Tarek Ahmed. Reservoir engineering handbook
Подождите немного. Документ загружается.

Vasquez-Beggs’ oil compressibility correlation and the Petrosky-Far-
shad c
o
expression can be incorporated in Equation 2-114 to give:
For the Vasquez-Beggs c
o
equation:
where
A = 10
-5
[-1,433 + 5 R
sb
+ 17.2 (T - 460) - 1,180 g
gs
+ 12.61 °API]
For the Petrosky-Farshad c
o
expression:
with the correlating parameter A as given by Equation 2-111
Crude Oil Viscosity
Crude oil viscosity is an important physical property that controls and
influences the flow of oil through porous media and pipes. The viscosity,
in general, is defined as the internal resistance of the fluid to flow.
The oil viscosity is a strong function of the temperature, pressure, oil
gravity, gas gravity, and gas solubility. Whenever possible, oil viscosity
should be determined by laboratory measurements at reservoir tempera-
ture and pressure. The viscosity is usually reported in standard PVT
analyses. If such laboratory data are not available, engineers may refer to
published correlations, which usually vary in complexity and accuracy
depending upon the available data on the crude oil.
According to the pressure, the viscosity of crude oils can be classified
into three categories:
• Dead-Oil Viscosity
The dead-oil viscosity is defined as the viscosity of crude oil at atmos-
pheric pressure (no gas in solution) and system temperature.
• Saturated-Oil Viscosity
The saturated (bubble-point)-oil viscosity is defined as the viscosity of
the crude oil at the bubble-point pressure and reservoir temperature.
rr
oob b
Ap p=-exp[ ( )]
..0 4094 0 4094
(2 -116)
rr
oob
b
A
p
p
=
Ê
Ë
Á
ˆ
¯
˜
È
Î
Í
Í
˘
˚
˙
˙
exp ln (2 -115)
108 Reservoir Engineering Handbook
Reservoir Eng Hndbk Ch 02b 2001-10-24 09:26 Page 108

• Undersaturated-Oil Viscosity
The undersaturated-oil viscosity is defined as the viscosity of the crude
oil at a pressure above the bubble-point and reservoir temperature.
Estimation of the oil viscosity at pressures equal to or below the bub-
ble-point pressure is a two-step procedure:
Step 1. Calculate the viscosity of the oil without dissolved gas (dead oil),
m
ob
, at the reservoir temperature.
Step 2. Adjust the dead-oil viscosity to account for the effect of the gas
solubility at the pressure of interest.
At pressures greater than the bubble-point pressure of the crude oil,
another adjustment step, i.e. Step 3, should be made to the bubble-point
oil viscosity, m
ob
, to account for the compression and the degree of under-
saturation in the reservoir. A brief description of several correlations that
are widely used in estimating the oil viscosity in the above three steps is
given below.
METHODS OF CALCULATING VISCOSITY
OF THE DEAD OIL
Several empirical methods are proposed to estimate the viscosity of
the dead oil, including:
• Beal’s correlation
• The Beggs-Robinson correlation
• Glaso’s correlation
These three methods are presented below.
Beal’s Correlation
From a total of 753 values for dead-oil viscosity at and above 100°F,
Beal (1946) developed a graphical correlation for determining the viscos-
ity of the dead oil as a function of temperature and the API gravity of the
crude. Standing (1981) expressed the proposed graphical correlation in a
mathematical relationship as follows:
Reservoir-Fluid Properties 109
Reservoir Eng Hndbk Ch 02b 2001-10-24 09:26 Page 109

with
a = 10
(0.43 + 8.33/API)
where m
od
= viscosity of the dead oil as measured at 14.7 psia and
reservoir temperature, cp
T = temperature, °R
The Beggs-Robinson Correlation
Beggs and Robinson (1975) developed an empirical correlation for
determining the viscosity of the dead oil. The correlation originated from
analyzing 460 dead-oil viscosity measurements. The proposed relation-
ship is expressed mathematically as follows:
m
od
= 10
x
- 1 (2-118)
where x = Y(T- 460)
-1.163
Y = 10
Z
Z = 3.0324 - 0.02023°API
An average error of -0.64% with a standard deviation of 13.53% was
reported for the correlation when tested against the data used for its
development. Sutton and Farshad (1980) reported an error of 114.3%
when the correlation was tested against 93 cases from the literature.
Glaso’s Correlation
Glaso (1980) proposed a generalized mathematical relationship for
computing the dead-oil viscosity. The relationship was developed from
experimental measurements on 26 crude oil samples. The correlation has
the following form:
where the coefficient a is given by:
a = 10.313 [log(T - 460)] -36.447
m
od
a
T API=-
-
[ . ( )] ( ) [log ( )]
.
3 141 10 460
10 3 444
(2 -119)
m
od
7
4.53
a
0.32
1.8(10 )
API
360
T 260
(2 - 117)=+
-
Ê
Ë
Á
ˆ
¯
˜
Ê
Ë
Á
ˆ
¯
˜
110 Reservoir Engineering Handbook
Reservoir Eng Hndbk Ch 02b 2001-10-24 09:26 Page 110

