Russell I. (ed.) Whisky. Technology, Production and Marketing
Подождите немного. Документ загружается.

[16:01 13/3/03 n:/3991 RUSSELL.751/3991-008.3d] Ref: 3991 Whisky Chapter 8 Page: 256 242-273
encourage dairying; in the East of Scotlan d, cereal growing has encouraged
beef production as a complementary enterprise to arable farming). In general
there has been a movement of co-products to the South and West, with many
also being used in Northwest England. The dried feeds can be distributed
throughout the British Isles, and are also exported, often from northern
ports, to the Benelux countries, Scandinavian and Ireland.
Agri-Food Market Analysis (2000) is a map showing brewing and distilling
in the UK, and illustrates the difficulties that can arise in more remote geo-
graphical locations, where distillery production can be relatively large com-
pared to livestock numbers.
Islay is a good example of the problems that can occur. The Dunlop cheese
plant sadly closed in the spring of 2000, and dairy cattle numbers declined
from about 700 to 150. The dairy herd and followers were major consumers for
the draff from the island’s distilleries. At capacity, the seven working distil-
leries on Islay and neighbouring Jura (Ardbeg, Bowmore, Bunnahabhain, Caol
Ila, Isle of Jura, Lagavulin and Laphroaig) produce around 36 000 t p.a. of
draff. This is sufficient for almost 3 t per head per annum for all the cattle
on the two islands. This is a very high rate of draff usage; the 1983 survey by
Lilwall and Smith found an average usage rate of 0.7 t per head per annum,
with maximum usage rates of 2.2 t and 1.2 t per head per annum for beef
fatteners and dairy specialists.
With the recent drastic reductions in the dairy herd there is therefore an
urgent need to increase beef enterprises to sustain both rural and distilling
sections of the local economy. If this is not done, then distilleries may be left
with the undesirable option of either regarding the draff as waste or transport-
ing it to the Scottish mainland; both at considerable cost.
256 Whisky: Technology, Production and Marketing
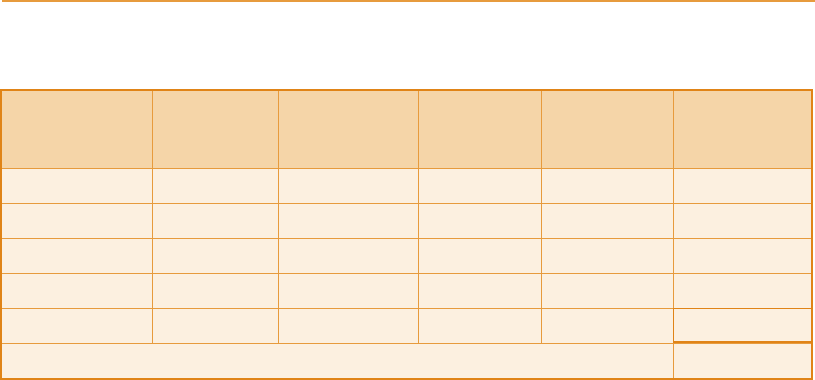
Table 8.8
Grain dried feeds – calculated production at 61 per cent utilization (Year 2000)
Distillery
(product name)
Capacity
(kla/annum)
Cereal and
yeast residue
1
(t dry matter)
% Recovery
2
% Dry matter Actual tonnes
of product
Dumbarton 35 000 17 200 97 90 18 500
Invergordon 38 000 18 700 97 90 20 000
North British 63 000 31 000 97 90 33 000
Port Dundas 39 000 19 200 97 90 21 000
Strathclyde 32 000 15 700 97 90
17 000
TOTAL 109 500
1
At assumed yield of 377 l alcohol/t cereal mashed.
2
Centrifuge processes assumed to be less efficient than filtration. Dried feeds (drying losses only) assumed to be
3percent.

[16:01 13/3/03 n:/3991 RUSSELL.751/3991-008.3d] Ref: 3991 Whisky Chapter 8 Page: 257 242-273
Although there may be difficulties in areas where draff transport is very
expensive, Scotland as a whole could use more draff and other moist feeds
were it possible to utilize the pot ale. Lilwall and Smith (1983) estimated 1979/
1980 draff usage at 416 000 t p.a., and considered that uptake could be more
than doubled. They stated that an improved marketing strategy of longer-term
allocation between drying plants and direct sale would increase volumes.
