Vlak J.M., de Gooijer C.D., Tramper J., Miltenburger H.G. (Eds.) Insect Cell Cultures: Fundamental and Applied Aspects
Подождите немного. Документ загружается.

272
Expression and characterization of E1
Our approach to express E1 with the baculovirus sys-
tem was based on our experiences with expression of
this protein with the PRV vector (Van Zijl et al
.,
1991).
Two versions of the E1 gene of classical swine fever
strain Brescia (Moormann et al
.,
1990), one encoding
E1 with a C-terminal membrane anchor (transmem-
brane region, TMR), and one encoding E1 without a
C-terminal TMR, were inserted into the PRV glycopro-
tein gX locus (Rea et al
.,
1985), and expressed in swine
kidney cells. E1 without TMR was secreted, whereas
E1 with TMR was retained in the membranes of the
endoplasmic reticulum. To express both versions of
the Brescia E1 gene in Sf21 insect cells, the sequences
encoding E1 with and without TMR were fused to the
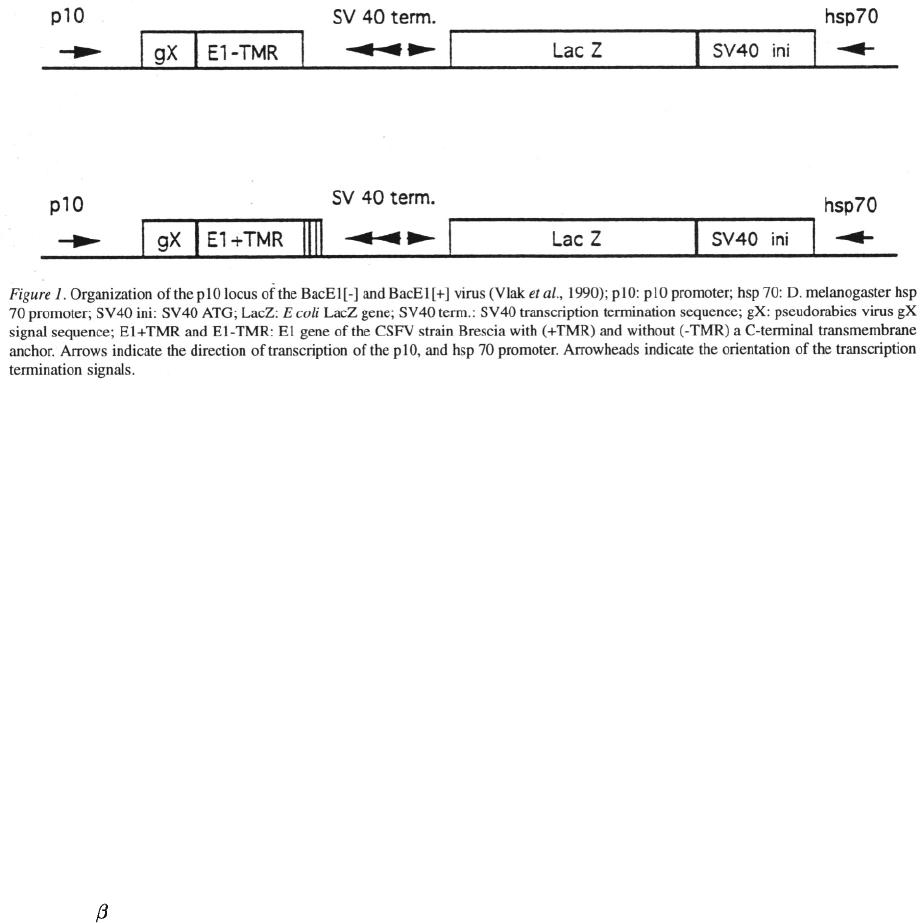
signal sequence of gX of PRV and inserted into the p10
locus of Autographa californica nuclear polyhedrosis
virus (AcNPV), using pAcAS3 (Vlak et al., 1990) as
transfer vector (Fig. 1). Polyhedrin-positive plaques
expressing -galactosidase were isolated and tested for
expression of E1 by immunostaining with an E1 spe-
cific monoclonal antibody (Wensvoort et al., 1989).