The above expression can be used within the range of 50–300°F for
the system temperature and 20–48° for the API gravity of the crude.
Sutton and Farshad (1986) concluded that Glaso’s correlation showed
the best accuracy of the three previous correlations.
METHODS OF CALCULATING THE
SATURATED OIL VISCOSITY
Several empirical methods are proposed to estimate the viscosity of
the saturated oil, including:
• The Chew-Connally correlation
• The Beggs-Robinson correlation
These two correlations are presented below.
The Chew-Connally Correlation
Chew and Connally (1959) presented a graphical correlation to adjust
the dead-oil viscosity according to the gas solubility at saturation pres-
sure. The correlation was developed from 457 crude oil samples. Stand-
ing (1977) expressed the correlation in a mathematical form as follows:
m
ob
= (10)
a
(m
od
)
b
(2-120)
with a = R
s
[2.2(10
-7
) R
s
- 7.4(10
-4
)]
b =
c = 8.62(10
-5
)R
s
d = 1.1(10
-3
)R
s
e = 3.74(10
-3
)R
s
where m
ob
= viscosity of the oil at the bubble-point pressure, cp
m
od
= viscosity of the dead oil at 14.7 psia and reservoir
temperature, cp
The experimental data used by Chew and Connally to develop their
correlation encompassed the following ranges of values for the indepen-
dent variables:
Reservoir-Fluid Properties 111
068
10
025
10
0 062
10
...
cd e
++
Reservoir Eng Hndbk Ch 02b 2001-10-24 09:26 Page 111