However, they did not address the central problems of dealing with fluctuat-
ing supplies, and the use of the pot ale released, were draff sales to increase.
This latter constraint means that in the longer term moist co-product sales
will decline from the current figure of 284 000 p.a. with an increase in dri ed
feeds.
Waste waters and effluents
The remaining co-products, which have no commercial value, must be pro-
cessed and/or disposed of economically and in compliance with environmen-
tal legislation. These comprise the second malt distillation residues – spent
lees, washing waters from cleaning process vessels and pipelines – together
with condensate where pot ale or spent wash is evaporated to produce syrup
or, by further processing, dark grains. In addition there may be liquors
produced by vapour scrubbing of dark grains plant dryer emissions, as
well as blowdown from steam boilers and purge-water from cooling systems.
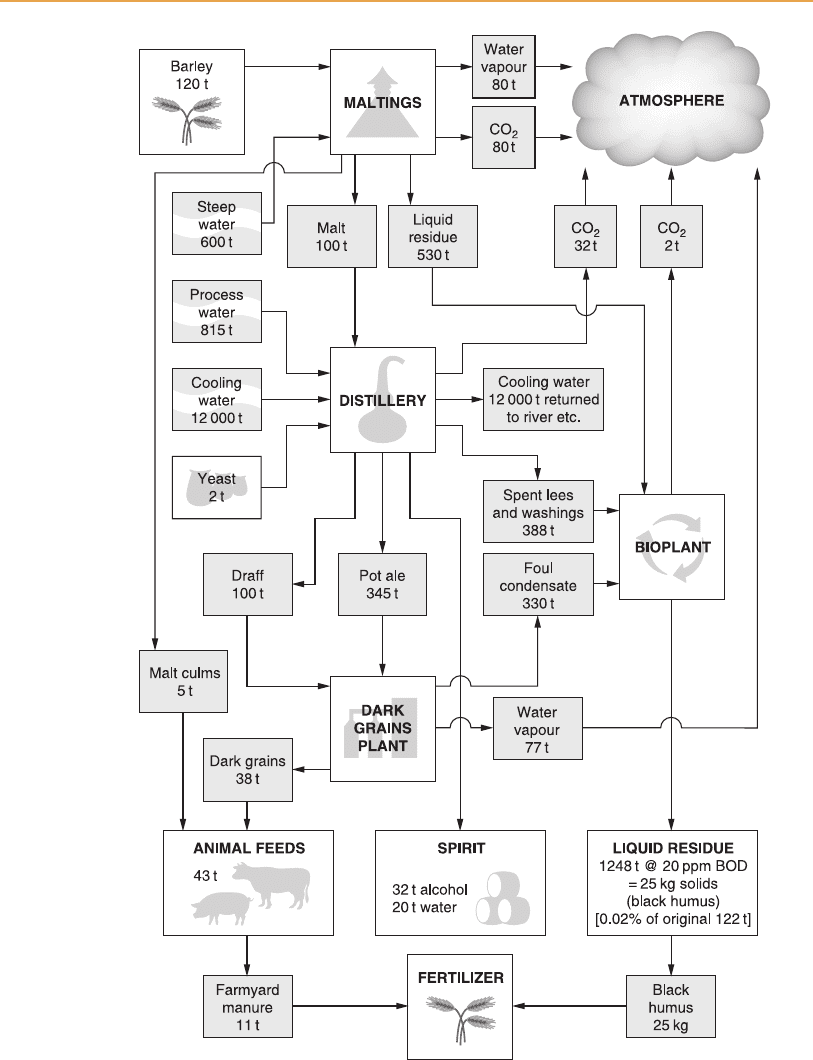
Figure 8.4 provides an illustration of the derivation and quantities of the
individual wastewaters produced during the making of malt whisky and
dark grains.
Several disposal routes are available, all now regulated by various pieces of
legislation, the principle one being the EC Urban Wastewater Treatment
Directive (UWWT), which controls both the direct discharge of wastewaters
into inland freshwaters, estuaries and the sea, and discharges made indirectly
via public sewage systems. In a few instances, wastew aters are spray irrigated
over farmland, or lagooned. This relatively new piece of European legislation
lays down the minimum degree of treatment necessary prior to direct dis-
charge into any of the ‘controlled’ waters mentioned above. In addition, the
regulatory authority enforcing the legislation – the Scottish Environment
Protection Agency – requires that several Environmental Quality Standards
(EQS) are achieved; notably those for dissolved copper and dissolved oxygen
in the receiving waters.
