Vij D.R. Handbook of Applied Solid State Spectroscopy
Подождите немного. Документ загружается.

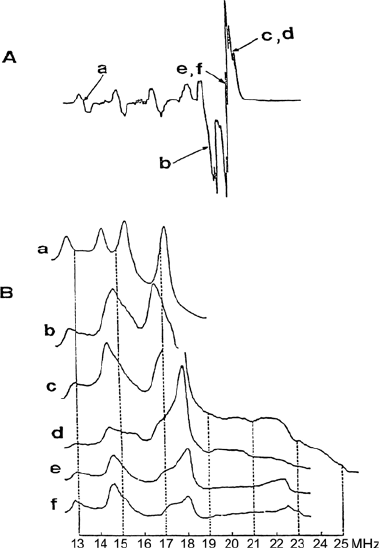
with the magnetic field perpendicular to the complex plane occur at low field.
By setting the magnetic field in the low field region of the EPR spectrum
(indicated by “a” in Figure 4.9a), the perpendicular orientation is selected.
The ENDOR spectrum taken at this field value (Figure 4.9b, line a) has a
crystal-like character. It consists of two
14
N hyperfine lines, each split by
quadrupolar coupling. Thus the nitrogen à and Q coupling tensor compo-
nents with respect to the symmetry axis of g and the Cu hyperfine tensors
were determined.
Figure 4.9 Cu(pic)
2
in Zn(pic)
2
.
4 H
2
O powder. (A) EPR spectrum. The magnetic field settings
chosen for the ENDOR spectrum are indicated by arrows. (B)
14
N ENDOR spectra referring to
the field settings indicated in the EPR spectrum. For the magnetic field set to a the ENDOR
spectrum has a crystal like character. c and d as well as e and f differ only by a small shift in
the field. From [7].
However, all the components of the hyperfine tensor cannot be determined
form the crystal-like spectra. Additionally, when the g and the hyperfine
tensors are not coaxial, the hyperfine component thus measured, in general,
does not correspond to a principal value.
Several theoretical approaches for analyzing ENDOR spectra taken at
arbitrary field values and corresponding to multiple orientations, have been
reported [8, 9]. Consequently, ENDOR data can be used to characterize quite
general cases of hyperfine coupling. The elements of the hyperfine tensor Ã
4. ENDOR Spectroscopy
176
and its relative orientation to the g tensor frame can be deduced from the
variation of the hyperfine coupling value at different field settings across the
EPR spectrum. These techniques are called angle or orientation selection.
In the angle selection method, ENDOR spectra are recorded at each point
in the EPR powder pattern including the turning points. From crystal-like
spectra approximate principal values are obtained. Simulation of selected
spectra is then carried out by varying the respective parameters. For a series
of metalloenzymes, ENDOR lines obtained with the angle selection method of
many nuclei including
1
H,
2
H,
13
C,
14,15
N,
17
O,
33
S,
57
Fe,
63,65
Cu, and
95,97
Mo
have given valuable information about the sites of the metal center and a
bound ligand or substrate [10].
The angle selection method has been applied to many different systems:
transition metal complexes [11] in which the g tensor anisotropy dominates;
nitroxide radical [12] with strongly anisotropic nitrogen hyperfine couplings;
and organic biradicals [13] in which the magnetic anisotropy results from the
electron-electron coupling (D) tensor.
Besides the requirement of considerable anisotropy of the magnetic
interaction in the system, another important condition must be fulfilled for
one to observe crystal-like spectra and apply the angle selection method:
Saturation of particular EPR transitions should be possible. This condition is
satisfied when the process causing transfer of saturation from one orientation
to another is slow compared to the spin-lattice relaxation rate. Such a process
is called spin diffusion.
Spin diffusion is slow relative to the spin-lattice relaxation in transition
metal complexes and biomolecules containing metal ions. In contrast, in the
case of radicals in organic molecular crystals (at low temperature) the spin-
lattice relaxation is slower than the spin-diffusion processes. Consequently,
the saturation is transferred to all other EPR transitions in a time short with
respect to T
1e
, the electronic spin-lattice relaxation time. As a result, powder-
type ENDOR spectra are observed [14]. At higher temperatures, however, the
spin-diffusion may be slower, making selective saturation possible [12].
Regardless of the type of ENDOR spectra observed in disordered solids,
they are much easier to interpret than EPR powder patterns.
