Rafferty J.P. (ed.) Minerals
Подождите немного. Документ загружается.

7 Minerals 7
112
determination of crystal structure possible. Prior to this, the
classification of silicates was based on chemical and physical
similarities, which often proved to be ambiguous. Although
many properties of a silicate mineral group are determined
by tetrahedral linkage, an equally important factor is the
type and location of other atoms in the structure.
Silicate minerals can be thought of as three-dimensional
arrays of oxygen atoms that contain void spaces (i.e.,
crystallographic sites) where various cations can enter.
Besides the tetrahedral (4-fold coordination) sites, 6-fold,
8-fold, and 12-fold sites are common. A correlation exists
between the size of a cation (a positively charged ion) and
the type of site it can occupy: the larger the cation, the
greater the coordination, because large cations have more
surface area with which the oxygen atoms can make con-
tact. Tetrahedral sites are generally occupied by silicon
and aluminum; 6-fold sites by aluminum, iron, titanium,
magnesium, lithium, manganese, and sodium; 8-fold sites
by sodium, calcium, and potassium; and 12-fold sites by
potassium. Elements of similar ionic size often substitute
for one another. An aluminum ion, for example, is only
slightly larger than a silicon ion, allowing substitution for
silicon in both tetrahedral and 6-fold sites.
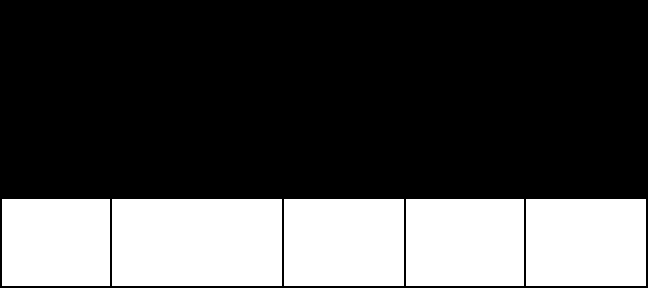
SIlICATe MINerAlS
name colour lustre M
ohs
hard-
ness
specific
gravity
Tectosilicates (three-dimensional networks)
feldspar (for other examples, see feldspar)
orthoclase flesh-red, white
to pale yellow,
red, green
vitreous 6–6½ 2.6
113
7
the Silicates 7
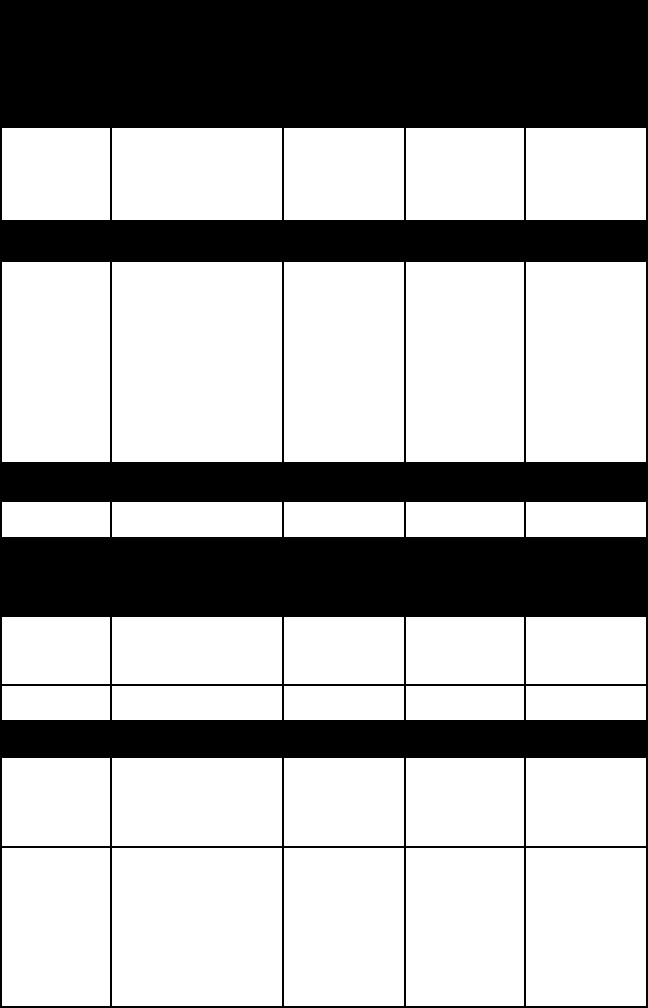
name colour lustre Mohs
hard-
ness
specific
gravity
feldspathoid (for other examples, see feldspathoid)
nepheline light-coloured;
reddish, green-
ish, brownish
vitreous to
greasy
5½–6 2.6–2.7
silica (for other examples, see silica mineral)
quartz variable vitreous
to greasy
(coarse-
grained);
waxy to
dull (fine-
grained)
7 (a hard-
ness
standard)
2.65
zeolite (for other examples, see zeolite)
chabazite white; flesh-red vitreous 4½ 2.0–2.1
Phyllosilicates (sheet structures)
clay (for other examples, see clay mineral)
chlorite green vitreous or
pearly
2–3 2.6–3.0
smectite 2.2–2.7
mica (for other examples, see mica)
apophyllite colourless,
white, pink, pale
yellow, or green
pearly
iridescent
4½–5 2.3–2.4
muscovite commonly white
or colourless;
light shades of
green, red, or
brown
vitreous
to silky or
pearly
2–2½ 2.8–3.0
7 Minerals 7
114
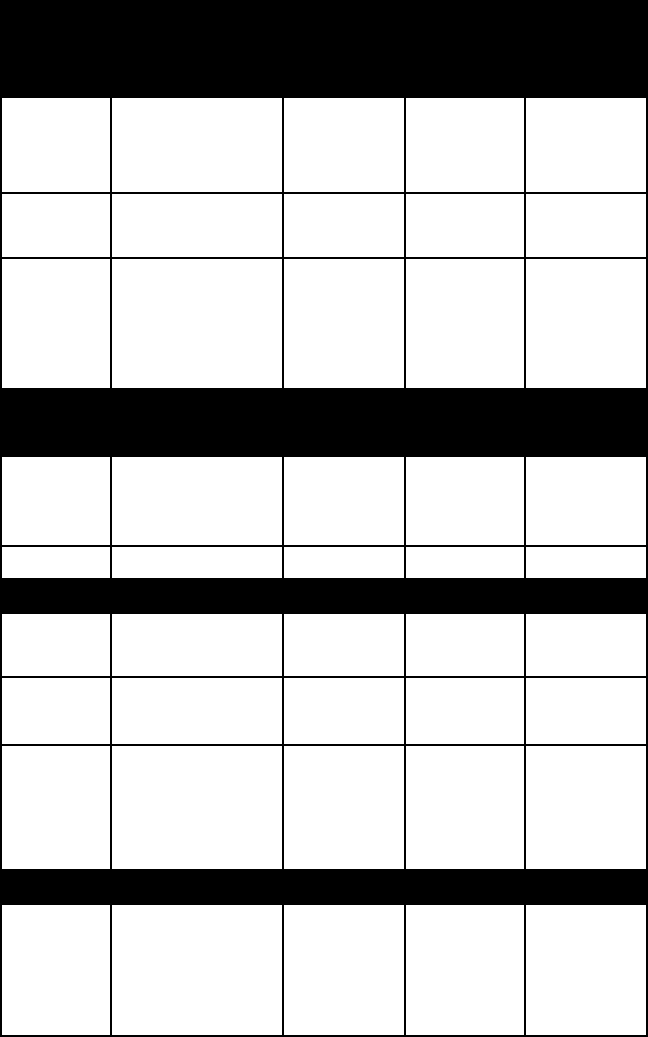
name colour lustre Mohs
hard-
ness
specific
gravity