At certain distilleries, located on estuaries and adjacent coastal waters
where good dispersive and mixing conditions prevail, pot ale or spent
wash are disposed off directly via long sea outfalls along with the weaker
wastewaters and, in some instances, cooling water, but only where the local
conditions allow the UWWT regulations and EQS achievement to be com-
plied with.
Disposal of wastewater via public sewerage systems normally necessitates
pre-treatment on site in ord er to meet Water Authority requirements, so that
they in turn are able to comply with UWWT regulations.
Chapter 8 Co-products 257
[16:01 13/3/03 n:/3991 RUSSELL.751/3991-008.3d] Ref: 3991 Whisky Chapter 8 Page: 258 242-273
258 Whisky: Technology, Production and Marketing
Figure 8.4
Weekly material flow for a medium-sized distillery with associated malt ings, dark-grains
plant and bioplant.

[16:01 13/3/03 n:/3991 RUSSELL.751/3991-008.3d] Ref: 3991 Whisky Chapter 8 Page: 259 242-273
At inland sites without access to public sewers, on site treatment is obliga-
tory before discharge to a watercourse that is, more often than not, one capable
of supporting salmonid fish. Consequently the degree of treatment required is
high, and the consent conditions imposed by the Scottish Environmental
Protection Agency (SEPA) are correspondingly stringent.
The nature of the wastewater lends itself to biological treatment provided
the acidity and lack of nutrients (nitrogen and phosphorus) are first rectified.
Figure 8.4 illustrates the quantities of sludge derived from the biological
treatment of wastewaters, which approximates to 0.5 kg dry matter for each
kilogram of biochemical oxygen demand (BOD) degraded.
The high concentration of copper in the spent lees gives rise to high levels
of copper in the surplus biomass produced during treatment, effectively
removing copper from the treated effluent. Subsequent applications of the
‘bioplant sludge’ to the farmland give rise to elevated levels of copper in the
soil, which must be monitored and managed carefully in order to comply
with the Code of Practice associated with the disposal of sludge to agricul-
tural land. The copper deficiency of much of the land in Scotland exempli-
fies the benefits of this practice, provided the appropriate precautions are
taken.
In anticipation of an overall tightening of environmental legislation relating
to potentially toxic elements, the industry has developed methods of removing
copper from spent lees in order to ensure that current disposal routes for
wastewater and surplus biomass are secure.
Copper in spent lees is present, for the most part as soluble Cu
2+
ions, which
lends itself to removal by ion exchange, electro-winning, or precipitation and
membrane filtration. In fact all three techniques have been investigated, and as
a result two successful methods have been developed; one based on a combi-
nation of cation exchange followed by electro-winning of the eluted soluble
copper concentrate, and the other using precipitation by sodium hydroxide
and ultra-filtration to concentrate the preci pitate. However, only the first
method recovers copper as a metal.
The other major emission from the whisky distillation process is of water
used for distillate cooling. Where water resources are sufficient, water is used
on a total loss basis amounting to 120 m
3
/t of malt mashed. At many sites,
however, water supp lies are limited, resulting in full or partial recirculation
through a cooling tower.
Limitations on the temperature are imposed under the Freshwater Fisheries
Regulations in order to protect freshwater fisheries, especially where salmo-
nids occur. Restrictions are placed on cooling water discharges such that a
receiving water does not become heated above a certain point, which poses
problems, especially during the summer months when ambient temperatures
are high and stream flows are low. Traditionally these months were avoided
by distilleries for whisky making, but production requirements have meant
that whisky has to be made when conditions are less than favourable in terms
of temperature and water availability. Consequently, steps must be taken to
reject heat prior to return to a watercourse – usually by means of a cooling
tower.
Chapter 8 Co-products 259

[16:01 13/3/03 n:/3991 RUSSELL.751/3991-008.3d] Ref: 3991 Whisky Chapter 8 Page: 260 242-273
Markets for co-products
The calculations above show the considerable volumes of co-products pro-
duced by the whisky industry.
The distillery uses the starch in the cereal to produce alcohol, and the resi-
due is thereby enriched by plant structural fibre, protein and oil, and yeast.
The requirements of the industry are for relatively large markets capable of
absorbing the fluctuating supplies of available co-product. Smaller niche mar-
kets do exist , but are of little economic significance. Possible markets therefore
include:
. Animal feeds
. Human food
. Fertilizer
. Fuel
. Production of biomass.