4.4 PULSED-ENDOR
The conventional CW-ENDOR method has a serious disadvantage: The
intensity of the ENDOR signals depends critically on the balance between the
relaxation and induced transition rates (saturation conditions for both EPR
and NMR transitions have to be satisfied). The experimental parameters [4],
in general, cannot be independently optimized. Thus, the ENDOR transitions
are often not observed because of unfavorable relaxation rates. Moreover,
even when all experimental variables are optimized, the ENDOR signal
177
4.4 Pulsed-ENDOR
intensity is only a few percent of the EPR signal. The pulsed ENDOR
techniques offer greater sensitivity, higher spectral resolution, as well as
reduced time of the measurement. The most important advantage of pulsed-
ENDOR (or sometimes called ESE-ENDOR or time-resolved ENDOR) over
CW is that there is no restriction to balance relaxation and induced transition
rates. Consequently, the temperature is not such a critical parameter in pulsed
ENDOR spectroscopy. Additionally, the pulsed technique permits a study of
transient radicals.
In a CW experiment both microwave (mw) and radio frequency fields are
applied continuously to the sample during the experiment. In a pulsed
ENDOR experiment the pulse sequence, composed of short, intense mw (of
the order of hundreds of nanoseconds) and rf (microsecond) pulses, is applied.
A pulse of an oscillating field is characterized by its frequency Z as well as its
time of duration t. Both of these parameters can be adjusted to invert the
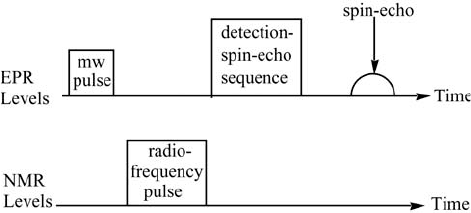
population of the respective levels. The general scheme (Figure 4.10) for a
population transfer pulsed-ENDOR experiment (Davies sequence) is as
follows:
Figure 4.10 Schematic representation of the population transfer pulsed-ENDOR (Davies)
method.
(1) First, a microwave S pulse is applied to invert the population of the
selected EPR transition.
(2) After a time, short compared to the electron spin-lattice relaxation time,
the RF S pulse is applied. Depending on the excitation frequency of
this pulse (NMR frequency), the pulse will or will not change the
population of the nuclear transitions.
(3) The effect of the RF pulse on the EPR transition is then detected by an
electron spin-echo sequence.
While varying the NMR frequency, the whole sequence is repeated (at a
rate slower than the spin-lattice relaxation rate). A plot of the amplitude of
the spin-echo vs. the NMR frequency constitutes the ENDOR spectrum.
There is another commonly used pulsed sequence referred to as Mims
ENDOR. In this technique the S pulse at the RF frequency is applied between
the second S/2 and third S/2 mw pulse (two S/2 pulses to invert EPR
4. ENDOR Spectroscopy
178
population) in contrast to the Davies ENDOR, where the RF pulse is applied
between the first S and first S/2 microwave pulses: The Mims ENDOR
experiment is particularly effective for weakly coupled nuclei but some blind
spots do occur where frequencies cannot be observed [15]. It is often
advantageous to combine data from both the Mims and the Davies ENDOR
response [16]. For nuclei with large magnetic moments such as
1
H and
19
F, Q
n
at the X-band is often larger (14 MHz) than the hyperfine field and so the
ENDOR response is centered at the Larmor frequency Q
n
of the nucleus and is
split by the hyperfine coupling A
n
and by quadrupole interactions. For nuclei
with smaller magnetic moments like
2
H or
14
N, the ENDOR response is
centered at half the hyperfine coupling frequency and split into two lines that
are separated by twice the Larmour frequency. Often times, Q
n
= 14 MHz at
the X-band is comparable to the magnitude of many nuclear hyperfine
couplings, which results in substantial overlap of the proton ENDOR signals
with signals from other nuclei. This problem is solved by recording ENDOR
spectra at higher (fields such as 35 (Q-band) [17] or 95 (W-band) [18] GHz
microwave frequencies where Q
n
increases proportionally to the external field
but A
n
is independent of field, and results in a separation of overlapping
ENDOR lines.
Several pulsed displays have been developed. As an example, Figure 4.11
shows (a) the overlapping
1
H and
14
N resonances in the Davies-type ENDOR
spectrum and (b) the selected
14
N resonances obtained by the hyperfine-
ENDOR technique are given in [19–21]. Before attempting these books, an
easier-to-understand review of the basic principles in pulsed-ENDOR and the
related electron spin-echo envelope modulation (ESEEM) spectroscopy has
been given [16]. An earlier review of pulsed-ENDOR techniques is given in
[15].