One plaque purified E1-TMR virus (BacE1[-]) and
one plaque purified E1+TMR virus (BacE1[+]) were
selected for further characterization by radio immuno-
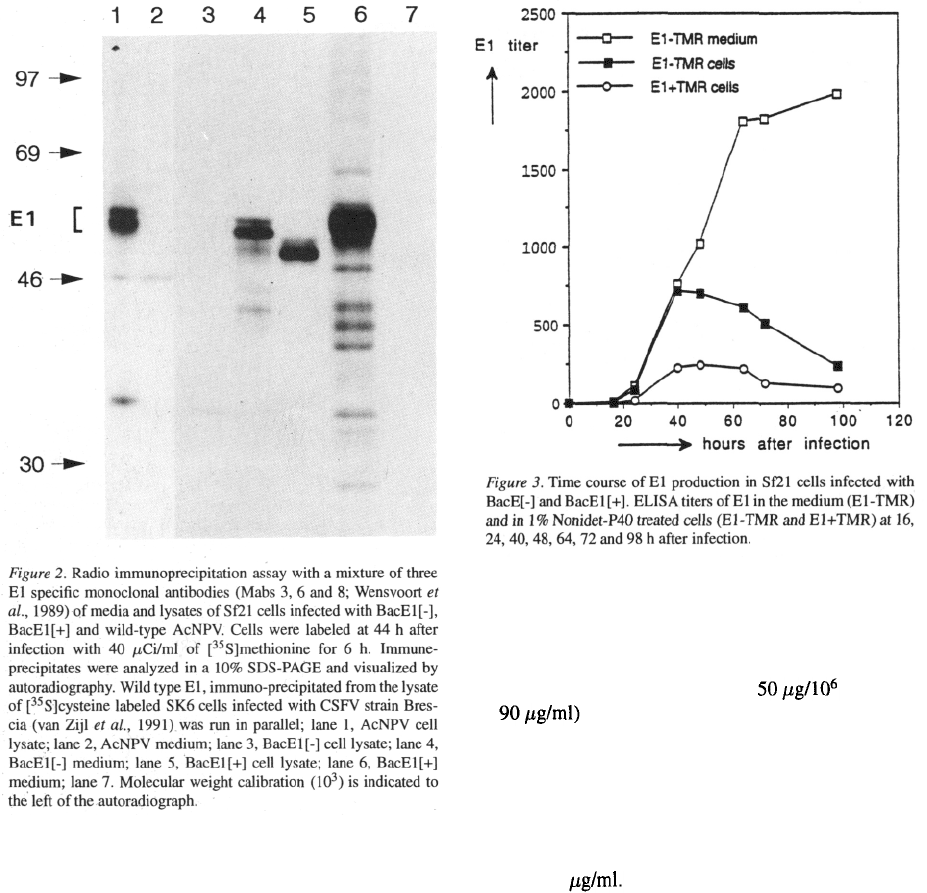
precipitation (Fig. 2).
The E1 protein precipitated from the BacE1 [+] cell-
lysate was similar in size to wild-type E1 (compare
lanes 1 and 6; wild-type E1 is a doublet with a molec-
ular mass of 51 to 54 kD (Wensvoort et al., 1990)). As
expected the E1 protein precipitated from the BacE1 [-]
lysate (lane 4) was slightly smaller (49 to 52 kD) than
wild type E1. E1-TMR was secreted from insect cells
(lane 5) with a somewhat lower molecular weight (46
to 48 kD) than cell-associated E1-TMR. No E1+TMR
protein was secreted from insect cells (lane 7).
The N-glycans of the cell-associated E1+TMR
were completely sensitive to endo H, indicating that
similarly as in CSFV infected cells, the E1+TMR pro-
tein is anchored in the membranes of the endoplasmic
reticulum or cis-Golgi region of insect cells (Hulst et
al
.,
1993). The N-linked glycans of the secreted E1
were partially resistant to endo H, indicating that a
part of the high mannose units were trimmed to a small-
er endo H resistant form (Kuroda et al
.,
1990). This
explains the lower molecular weight of the secreted
E1 -TMR (lane 5) compared to cell associated E1 -TMR
(lane 4).
Similarly as in CSFV infected cells (Wensvoort et
al., 1990; Thiel et al
.,
1991), E1 secreted from insect
cell was efficiently dimerized. Furthermore, E1+TMR
and both the secreted and cell-associated forms of E1 -
TMR reacted identically as native E1 in an ELISA
with four E1 specific monoclonal antibodies, which
each recognize a discontinuous antigenic domain on E1
(van Rijn et al., 1993). This indicated that E1 expressed
in insect cells is antigenically indistinguishable from
native E1.
Production level of E1
The level of expression of E1 in Sf21 cells and in
the medium, determined at different time points after
infection in an E1 specific ELISA (Wensvoort et al
.,
273
1988), showed that the total amount of E1-TMR (cell-
associated plus secreted forms) synthesized in Sf21
cells is about 10 times higher than for E1+TMR
(Fig. 3.) Because no significant growth differences
between BacE1[-] and BacE1[+] virus were observed
and the level of E1 specific RNA present in Sf21 cells
infected with both viruses was almost identical (Fig. 4),
this lower production level of E1+TMR is probably
due to inhibition of protein synthesis as a result of the
accumulation of E1+TMR in the membranes of the
endoplasmic reticulum (see above).