All the above markets are large, and their respective potential can be com-
pared using dark grains as an example.
Animal feed
The UK market for animal feed is large, with some 20 million tonnes worth
£2.2 billion being purchased in 2000 (Dean, 2001).
The percentages of purchased compounded (manufactured) feed for each
class of livestock are as follows (Feed Facts Quarterly, 2000):**37
1999 volumes (%)
Cattle and calves 38 per cent
Pigs 21 per cent
Poultry 27 per cent
Sheep 7 per cent
Others (including horse feeds) 7 per cent
In terms of market size, the ruminant (cattle and sheep) market is the largest
single sector. It is also nutritionally less demanding than the others. Ruminants
have evolved since the appearance of grass in the Miocene period some 20
million years ago, and use pre-gastric fermentation where micro-organisms
degrade the plant cell wall. The ability to ruminate (i.e. regurgitate, rechew
and reswallow) the feed reduces the particle size and aids this breakdown. In
addition, the presence of large numbers of micro-organi sms allows the use of a
wider range of protein sources and non-protein nitrogens (Van Soest, 1982).
Monogastric animals and birds lack the enzymes required to break down
plant structural carbohydrate, and also req uire protein with a higher content
of essential amino acids.
260 Whisky: Technology, Production and Marketing
[16:01 13/3/03 n:/3991 RUSSELL.751/3991-008.3d] Ref: 3991 Whisky Chapter 8 Page: 261 242-273
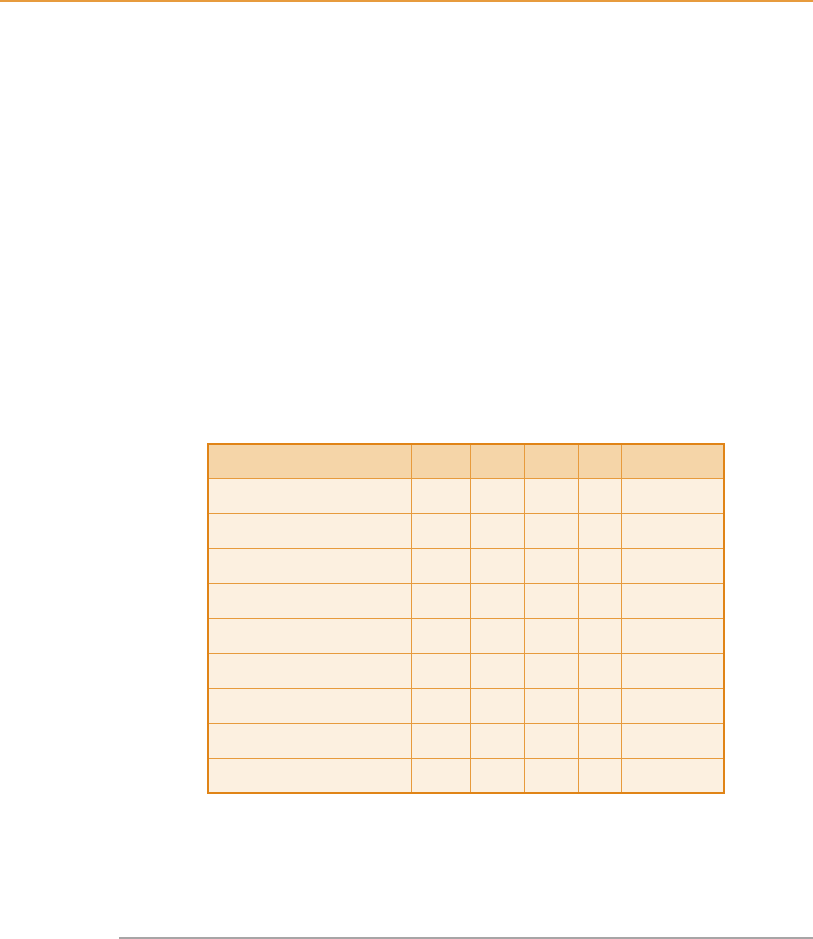
The essential amino acids of wheat dark grains, extracted soya bean meal
and fish meal are compared in Table 8.9.
In modern pig and poultry diets the principle limiting amino acids are
commonly lysine, threonine and methionin e. Wheat dark grains are relatively
low in these amino acids, and are particularly deficient in lysine.