A review of ESE-ENDOR techniques, tailored to the study of magnetic
nuclei coupled to paramagnetic metals and radicals, has been published with
illustrative examples from metal and radical centers in photosystem II [22].
The pulsed-Davies ENDOR experiment for S = ½ and I = ½ was developed
for W band (95 GHz) ENDOR effects observed at low temperature [23].
Two-dimensional TRIPLE experiments have been carried out on
orientationally disordered disordered samples and shown it can resolve
overlapping powder patterns. This makes it possible to resolve and determine
the relative orientation of the hyperfine tensors and the relative signs of the
hyperfine couplings [24].
4.4 Pulsed-ENDOR
selective ENDOR techniques [19]. More complete details of the pulsed-
179
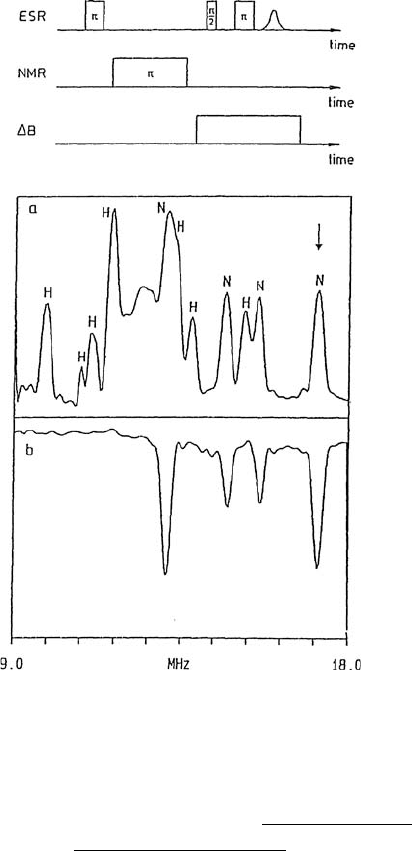
Figure 4.11 Cu(pic)
2
/Zn(pic)
2
single crystal. (a) Conventional (Davies-types) pulsed-ENDOR
spectrum with
1
H and
14
N lines using the pulse sequence given above. (b) Hyperfine-selective
14
Commercial ENDOR spectrometers at X-, Q- and W-bands are available
from Bruker BioSpin Corp. in North America (epr@bruker.com
) and Bruker
Biospin GmbH in Europe (erp@bruker-biospin.de)
. The CW-ENDOR X-band
spectrometer is known as an ELEXSYS E-560-D, which uses a TM cavity
with vertical access. The pulsed-EPR at X-band is known as E-560-D-P using
a TE cavity (cylindrical), vertical access. The pulsed-ENDOR system at Q-
band (34 GHz) is the ELEXSYS E-580-Q and the pulsed-ENDOR system at
W-band is known as the ELEXSYS E-680, while the CW-ENDOR at W-band
is the E-600.
4.5 APPLICATIONS
ENDOR measurements can be used extensively to gain resolution in a
complex spectrum, to separate overlapping EPR spectra, and to identify the
4. ENDOR Spectroscopy
N ENDOR spectrum. Figure 4.9 shows powder spectra. From [19].
180
nuclei responsible for the coupling. This technique has been applied to
various systems. In the following section, examples are given for studying (a)
organic radicals in organic host crystals, (b) radicals trapped in xenon, argon,
or Freon matrices, (c) triplet state radicals in crystals or polycrystalline
samples, (d) free radicals in biological systems, (e) polymeric systems, (f)
inorganic radicals in irradiated inorganic single crystals, (g) paramagnetic
complexes in organic single crystals, (h) F and H centers found in inorganic
host lattices, (i) paramagnetic inorganic ions in organic crystals, (j) transition
metal ion complexes observed in frozen solution and powders, (k) defects and
complexes on surfaces such as silica alumina, zeolite, and nafion, (l) impurity
centers in semiconductor host crystals, (m) spin centers in silicon and borate
systems, (n) paramagnetic centers in cubic host crystal and (o) perovskite-type
materials. Selected examples are given in each area with references noted.
A detailed tabulation of the published ENDOR data for single crystals,
polycrystalline solids and biological materials for 58 isotropic species through
1993 has been given in the Handbook of Electron Spin Resonance [25].
Earlier ENDOR studies in disordered matrices, crystalline systems, hemes and
hemoproteins, iron-sulfur proteins, radiation biophysics, polymer studies, and
triplet state systems in solids were reviewed in Dorio and Freed (eds.) [26].