Pressure, psia: 132–5,645
Temperature, °F: 72–292
Gas solubility, scf/STB: 51–3,544
Dead oil viscosity, cp: 0.377–50
The Beggs-Robinson Correlation
From 2,073 saturated oil viscosity measurements, Beggs and Robinson
(1975) proposed an empirical correlation for estimating the saturated-oil
viscosity. The proposed mathematical expression has the following form:
m
ob
= a(m
od
)
b
(2-121)
where a = 10.715(R
s
+ 100)
-0.515
b = 5.44(R
s
+ 150)
-0.338
The reported accuracy of the correlation is -1.83% with a standard
deviation of 27.25%.
The ranges of the data used to develop Beggs and Robinson’s equa-
tion are:
Pressure, psia: 132–5,265
Temperature, °F: 70–295
API gravity: 16–58
Gas solubility, scf/STB: 20–2,070
METHODS OF CALCULATING THE
VISCOSITY OF THE UNDERSATURATED OIL
Oil viscosity at pressures above the bubble point is estimated by first
calculating the oil viscosity at its bubble-point pressure and adjusting the
bubble-point viscosity to higher pressures. Vasquez and Beggs proposed
a simple mathematical expression for estimating the viscosity of the oil
above the bubble-point pressure. This method is discussed below.
The Vasquez-Beggs Correlation
From a total of 3,593 data points, Vasquez and Beggs (1980) proposed
the following expression for estimating the viscosity of undersaturated
crude oil:
112 Reservoir Engineering Handbook
Reservoir Eng Hndbk Ch 02b 2001-10-24 09:26 Page 112
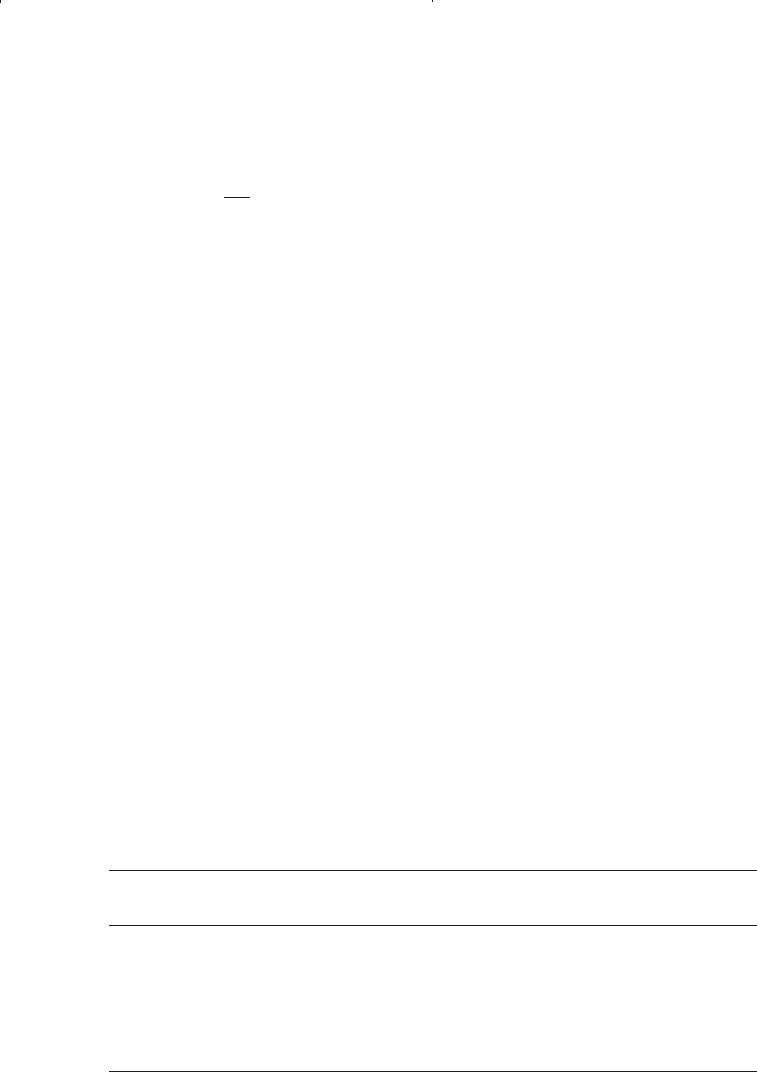
where
m = 2.6 p
1.187
10
a
with
a =-3.9(10
-5
) p - 5
The data used in developing the above correlation have the following
ranges:
Pressure, psia: 141–9,151
Gas solubility, scf/STB: 9.3–2,199
Viscosity, cp: 0.117–148
Gas gravity: 0.511–1.351
API gravity: 15.3–59.5
The average error of the viscosity correlation is reported as -7.54%.
Example 2-34
In addition to the experimental PVT data given in Example 2-29, the
following viscosity data are available:
Dead Oil Saturated Oil Undersaturated Oil
Oil # m
od
@ T m
ob
, cp m
o
@ p
1 0.765 @ 250°F 0.224 0.281 @ 5000 psi
2 1.286 @ 220°F 0.373 0.450 @ 5000 psi
3 0.686 @ 260°F 0.221 0.292 @ 5000 psi
4 1.014 @ 237°F 0.377 0.414 @ 6000 psi
5 1.009 @ 218°F 0.305 0.394 @ 6000 psi
6 4.166 @ 180°F 0.950 1.008 @ 5000 psi
Using all the oil viscosity correlations discussed in this chapter, please
calculate m
od
, m
ob
, and the viscosity of the undersaturated oil.
mm
oob
b
m
p
p
=
Ê
Ë
Á
ˆ
¯
˜
(2 -123)
Reservoir-Fluid Properties 113
Reservoir Eng Hndbk Ch 02b 2001-10-24 09:26 Page 113
Solution
Dead-oil viscosity
Oil # Measured m
od
Beal’s Beggs-Robinson Glaso’s
1 0.765 0.322 0.568 0.417
2 0.286 0.638 1.020 0.775
3 0.686 0.275 0.493 0.363
4 1.014 0.545 0.917 0.714
5 1.009 0.512 0.829 0.598
6 4.166 4.425 4.246 4.536
AAE 44.9% 17.32% 35.26%
Saturated-oil viscosity
Oil # Measured m
ob
Chew-Connally Beggs-Robinson
1 0.224 0.313* 0.287*
2 0.373 0.426 0.377
3 0.221 0.308 0.279
4 0.377 0.311 0.297
5 0.305 0.316 0.300
6 0.950 0.842 0.689
AAE 21% 17%
*Using the measured m
od
.
Undersaturated-oil viscosity
Oil # Measured m
o
Beal’s Vasquez-Beggs
1 0.281 0.273* 0.303*
2 0.45 0.437 0.485
3 0.292 0.275 0.318
4 0.414 0.434 0.472
5 0.396 0.373 0.417
6 1.008 0.945 1.016
AAE 3.8% 7.5%
Using the measured m
ob
.
114 Reservoir Engineering Handbook
Reservoir Eng Hndbk Ch 02b 2001-10-24 09:26 Page 114