The relative poor amino acid profile of the distillers’ feeds, together with
their elevated cereal fibre content, means that they generally find their highest
economic value in ruminant feeds. Small volumes of pot ale, pot ale syrup and
grain dark grains are used in other species, where they have desirable parti-
cular attributes (for example, the low fibre in pot ale syrup makes it suitable
for pigs, and the high xanthophyll pigment content in maize dark grains
makes them attractive for pigmenting egg yolks).
Human food
The nutritional properties outlined above make entry into the human food
market problematic, and there have been a number of studies of co-product
use (Tsen, 1982; Cooler et al., 1986; O’Palka, 1987) in both baked goods and in
canned meat products.
Although the co-produ cts could be included at low levels without signifi-
cant adverse effect, at higher levels the lack of functional gluten, the dark
colour and flavour adversely affected product quality. It has to be concluded
that, without the use of low temperature drying, the removal of at least some
Chapter 8 Co-products 261
Table 8.9
Comparison of essential amino acids (g/16gN)
(Sources: ARC, 1981; MAFF, 1990)
WDG SBM FM IP WDG % IP
Arginine 3.31 7.90 5.10 – –
Histidine 3.24 3.18 1.89 2.3 141
Isoleuc ine 3.80 5.11 3.64 3.8 100
Leucine 8.50 8.19 6.69 7.0 121
Lysine 2.15 6.77 5.67 7.0 31
Methionine þ cystine 2.98 2.80 3.78 3.5 85
Phenylalanine þ tyrosine 7.97 9.32 7.16 6.7 119
Threonine 3.31 4.15 4.39 4.2 79
Valine 4.83 5.84 5.34 4.9 98
WDG, wheat dark grains; SBM, extracted soya bean meal; FM, white
fish meal; IP, ideal protein (pigs).
[16:01 13/3/03 n:/3991 RUSSELL.751/3991-008.3d] Ref: 3991 Whisky Chapter 8 Page: 262 242-273
cereal fibre and the production of lower copper products, their monetary
values in human food applications will remain low.
Fertilizer
As noted above, dilute liquid co-products have been used as fertilizers for
many years, and numerous studies have been conducted by the Scottish
Agricultural College. Use on hills encourages grass growth at the expense of
heather (Bucknall et al., 1979), and applications of over 1000 m
3
/ha per year
have converted rough grazing into productive grassland. They have also been
used on grassland and in forestry, but such use has only been on a limited
scale as access problems through the winter and the dangers of run-off result
in limited widespread adoption.
Recently, year-round application of pot ale to grassland has been practised at
those sites remote from access to further processing. Rigorous control of soil
copper levels and avoidance of run off, either directly or via field drainage
systems, have had to be established in order to satisfy the regulatory authorities.
The dried co-products can also be used as a fertilizer, but here the primary