On the basis of this ELISA, it was estimated that
75% of the E1-TMR protein was secreted from insect
cells. The concentration of E1 secreted in the medium,
determined by ELISA using a pure E1 preparation as
standard, was approximately 30 to cells (50
to when Sf21 cells were grown as monolay-
ers in serum-free medium ( SF900; Gibco BRL).
In a pilot experiment, Spodoptera exiqua larvae
were infected with BacE1[-] and BacE1[+] virus. As
expected, only the E1 -TMR protein was secreted in the
hemolymph fluid of the larvae. The production level of
E1-TMR in the hemolymph fluid was approximately
300 to 400 Analysis of the E1 -TMR protein by
SDS-PAGE and Western blotting showed that 50% of
the E1 was C-terminally cleaved probably by a host
protease to a smaller protein with a molecular weight
of 29 to 31 kD (Fig. 5). Because the neutralizing epi-
topes on E1 are positioned in the N-terminal half of
the protein (Van Rijn et al., 1993), E1 produced in
larvae could still be suitable as a subunit vaccine. To
improve production of E1, further experiments with
larvae producing more hemolymph fluid, are needed.
Furthermore, vaccination experiments in pigs with E1
secreted in the hemolymph fluid should be performed
to establish whether E1 produced in larvae is as effi-
cacious in inducing protection against CSFV as E1
produced in insect cells.
274
Vaccination of pigs with E1 produced in insect cells
For the first vaccination trial with E1 produced with the
baculovirus system, E1-TMR purified from the medi-
um of insect cells by immunoaffinity chromatography,
was used (Hulst et al., 1993). Because no data were
available regarding the dose of E1 required for the
induction of a protective immune response in pigs by a
dead subunit vaccine, groups of pigs were vaccinated
with high doses, i.e., 20 and of E1. The vaccine
was applied in a double water-oil emulsion (Herbert,
1965; Barterling & Vreeswijk, 1991). After vaccina-
tion all pigs developed high neutralizing antibody liters
against classical swine fever virus (Table 1). In fact, no
significant differences in liters were observed between
pigs vaccinated with 20 or of E1 42 days after
inoculation. After intranasal challenge with 100
(50% lethal dose) of classical swine fever virus strain
Brescia, all pigs vaccinated with E1 were complete-
ly protected and did not show any signs of disease or
viraemia. In contrast, the non-vaccinated pigs devel-
oped fever from day 4 and 5 on, became recumbent at
day 6, and were killed when moribund at day 8 post
challenge.
The results of this limited vaccination experiment
with E1 are highly promising in several aspects. Firstly,
after vaccination with the conventional Chinese (C)-
strain vaccine, pigs with neutralizing antibody titers of
30 or higher are protected from classical swine fever,
and do not transmit the virus to seronegative contact
pigs, after challenge with virulent CSFV (Terpstra et
al
.,
1988). Pigs inoculated once with of E1
produced in insect cells, develop neutralizing antibody
titers of 3000 and higher, indicating that a much lower
dose of E1 might be sufficient to induce protection
against CSF. Preliminary results with lower doses of
E1, directly prepared from serum-free SF900 insect
cell medium, confirm that the dose needed to protect
pigs, and to prevent transmission of classical swine
fever virus, is significantly lower than E1 (the
50% protective dose is about E1).
Secondly, the rise of antibody titers against E1 pro-
ceeded much faster in pigs vaccinated with E1 pro-
duced in insect cells (dead E1) than in pigs infected
275
with low-virulent field strains of classical swine fever
virus or with the PRV-E1 recombinant viruses (Van Zijl
et al
.,
1991; G. Wensvoort, personal communication).
All these observations indicate that E1 expressed by the
baculovirus-insect cell system may be a very effective
dead subunit vaccine.
Thirdly, pigs infected with field virus can be dis-
criminated from pigs vaccinated with E1 on the basis of
presence (field virus infection) or absence (vaccination
with E1) of antibodies directed against other immuno-
genic structural or non-structural viral proteins. Such a
discriminating serological test has to be developed and
should accompany the E1 subunit vaccine in a vaccina-
tion campaign for a controlled eradication of classical
swine fever during outbreaks of the disease.
Application of CSFV proteins expressed with a
baculovirus vector in diagnostic tests
The pestiviruses CSFV, BVDV and BDV are struc-
turally, antigenically, and genetically, closely related.
The genomic organization of these viruses are similar.