Surface/Interfacial Tension
The surface tension is defined as the force exerted on the boundary
layer between a liquid phase and a vapor phase per unit length. This
force is caused by differences between the molecular forces in the vapor
phase and those in the liquid phase, and also by the imbalance of these
forces at the interface. The surface can be measured in the laboratory and
is unusually expressed in dynes per centimeter. The surface tension is an
important property in reservoir engineering calculations and designing
enhanced oil recovery projects.
Sugden (1924) suggested a relationship that correlates the surface ten-
sion of a pure liquid in equilibrium with its own vapor. The correlating
parameters of the proposed relationship are molecular weight M of the
pure component, the densities of both phases, and a newly introduced
temperature independent parameter P
ch
. The relationship is expressed
mathematically in the following form:
where s is the surface tension and P
ch
is a temperature independent para-
meter and is called the parachor.
The parachor is a dimensionless constant characteristic of a pure com-
pound and is calculated by imposing experimentally measured surface
tension and density data on Equation 2-124 and solving for P
ch
. The
Parachor values for a selected number of pure compounds are given in
Table 2-1 as reported by Weinaug and Katz (1943).
Table 2-1
Parachor for Pure Substances
Component Parachor Component Parachor
CO
2
78.0 n-C
4
189.9
N
2
41.0 i-C
5
225.0
C
1
77.0 n-C
5
231.5
C
2
108.0 n-C
6
271.0
C
3
150.3 n-C
7
312.5
i-C
4
181.5 n-C
8
351.5
s
rr
=
-
È
Î
Í
˘
˚
˙
P
M
ch L v
()
4
(2 -124)
Reservoir-Fluid Properties 115
Reservoir Eng Hndbk Ch 02b 2001-10-24 09:26 Page 115

Fanchi (1985) correlated the parachor with molecular weight with a
simple linear equation. This linear is only valid for components heavier
than methane. Fanchi’s linear equation has the following form:
(P
ch
)
i
= 69.9 + 2.3 M
i
(2-125)
where M
i
= molecular weight of component i
(P
ch
)
i
= parachor of component i
For a complex hydrocarbon mixture, Katz et al. (1943) employed the
Sugden correlation for mixtures by introducing the compositions of the
two phases into Equation 2-124. The modified expression has the follow-
ing form:
with the parameters A and B as defined by:
where r
o
= density of the oil phase, lb/ft
3
M
o
= apparent molecular weight of the oil phase
r
g
= density of the gas phase, lb/ft
3
M
g
= apparent molecular weight of the gas phase
x
i
= mole fraction of component i in the oil phase
y
i
= mole fraction of component i in the gas phase
n = total number of components in the system
Example 2-35
The composition of a crude oil and the associated equilibrium gas is
given below. The reservoir pressure and temperature are 4,000 psia and
160°F, respectively.
A
M
B
M
o
o
g
g
=
=
r
r
62 4
62 4
.
.
s
14
1
=-
[]
=
Â
()( )PAxBy
ch i i i
i
n
(2 -126)
116 Reservoir Engineering Handbook
Reservoir Eng Hndbk Ch 02b 2001-10-24 09:26 Page 116

Component x
i
y
i
C
1
0.45 0.77
C
2
0.05 0.08
C
3
0.05 0.06
n-C
4
0.03 0.04
n-C
5
0.01 0.02
C
6
0.01 0.02
C
7+
0.40 0.01
The following additional PVT data are available:
Oil density = 46.23 lb/ft
3
Gas density = 18.21 lb/ft
3
Molecular weight of C
7+
= 215
Calculate the surface tension.
Solution
Step 1. Calculate the apparent molecular weight of the liquid and gas phase:
M
o
= 100.253 M
g
= 24.99
Step 2. Calculate the coefficients A and B:
Step 3. Calculate the parachor of C
7+
from Equation 2-125:
(P
ch
)
C
7+
= 69.9 + (2.3) (215) = 564.4
B ==
18 21
62 6 24 99
0 01168
.
(.)(.)
.
A ==
46 23
62 4 100 253
0 00739
.
(.)( . )
.
Reservoir-Fluid Properties 117
Reservoir Eng Hndbk Ch 02b 2001-10-24 09:26 Page 117