constraints have been economic rather than logistical. A recent study,
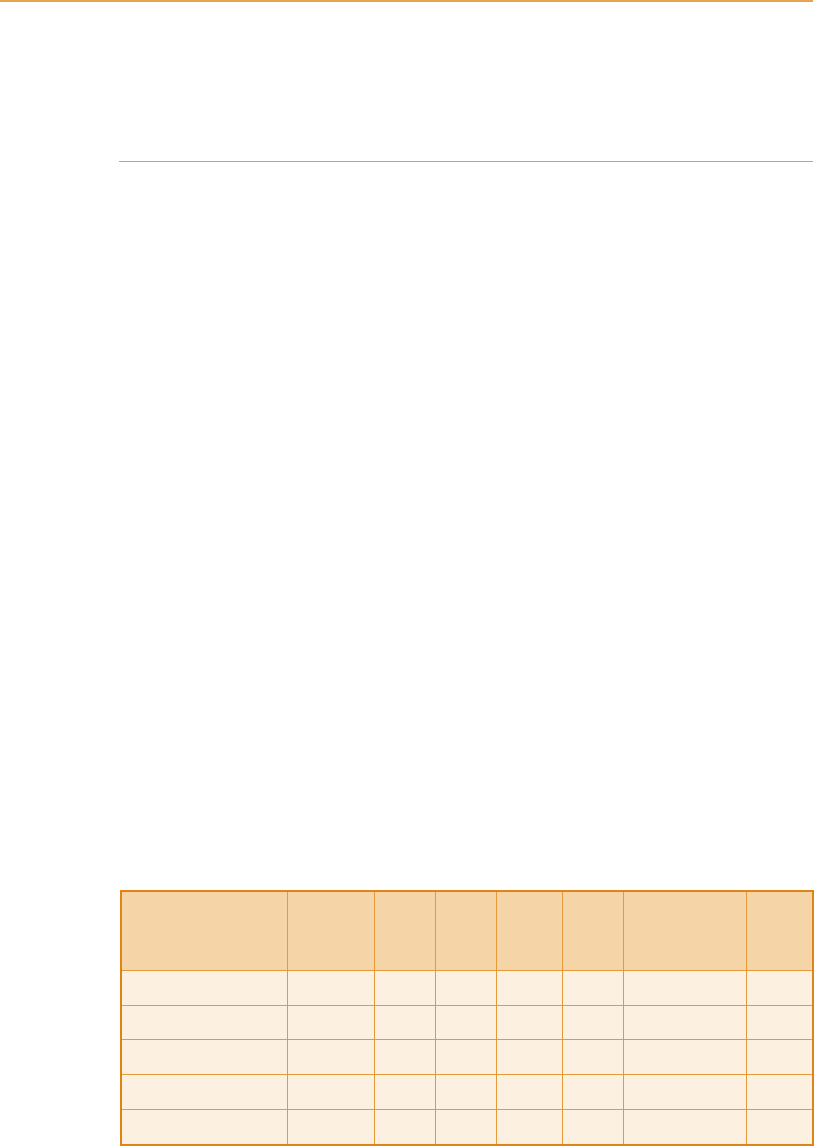
(Bingham and Sinclair, 2000, personal communication) chemically analysed
typical co-products (see Table 8.10).
Scottish Agricultural College experiments showed a positive plant response
to the above co-products, but in comparison with commercially available fer-
tilizers they are very dilute nutrient sources.
From quoted market information it can be calculated that the value of nitro-
gen, phosphor and potassium in a range of inorganic fertilizers (5 : 24 : 24,
13 : 13 : 20 and 20 : 10 : 10) is currently £3.78, £1.25 and £2.87 per unit of N,
P
2
O
5
and K
2
O respectively (Bojduniak, 2001a, 2001b).**38
262 Whisky: Technology, Production and Marketing
Table 8.10
Chemical analysis of co-product
(Source: United Distillers & Vintners (Distilling) Ltd)
Product Dry
matter
(%)
pH N
(%)
P
2
O
5
(%)
K
2
O
(%)
Cu (mg/kg
dry matter)
Farm
value
(£/t)
Steeped barley 40 3–5 0.82 0.73 0.48 2 5.40
Malt draff 22 3–5 0.70 0.47 0.19 4 3.79
Barley dark grains 90 N/A 3.89 3.71 2.21 50 25.74
Malt residuals 90 N/A 3.31 2.76 3.75 15 26.77
Pot ale syrup 45 3–5 2.45 4.53 2.49 29 22.12

[16:01 13/3/03 n:/3991 RUSSELL.751/3991-008.3d] Ref: 3991 Whisky Chapter 8 Page: 263 242-273
The monetary value of the co-product nutrients is given in the last column
of Table 8.10. With a range of typical value from below £4/t to £27/t, sale of
the products in competition with inorganic fertilizers is unattractive.
Research carried out by Diageo**39 some years ago demonstrate d that a peat
substitute could be produced from draff by composting. However, the rela-
tively small market, combined with high transport and marketing costs, has so
far not resulted in any sustained commercialization.
Fuel
The use of fossil fuel to dry the co-products first so that they can then them-
selves be used as fuel would at first seem perverse. However, sugar cane
residues (‘bagasse’) has been used as a fuel for many years (Morrison, 1947),
and more latterly as a source of energy for fuel alcohol production.
Using gross energy figures for the dried animal feeds (Gizzi, 2001) and DTI
data (Department of Trade and Industry, 2001) on the cost and gross energy of
coal for UK power generation, it can be calculated that the value of barley,
wheat and maize dark grains are £21.71/t, £22.01/t and £23.06/t respectively.
These values assume that the dark grains could be used as a direct replace-
ment for coal with no other modification (in practice at least some changes
would probably be necessary, as, for example, the dark grains could not be
exposed to the weather).
Again, except as a last resort the use of the feeds as fuel is very unlikely to be
competitive.