Sera induced in pigs infected with CSFV and in pigs
and ruminants infected with BVDV, cross-react in neu-
tralization assays (Wensvoort et al
.,
1989). Therefore,
a serological test which specifically detects antibod-
ies in field sera directed against CSFV specific epi-
topes, is essential to differentiate between pigs infected
with CSFV and pigs infected with BVDV or BDV. For
CSFV, such a serological test (Wensvoort et al., 1988)
is available commercially for several years already. In
this diagnostic ELISA, which detects antibodies in field
sera, which are specifically directed against conserved
(present on all CSFV strains) epitopes on E1 of CSFV
(Wensvoort, 1989), native E1 was used as antigen.
This antigen, however, has now been replaced by E1
synthesized in insect cells. Replacement of native El
appeared to have several advantages. Instead of inten-
sive large scale isolation of native E1 from porcine
kidney cells infected with CSFV, serum-free medium
of Sf21 cells infected with BacE1[-], grown in small
culture flasks (75 ), can be used directly in this
assay. Because of the constant quality of the E1 pro-
duced in insect cells, 75% less inconclusive results with
field sera were scored in this ELISA. Furthermore, E1
synthesized in insect cells improved the sensitivity of
the test (R. Bloemraad, personal communication).
Animals infected with pestiviruses also raise anti-
bodies against envelope glycoprotein E2 (Kwang et al.,
1992). Glycoprotein E2 of CSFV, recently identified as
a ribonuclease, can also be expressed in large amounts
in insect cells (Hulst et al
.,
1994). The ribonuclease
specific activity of E2 produced in insect cells is com-
parable to that of native E2 (Schneider et al
.,
1993),
indicating that the conformation of E2 synthesized in
insect cells is indistinguishable from native E2. There-
fore, E2 synthesized in insect cells could also be suit-
able as antigen in an serological test for the detection
of field infections. Preliminary results in our laborato-
ry show that such a test can indeed be developed on
the basis of E2. Nevertheless, before a reliable, sensi-
tive, and CSFV specific serological test based on E2
produced in insect cells can be employed commercial-
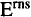
276
ly, monoclonal antibodies, specifically directed against
conserved epitopes (present on all CSFV strains) on
E2 of CSFV, have to be produced. For the production
of these monoclonal antibodies, properly synthesized,
and processed viral proteins synthesized in insect cells
should be very suitable.
Because animals infected with pestiviruses also
raise antibodies to the non-structural viral protein, p80
(Donis & Dubovi, 1987), a diagnostic test for the detec-
tion of field infections could also be developed on the
basis of this protein. Using p80 expressed in insect
cells as antigen in an ELISA, Petric et al. (1992) and
Vanderheijden et al. (1993), showed that anti-BVDV
antibodies could be detected in field sera from cattle
infected with BVDV. Because the degree of homolo-
gy between the amino acid sequences encoding p80
of BVDV, BDV and CSFV is high (more than 86%;
Moormann et al
.,
1990), sera from pigs infected with
BVDV and BDV cross-react with CSFV p80. There-
fore, a serological test on the basis of p80 will not
be suitable to differentiate between pigs infected with
CSFV, BVDV and BDV.
Concluding remarks
The baculovirus-insect cell expression system has pro-
vided us with properly synthesized envelope proteins
E1 and E2 of CSFV. Because the production level in
insect cells of these proteins is high, we are able to
apply these expression products for various purposes.
Nevertheless, in the literature their are many exam-
ples that the production levels of viral proteins in the
baculovirus insect cell system can be poor. In our lab-
oratory, the production levels of the structural proteins
of porcine reproductive and respiratory syndrome virus
(Lelystad virus) in insect cells were poor compared to
CSFV E1 and E2 (J. Meulenberg, personal communi-
cation). Instability of the messenger RNAs of the viral
proteins in the nuclei of insect-cells or instability of the
proteins themselves, as a result of a different process-
ing or routing in insect cells compared to mammalian
cells, may be responsible for this low level of pro-
duction. It therefore should be recognized that RNA
viruses like CSFV and Lelystad virus replicate in the
cytoplasm of the mammalian cell. For the production of
proteins of these kind of viruses, eukaryotic expression
systems based on vectors replicating in the cytoplasm,
like the semliki forest virus vector system (Liljeström
& Garroff, 1991), or the vaccinia virus vector system
(Mackett et al
.,
1982), might be more suitable.
Acknowledgments
We thank Just Vlak and John Martens for providing the
Spodoptera exiqua larvae and for their help to infect
the larvae with the baculovirus-E1 recombinants. The
data of the diagnostic ELISA using E1 were kindly
provided by Rinus Bloemraad.