4.5.1 Organic Radicals in Organic Host Crystals
Often the crystals are irradiated at a low temperature and then the radicals
identified as the temperature is raised. Electron attachment and proton
abstraction occur at different temperatures so intermediate radicals can be
formed. An example of the principal values of the hyperfine couplings for the
D- and E-protons and the
14
N couplings for the radical formed [27] upon X-
irradiation of deoxycytidine-5'-phosphate-hydrate at 11 K and measured by
X-band ENDOR techniques at 6 K is given in Table 4.3. Two Mrad/hour of
irradiation was applied for 2–4 hours. Because of the increased resolution, the
couplings can be determined more precisely and it is possible to detect the
presence of several radicals. Radicals I and II
a
and II
b
were identified at 6 K.
Typically the primary radical is formed at the lowest temperature as a result of
reaction with the electrons formed from the X-irradiation. However, as the
temperature is raised, the unstable primary radical decays—forming other
products which may or may not be radicals. An example [28] where different
radicals were identified as a function of temperature occurred upon 4.2 K
X-irradiation of a single crystal of citric acid (2-hydroxy-1,2,3-propane
tricarboxylic acid), partially deuterated and warmed to 100 K. ENDOR
analysis achieved upon cooling the radicals formed at 100 K to 4.2 K, thus
quenching the reaction, are given in Table 4.4.
Radical I is formed by the loss of a carboxylic acid group from the 2-
position and radical II is due to electron attachment to the carboxylic acid
group located at the 3-position. Upon warming to 300 K, radical II decays and
181
4.5 Applications
radical III is detected where loss of the Į-proton from the 3-position is
detected. Two nonequivalent crystal sites are detected for radical III and the
Į-couplings for all sites have been determined. It was also found [28] that
radical I was stable at 300 K; demonstrating that upon decay of radical I,
radical III is an end-product of the decay of radical II. Secondary radical
products stable at room temperature have been studied to examine whether the
free radical position in the crystal is the same as that of the undamaged
molecules. The ENDOR resolution permits small couplings (on the order of
1 MHz due to distant protons) to be precisely measured. From the resulting
proton tensor, dipolar distances can be estimated and thus proton-couplings
from neighboring molecules and hydrogen-bonded protons can be assigned
based on crystal structure data.
Table 4.3 ENDOR hyperfine coupling parameters at 6 K of 11 K X-irradiated deoxycytidine
5'-phosphate-H
2
O (5' dCMP) single crystal) [27].
Principal Values (mT)
Radical I Radicals IIa and IIb
–2.243 ± 0.008 –2.260 ± 0.005
H
D
–1.592 ± 0.005 H
D1
–0.732 ± 0.010
–0.651 ± 0.005 –1.184 ± 0.008
a
iso
=
–1.495 a
iso
=
– 1.392
1.381 ± 0.004 –2.275 ± 0.003
H
E
1.439 ± 0.004 H
D2
–0.587 ± 0.003
1.623 ± 0.004 –1.190 ± 0.010
a
iso
= 1.481 a
iso
=
–1.350
1.520
N
1
+ N
3
0.615
0.011
a
iso
= 0.715
Radical I
Radical IIa Radical IIb
4. ENDOR Spectroscopy
182
Table 4.4 Citric acid single crystal, partially deuterated. X-irradiated at 4.2 K [28]. 4.2 K
ENDOR measured E-proton couplings for radicals I and II after crystal warmed to (100 K).
Radical I Radical II Radical I Radical III
4.2 K 4.2 K Stable Conformation Stable
Room
Room Temperature Temperature
Hyperfine Hyperfine Hyperfine
Tensors (MHz) Tensors (MHz) Tensors (MHz)
113.9 90.7 105.2
E
1
106.7 E
5
80.5 E
1
96.1 D' –94.2
102.3 79.4 92.0 (site 1) –58.6
107.5 23.1 82.0 –31.9
E
2
98.9 E
6
16.7 E
2
70.2
97.5 1.2 68.0
40.0 77.6 –92.3
E
3
26.0 70.3 D" –58.3
24.2 E
3
64.7 (site 2)
31.0
In Table 4.5 is given an example [29] where potassium hydrogen malonate
single crystals were Ȗ-irradiated at room temperature and
1
H and
13
C
––
Radical III
E
4
3
2
.
0
1
9
.
3
1
7
.
8
C–C–C–C–C
HH
O
D
O
O
H
O
O
D
D
C
ODO
Radical II
E
4
27.1
12.7
11.3
C–C–C–C–C
HH
O
D
O
O
H
O
–
O
D
D
C
ODO
Radical I
C–C–C–C–C
HH
O
D
O
O
H
O
O
D
D
H
H
couplings were measured for the OOC HCCOO radical in the host lattice.