In conclusion, of all the potential large market s examined use as ruminant
feed remains the most attractive – as has historically been the case.
Although such dependence on a single market has its dangers (as the recent
bovine spongiform encephalopathy (BSE), 2001 foot and mouth disease epi-
demic and low UK milk prices all illustrate), other major markets have not yet
proved more economically attractive.
Fuel may, however, be derived indirectly from pot ale and spent wash by
means of anaerobic digestion, whereby a mixed culture of bacteria break down
the soluble organic components into methane and carbon diox ide.
Unfortunately the digestion process requires the prior removal of the dead
yeast cells, since their refractory nature prevents degradation, which would
give rise to accumulation in the reactor and eventual process failure. Methods
for removing dead yeast cells include high-speed centrifugation, and coa gula-
tion with carboxymethyl cellulose followed by belt pressing and membrane
ultra-filtration. Equipment is costly to install, but the recovered yeast, at around
20 per cent dry matter, could be incorporated wi th wet draff for direct feeding,
or transported to a dark grains plant for processing there.
Yields of me thane would be equivalent to 0.34 m
3
/kg chemical oxygen
demand (COD) destroyed, which, for a clarified pot ale with a COD of
45 kg/m
3
and 90 per cent degradati on by anaerobic digestion, would produce
around 14 m
3
/m
3
– equivalent to approximately 14 l of heavy fuel oil.
Unfortunately, the digestion process releases most of the organically bound
Chapter 8 Co-products 263

[16:01 13/3/03 n:/3991 RUSSELL.751/3991-008.3d] Ref: 3991 Whisky Chapter 8 Page: 264 242-273
nitrogen and phosphorus as inorganic ammonium and phosphate salts – typi-
cally 1500 mg/l and 650 mg/l respectively. Consequently the only viable dis-
posal route is by application to farmland, where the nutrients would be useful
fertilizer additions – especially for grassland.
Several schemes have been trialled in the past, which confirm the effective-
ness of anaerobic digestion, but to date no full-scale system has been installed.
Instead, at sites economically remote from dark-grains plants or without
access to well-sited marine outfalls, pot ale is appli ed directly to grassland
at carefully controlled rates as a fertilizer.
Production of biomass
The production of microbial biomass from pot ale and spent wash is an attrac-
tive proposition at first glance, beari ng in mind the relatively large amounts of
amino acids and unfermented sugars present in these two co-products.
Indeed, over the years several attempts have been made to grow selected
micro-organisms for harve sting as animal foodstuffs.
For some years, product formulated from evaporated spent wash was mar-
keted as a culture medium for lactobacilli (trade name ‘Kickstart’) and sold in
the farm market. The concentrated cult ure medium was diluted on farms, and
a vial of freeze-dried lactobacilli was added and grown. The resulting bacterial
culture was then used as an inoculant on grass to improve silage quality.
Although used successfully, the project came to an end with the closure of
Cambus Distillery in 1993.
Other notable work was undertaken by Wm Grant & Sons, where a fungi
imperfecti and a yeast were grown in sequence to exp loit these amino acids
and sugars. Unfortunately, transferring the process from laboratory pilot to
full-scale use gave rise to insurmountable problems, and the scheme was
abandoned.
Another process, trialled by Long John Distillers in the 1980s at Tormore
Distillery, utilized the so called Malimo process. Aspergillus niger was the
organism selected following screening for best results. Again , problems
were encountered that led to abandonment of the process.
Work in Northern Ireland in the 1980s (Barker et al., 1983), where mixed
cultures of filamentous fungi and yeasts were grown on pot ale, also showed
promise. However, as with other biomass production projects, process and EU
approval difficulties have not yet allowed commercialization.