Addendum in proof
In this paper we still used the old pestivirus protein
nomenclature. A new nomenclature of these proteins
will, however, be proposed to the International Com-
mittee on Taxonomy of Viruses by the Flaviviridae
Study Group. In recent papers this new nomenclature
has already been used by several research groups. The
nomenclature of pestivirus proteins used in this paper
compared to the new one is as follows: E1 is renamed
E2, E2 is renamed , E3 is renamed El, and p80 is
renamed NS3.
References
Barteling S & Vreeswijk J (1991) Developments in foot and mouth
disease vaccines. Vaccine 9: 75–88.
Donis R & Dubovi E (1987) Molecular specificity of the antibody
responses in cattle naturally and experimentally infected with
cytopathic and noncytopathic bovine viral diarrhea virus bio-
types. American Journal of Veterinary Research 48: 1549–1554
Francki R, Fauquet C, Knudson D & Brown F (1991) Flaviviridae
Arch. Virol. Suppl. 2: 223–233.
Herbert W (1965) Multiple emulsions: A new form of mineral-oil
antigen adjuvant. Lancet 11: 771
Hulst M, Westra D, Wensvoort G & Moormann R (1993) Glyco-
protein E1 of hog cholera virus expressed in insect cells protects
swine from hog cholera. J. Virol. 67: 5435–5442.
Hulst M, Himes G, Newbigin E & Moormann R (1994) Glycoprotein
E2 of classical swine fever virus: expression in insect cells and
identification as a ribonuclease. Virology 200: 558–565.
Kuroda K, Geyer H, Geyer R, Doerfler W & Klenk H (1990) The
oligosaccharides of influenza virus hemagglutinin expressed in
insect cells by a baculovirus vector. Virology 174: 418–429.
Kwang J, Littledike E, Donis R & Dubovi E (1992) Recombinant
polypeptide from the gp48 region of the bovine viral diarrhoea
virus (BVDV) detects serum antibodies in vaccinated and infected
cattle. Vet. Microbiol. 32: 281–292.
Liljeström P & Garoff H (1991) A new generation of animal cell
expression vectors based on the Semliki forest virus replicon.
Biotechnology 9: 1356–1362.
Mackett M, Smith G & Moss B (1982) Vaccinia virus expression
vector. PNAS 79: 7415–7419.
Moennig V & Plagemann (1992) The pestiviruses. Adv. Virus Res.
41:53–98
Moormann R, Warmerdam P, van der Meer B, Schaaper W,
Wensvoort G & Hulst M (1990) Molecular cloning and nucleotide
277
sequence of hog cholera virus strain Brescia and mapping of the
genomic region encoding envelope protein El. Virology 177:
184–198.
Petric M, Yolken R, Dubovi E, Wiskerchen M & Collett M (1992)
Baculovirus expression of pestivirus non-structural proteins. J.
Gen. Virol. 73: 1867–1871.
Rea T, Timmins G, Long G & Post L (1985) Mapping and sequence
of the gene for the pseudorabies virus glycoprotein which accu-
mulates in the medium of infected cells. J. Virol. 54: 21–29.
Schneider R, Unger G, Stark R, Schneider-Scherzer E & Thiel H-J
(1993) Identification of a structural glycoprotein of a RNA virus
as a ribonuclease. Science 261: 1169–1171.
Terpstra C & Wensvoort G (1988) The protective value of vaccine
induced neutralizing antibody titres in swine fever. Vet. Microbi-
ol. 16: 123–128.
Thiel H-J, Stark R, Weiland E, Rümenapf T & Meyers G (1991) Hog
cholera virus: Molecular composition of virions from a pestivirus.
J. Virol. 65: 4705–4712.
Vanderheijden N, De Moerloze L, Vandenbergh D, Chappuis G,
Renard A & Lecomte C (1993) Expression of the bovine viral
diarrhoea virus Osloss p80 protein: its use as antigen for cattle
serum antibody detection. J. Gen. Virol. 74: 1427–1431.
Van Rijn P, van Gennip R, de Meyer E & Moormann R (1993)
Epitope mapping of envelope glycoprotein El of hog cholera
virus strain Brescia J. Gen. Virol. 74: 2053–2060
Van Zijl M, Wensvoort G, de Kluyver E, Hulst M, van der Gulden H,
Gielkens A, Berns A & Moormann R (1991) Live attenuated
pseudorabies virus expressing envelope glycoprotein El of hog
cholera virus protects swine against both Pseudorabies and Hog
Cholera. J. Virol. 65: 2761–2765.
Vlak J, Schouten A, Usmany M, Belsham G, Klinge-Roode M,
Maule A, van Lent J & Zuidema D (1990) Expression of
cauliflower mosaic virus gene I using a baculovirus vector based
upon the p10 gene and a novel selection method. Virology 179:
312–320.
