential applications for MEKC include the majority of HPLC
reversed-phase applications, MEKC offers increased resolution at the possible
expense of sensitivity. It
is
important to note that while
CE
separations are more
sensitive in terms of the absolute sensitivity of analyte detected, HPLC is more
sensitive in terms of the absolute concentration of analyte. In a CE separation 1-
10
nl of sample are injected, this equates to picogram quantities of the analyte
References
pp.
425-426
410
Chapter
I4
2
1
3
5h
4
-uv
5
t""l~"l~"'llll'
5.00
10.00
15.00
0.00
Mimrtea
Fig. 14.7. Separation
of
preservatives in the
MEKC
mode.
Peak
1,
phenoxy ethanol;
2,
methyl-
paraben;
3,
sorbic acid; 4, ethyl-paraben;
5,
propyl-paraben;
6,
isobutyl-paraben;
7,
n-butyl-
paraben. Electrolyte:
25
mM
phosphate, 25
mh4
borate;
5%
acetonitrile
(pH
8.0),
50
mM
SDS.
Detection:
UV
254
nm.
Capillary
50
pm
X
60
cm.
compared to the nanogram or microgram amounts injected via HPLC (where
routinely
5-50
pl
of sample are injected).
Despite this obvious limitation, MEKC offers a number
of advantages com-
pared with reversed-phase HPLC, for instance MEKC gives better separation
efficiency with
a
different selectivity (because the separation
is
based on both
free-zone and MEKC) from much smaller (nanoliter) injection volumes. There is
considerably less waste with MEKC due to the low consumption of solvents
compared with HPLC. MEKC should be considered, however, as
a
complemen-
tary technique to HPLC as gradient separations cannot at present be carried
out
Capillary electrophoresis in the petroleum industry
41
1
by MEKC (although MEKC can replace the need for gradient HPLC
[22])
and
the detection limits for HPLC (in terms of injected concentration) are better than
CE. HPLC is also usually a better choice if the analytes are only soluble in or-
ganic solvents (such as long-chain fatty acids). Although anionic surfactants
such as SDS are usually preferred, cationic surfactants such as CTAB can also
be used
[23].
14.2.3
Gel filled capillary electrophoresis (GFCE)
This technique is a direct extrapolation
of
classical electrophoresis in that the
main separation mechanism is based on differences in analyte size as they mi-
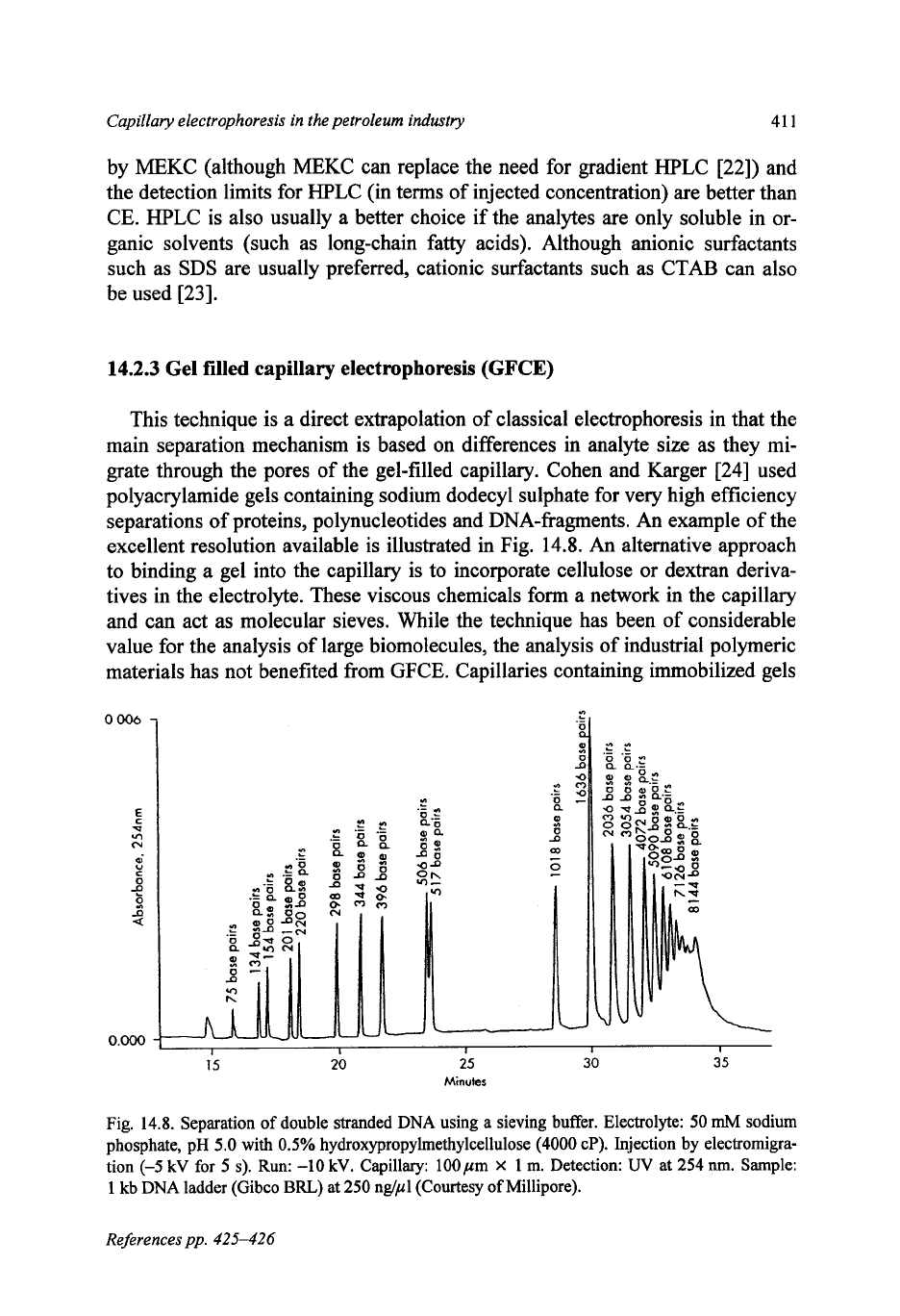
grate through the pores of the gel-filled capillary. Cohen and Karger
[24]
used
polyacrylamide gels containing sodium dodecyl sulphate for very high efficiency
separations of proteins, polynucleotides and DNA-fragments. An example of the
excellent resolution available is illustrated in Fig.
14.8.
An
alternative approach
to binding a gel into the capillary is to incorporate cellulose or dextran deriva-
tives in the electrolyte. These viscous chemicals form a network in the capillary
and can act as molecular sieves. While the technique has been of considerable
value for the analysis of large biomolecules, the analysis of industrial polymeric
materials has not benefited from GFCE. Capillaries containing immobilized gels
Om
1
15 20
25
30
35
Minutes
Fig.
14.8.
Separation
of
double stranded DNA using a sieving buffer. Electrolyte:
50
mM
sodium
phosphate, pH
5.0
with
0.5%
hydroxypropylmethylceliulose
(4000
cP). Injection by electromigra-
tion
(-5
kV
for
5
s).
Run:
-10
kV. Capillary: 100pm
X
1
m.
Detection:
UV
at
254
nm.
Sample:
1
kb DNA ladder (Gibco
BRL)
at
250
nglpl (Courtesy
of
Millipore).
References pp. 425-426