Nutritional characteristics
Distillers’ feeds have been used throughout the world to great effect, and
scientific study of them has also been extensive. A 50-year world literature
search of Nutrition Abstracts and Reviews (Commonwealth Agricultural
Bureau, 1974, 1978, 1980) and, since 1986, the Dialog database, produced
264 Whisky: Technology, Production and Marketing
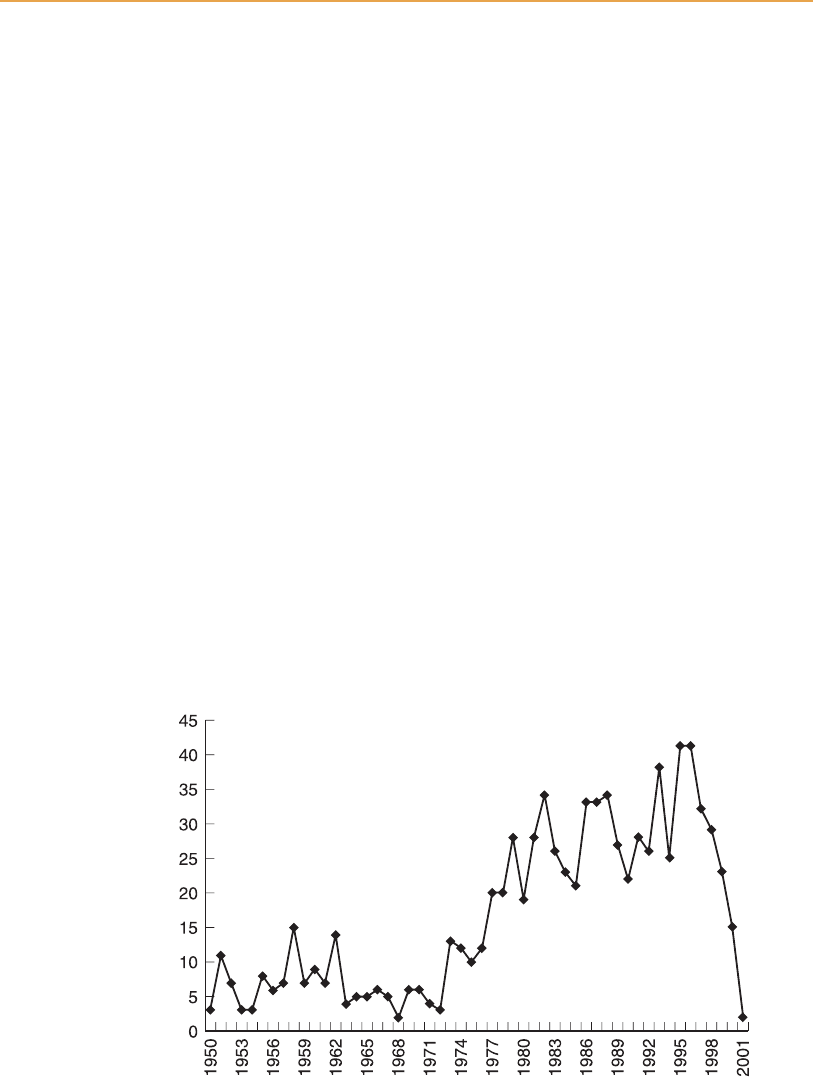
[16:01 13/3/03 n:/3991 RUSSELL.751/3991-008.3d] Ref: 3991 Whisky Chapter 8 Page: 265 242-273
861 articles on brewing and distilling co-products used in animal feeds (see
Figure 8.5). It may be that the sharp ann ual increase from 1973 to 1983 was
partly due to rising fuel costs and environmental pressures stimulating
research interest.
The recent decline in numbers of publications over the last four years has no
particular significance, except that as the feeds have become thoroughly
understood then further research is progressively less cost-effective.
Most recently, both the moist and dried co-products have been extensively
reviewed (by Crawshaw, 2001 and Gizzi, 2001, respectively). To avoid repeti-
tion, only nutrients of major economic significance are considered below.
Using April 2001 raw material prices (Agricultural Supply Industry,
2001;**40 Bojduniak, 2001a, 2001b) to formulate a typical compound dairy
feed (with energy and protein levels of 12.4 MJ/kg and 20 per cent of dry
matter), then the marginal value**41 of, for example, barley distillers’ dark
grains is £93.49/t. In this typical example the nutrients of value were metabo-
lizable energy, protein, phosphorus and salt. Table 8.11 illustrates their rela-
tive importance.
Table 8.11 shows that the energy content of the co-products is the most
important single determinant of value, followed by protein. Other nutrients
are far less significant. It is for this reason that the ruminant metabolizable
energy value (ME) of the co-products has been particularly well researched,
despite the high cost of such trials in animals.
In the UK alone there have been 26 in vivo ME determinations for the dried
feeds (Gizzi, 2001) and 8 for brewers’ grains and draff. This number compares
well with major competing feeds, such as extracted soya bean mea l or maize
gluten feed (MAFF, 1990).
Chapter 8 Co-products 265
Figure 8.5
Brewing and distilling feeds: scientific publications 1950–2000.