Wensvoort G, Terpstra C & de Kluyver E (1989) Characterization of
porcine and some ruminant pestiviruses by cross-neutralization.
Vet. Microbiol. 20: 291–306.
Wensvoort G, Bloemraad R & Terpstra C (1988) An enzyme immuno
assay employing monoclonal antibodies and detecting specifical-
ly antibodies to classical swine fever virus. Vet. Microbiol. 17:
129–140.
Wensvoort G (1989) Topographical and functional mapping of epi-
topes on hog cholera virus with monoclonal antibodies. J. Gen.
Virol. 70: 2865–2876.
Wensvoort G, Boonstra J & Bodzinga B (1990) Immuno-affinity
purification of envelope protein El of hog cholera virus. J. Gen.
Virol. 71: 531–540.
Address for correspondence: Rob J.M. Moormann, Department of
Mammalian Virology, Institute for Animal Science and Health (ID-
DLO), P.O. Box 65, NL–8200 AB Lelystad, the Netherlands.
This page intentionally left blank.

Cytotechnology
20:
279–288, 1996.
279
© 1996 Kluwer Academic Publishers. Printed in the Netherlands.
Production of multidomain complement glycoproteins in insect cells
Péter Závodzky & Sándor Cseh
Institute of Enzymology, Biological Research Center, Hungarian Academy of Sciences, Budapest, Pf. 7., H-1518,
Hungary
Key words: baculovirus, complement activation, genetic engineering, mosaic protein, serine-protease, zymogen
Abbreviations: AcMNPV – Autographa californica nuclear polyhedrosis virus; C1 – first component of comple-
ment; Clq, Clr, Cls – subcomponents of Cl; CCP – complement control protein; EGF – epidermal growth factor;
High5 – Trichoplusia ni cell line; SCR – short concensus repeat; Sf9 – Spodoptera frugiperda 9 cell line.
Introduction
The scarcity of sources makes the isolation of most
human proteins, peptides and proteinaceous materi-
als impossible or impractical. On the other hand there
exists a great demand for complex mammalian gly-
coproteins for research, diagnosis or therapy. Glyco-
proteins of various complexity have been expressed
in various laboratories (see proceddings of the Work-
shop on baculovirus and recombinant protein produc-
tion processes, edited by Vlak et al
.,
1992) using the
baculovirus expression system. Since insect cells do
almost all of the posttranslational modifications asso-
ciated with mammalian cells, the expressed proteins
are expected to be near-authentic concerning their bio-
logical activity and antigenicity. To produce complex,
glycosylated and processed proteins we have the option
of choosing from a number of different expression sys-
tems, each of which has a particular inherent set of
advantages and disadvantages. Bacterial systems pro-
vide important advantages, like the ease of use, low
cost and high level of expression, but impose a num-
ber of limitations for synthesis of eukaryotic proteins
(folding, proteolytic processing, glycosylation, secre-
tion, subunit assembly, etc.). For this reason eukaryotic
cells are preferred for expression of mammalian genes.
There are several host-vector systems for heterologous
gene expression in yeast or mammalian cells. The dis-
advantages of the mammalian expression systems are
the high cost and complexity of the cell culture main-
tenance and the difficulty of manipulating large viral
genomes, as well as the relatively low level of expres-
sion, that might be a high price for the correct folding
and full biological activity. Insect cell based expres-
sion systems are advantageous, since they are cost
effective, simple in handling while performing glyco-
sylation, fatty acid acylation, phosphorylation, disul-
fide formation, C-terminal amidation, hydroxylation
of aspartate residues, signal peptide cleavage, folding
and secretion. Therefore insect cells seem to be suit-
able for efficient expression of recombinant complex
mammalian glycoproteins in biologically active form.
Because of its complexity human complement
protein, Cl is an ideal object to test the potential
of baculovirus-insect cell expression for producing
sophisticated mammalian proteins.
Complement is a complex multicomponent system
having many similarities with blood coagulation and
fibrinolysis. The classical pathway of complement acti-
vation is initiated by the reaction of the first component,
Cl, with immune complexes. This recognition leads to
removal of the invading bacteria, virus or toxin.