–
183
4.5 Applications

Table 4.5 J-irradiated potassium hydrogen malonate (KH) single crystal [29].
1
H and
13
C
hyperfine tensors (MHz) from ENDOR measurements at room temperature.
D-
1
H
13
C
_____________________________________________________________________
–27.4 23.2
–
OOCCHCOO
–
–86.8 33.0 radical
_____________________________________________________________________
Hyperfine tensor of the distant protons.
a
Principal Values (MHz)
_____________________________________________________________________
Distance
Proton
b
Isotropic Dipolar R
C...H
(Å)
_____________________________________________________________________
A 4.32 3.32
B 0.04 4.28 –1.83 3.33
C 0.07 1.95 4.33
D 0.10 1.79 0.66 4.45
E 1.83 0.61 4.42
_____________________________________________________________________
a
The signs of the couplings are not obtained from the experiment.
b
the crystal structure data.
In this study, it was found that upon loss of an Į-hydrogen, the resulting
free radical retained the same position as the parent molecule. Often this is
not the case, and considerable movement upon radical formation occurs, all
dependent on the intermolecular packing arrangements of the original crystal.
ENDOR measurements of nuclei other than
1
H and
13
C can also be carried
out. Studies of deuterium [30], nitrogen [27]
23
Na [31], and chlorine-35 [32]
hyperfine coupling constants, as well as their?coupling constants have been
reported. From such data, structural relationships can be deduced. For
example the relationship between deuterium quadrupole constants and the N-
D and D…O bond lengths in a perdeuterates sulphamic acid (ND
3
SO
3
) single
crystal were reported [30]. Example of the couplings for D and
35
Cl deduced
are given in Table 4.6.
ENDOR measurements at 1.6 K have been used to deduce the exchange-
able dissociation proton coupling, the D-proton, the exchangeable O-H proton
in the undamaged structure and in the alkoxy radical, as well as the Jand E
proton, and G-methyl protons in a crystal of rhamnose X-irradiated at
4. ENDOR Spectroscopy
–1.14 –2.36 –1.96
Protons of neighboring molecules and hydrogen-bonded protons; protons assignment refers to
–2.32
–0.89 –1.07
–1.21
–56.3 207.8
–1.14
–0.04
184

guanosine 5'-monophosphate single crystals have been reported [34]. 4.2 K
[33]. Examples of radicals formed at 10 K and 65 K in X-irradiated
Table 4.6 Perdeuterated sulphamic acid (ND
3
SO
3
) single crystal.
2
H ENDOR at 4.2 K [30].
X-irradiated phenancyl chloride:
1
H and
35
Cl hyperfine coupling constants and
35
Cl quadrupole
coupling constant (MHz), ENDOR at 123 K [32].
Deuteron Quadrupole Tensors Phenancyl chloride Single Crystal
Deuteron Eigenvalues of Q
ii
D
1
z 257.4 (–)26.5
y –169.6 A(
1
H) (–)52.5 a
iso
= –5.30
x –87.8 (–)80.0
e
2
qQ/h = 171.6
K = 0.370
z 273.0 (–)08.3
y –163.9 A(
35
Cl) (–)15.8 a = 7.3
D
2
x –109.1 45.6
e
2
qQ/h = –182.0
K = 0.201
z 266.0 5.3 O
y –182.2 Q(
35
Cl) 5.9 »»
D
3
x –83.8 –11.2 CCHCl
e
2
qQ/h = 177.3 radical
K = 0.318
_________________________________________________________
a
Errors in tensor components are ± 2 kHz.
Radicals formed in J-irradiated organic single crystals often exhibit
internal motions, methyl groups rotate rapidly and t-butyl group rotate slowly
on an ENDOR time scale. This motion is reflected in the temperature
dependence of the electron spin relaxation times as detected by pulsed-EPR
measurements [35]. Typical examples are given by the study of irradiated
4-methyl-2,6-di-t-butylphenol [35] and irradiated 10-nonadecanone in urea
[36] where wobbling of the methylene groups and rotational motion of the
radical in the channel were detected. ENDOR studies can be useful for
determining the radiation chemistry of co-crystallized DNA base pairs [37,
38]. Varying temperature can be useful to follow the interconnecting of the
planar and puckered conformers of the adduct pyrroline ring [39] by ENDOR
measurements.
(kHz)
iso
185
4.5 Applications