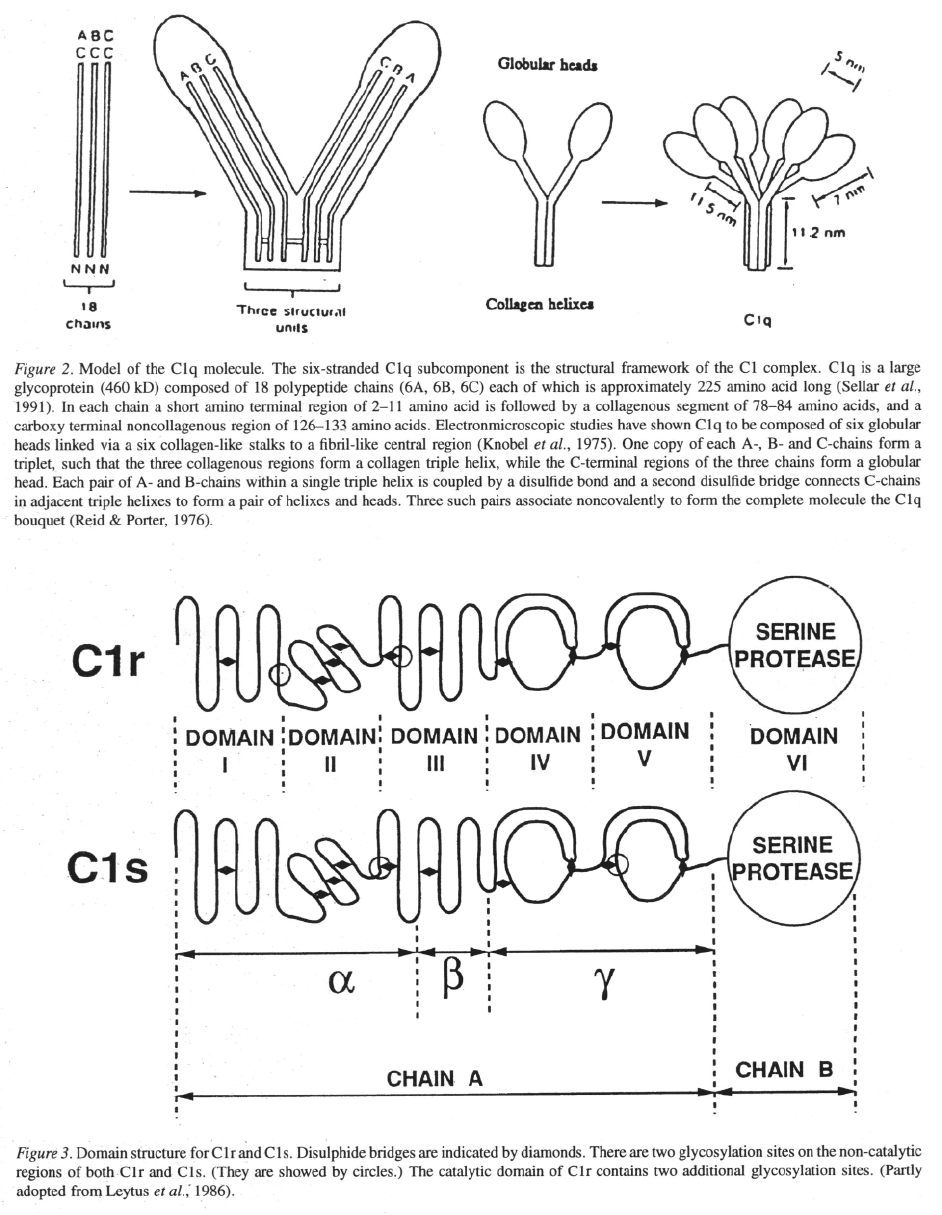
The first component of complement is a supramole-
cular complex composed of five subcomponents: one
Clq, two Clr and two Cls. A specific inhibitor protein,
Cl-inhibitor, regulates the activation and function of
the C1 complex (reviewed by Arlaud et al
.,
1987; Schu-
maker et al
.,
1987). Cl is a complex multifunctional
molecule, and the complexity of its function and reg-
ulation is also reflected in the structure of its subunits:
Clq is the structural framework of the Cl complex
(Reid & Porter, 1976; Borsos et al
.,
1980) and its inter-
esting flower boquet like structure is shown in Figure
1 and Figure 2. The dependent C1sC1rC1rC1s
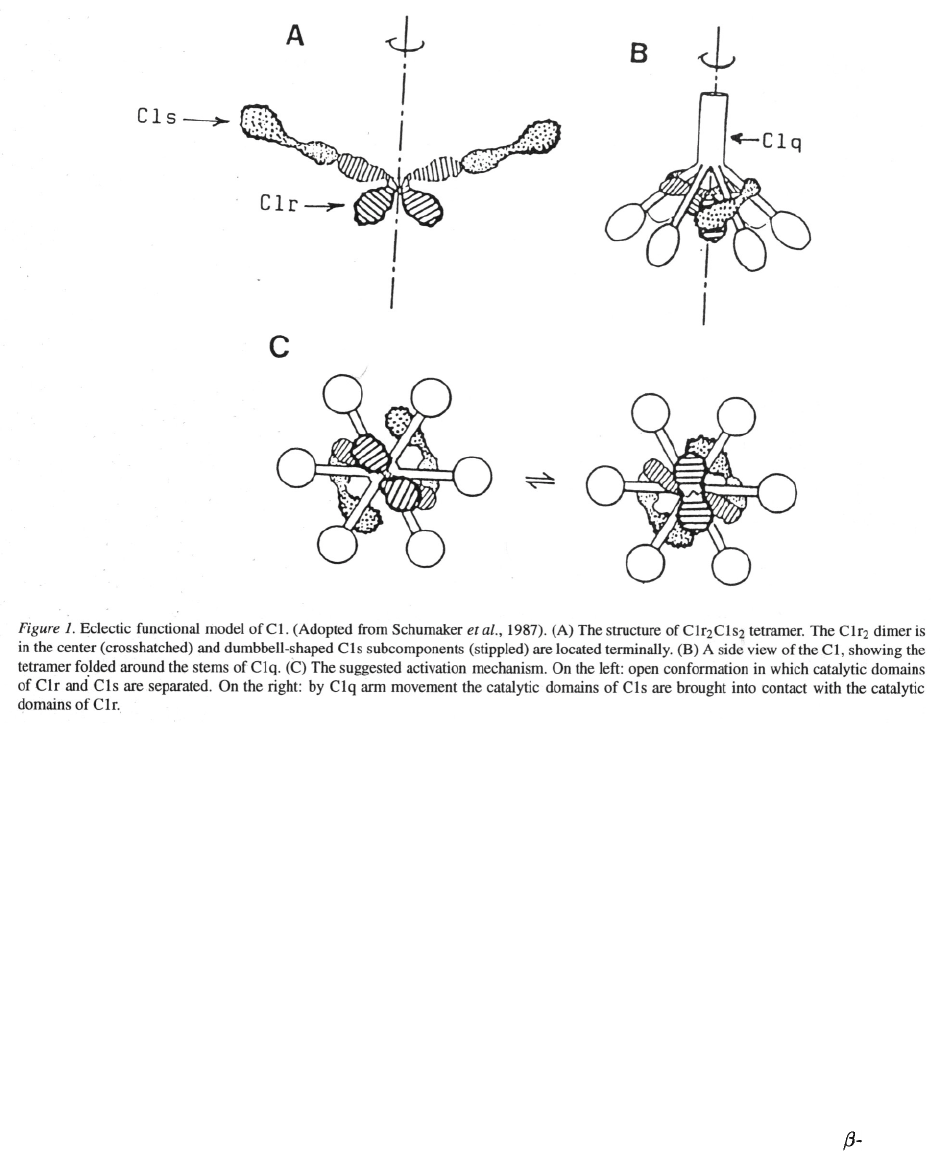
280
tetramer is responsible for the enzymatic activity of
C1 (Tschopp et al., 1980). C1r and C1s are zymogen
serine-proteases in the inactive C1 and the tetramer is
folded around the collagenous stems of C1q (Figure 1).
When C1 binds – through the C1q heads – to immune
complexes, the cone formed by the spreading arms will
be distorted. This distortion might be the activitation
signal that induces a conformational change convert-
ing the C1r proenzyme into an enzyme. The activated
C1r in turn activates C1s by the cleavage of an Arg-Ile
bond. The following initiation of the complement cas-
cade is sustained through the proteolytic action of C1s
on C4 and C2.
The C1r and C1s zymogens are single chain gly-
coproteins (86–78 kD) containing 688 (C1r) and 673
(C1s) amino acid residues (Leytus et al
.,
1986; Tosi
et al
.,
1987). There is strong relationship between the
two molecules: sequence comparison revealed 40%
amino acid identity and conservation of all the cys-
teine residues (Tosi et al., 1987). Both C1r and C1s
have been cloned and sequenced and have mosaic like
structures typical of plasma serine-proteases (Leytus
et al
.,
1986; Tosi et al
.,
1987; Mackinnon et al
.,
1987;
Patthy et al
.,
1994). Both C1r and C1s can be divid-
ed into six structural motifs (domains or modules),
including two pairs of internal repeats (I/III and IV/V),
a single copy of motif II and the trypsin like serine-
protease module (Figure 3).
Modules I and III are homologous domains, they
may be involved in a function that is highly specif-
ic to C1r and C1s (for example: Clr-Cls interaction,
tetramer-C1q binding). Module II shows homology
with epidermal growth factor (EGF). In general such
domains are exposed to the extracellular environment
and participate in protein-protein interactions. This
domain harbours an unusual amino acid, hydroxy
asparagine in position 150 in C1r and 134 in C1s. The
level of hydroxylation in native human C1r is 100%
281