prehnite pale green to
gray, white, or
yellow
vitreous 6–6½ 2.9–3.0
pyrophyl-
lite
white and vari-
ous pale colours
dull and
glistening
1–2 2.6–2.9
talc colourless;
white; pale or
dark green;
brown
pearly 1 (a hard-
ness
standard)
2.6–2.8
Inosilicates (chain structures)
amphibole (for other examples, see amphibole)
common
horn-
blende
pale to dark
green
glassy 5–6 3.0–3.4
mullite white 3.0
pyroxene (for other examples, see pyroxene)
augite brown, green,
black
vitreous 5½–6 3.2–3.5
rhodonite pink to brown-
ish red
vitreous 5½–6½ 3.6–3.8
wollas-
tonite
white; also
colourless, gray,
or very pale
green
vitreous 4½–5 2.9–3.1
Cyclosilicates (ring structures)
axinite clove- or
lilac-brown;
pearl-gray;
yellowish
highly
glassy
6½–7 3.3–3.4
115
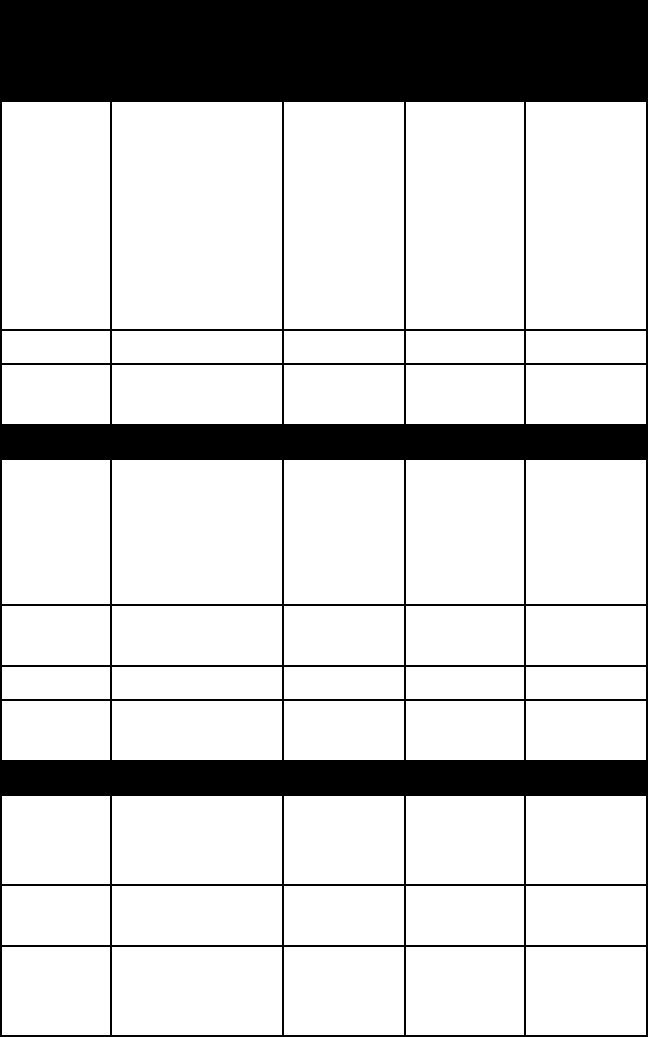
name colour lustre Mohs
hard-
ness
specific
gravity
beryl various greens;
variable, includ-
ing deep-green
(emerald),
blue-green (aqua-
marine), pink
(morganite), yel-
low (heliodore)
vitreous 7½–8 2.7–2.8
cordierite various blues vitreous 7 2.5–2.8
tourmaline extremely
variable
vitreous to
resinous
7–7½ 3.0–3.2
Sorosilicates (double tetrahedral structures)
hemimor-
phite
white, some-
times tinted
bluish or green-
ish; yellow to
brown
vitreous 5 3.4–3.5
melilite colourless; gray-
ish green; brown
vitreous to
resinous
5–6
gehlenite 3.1
åkerman-
ite
2.9
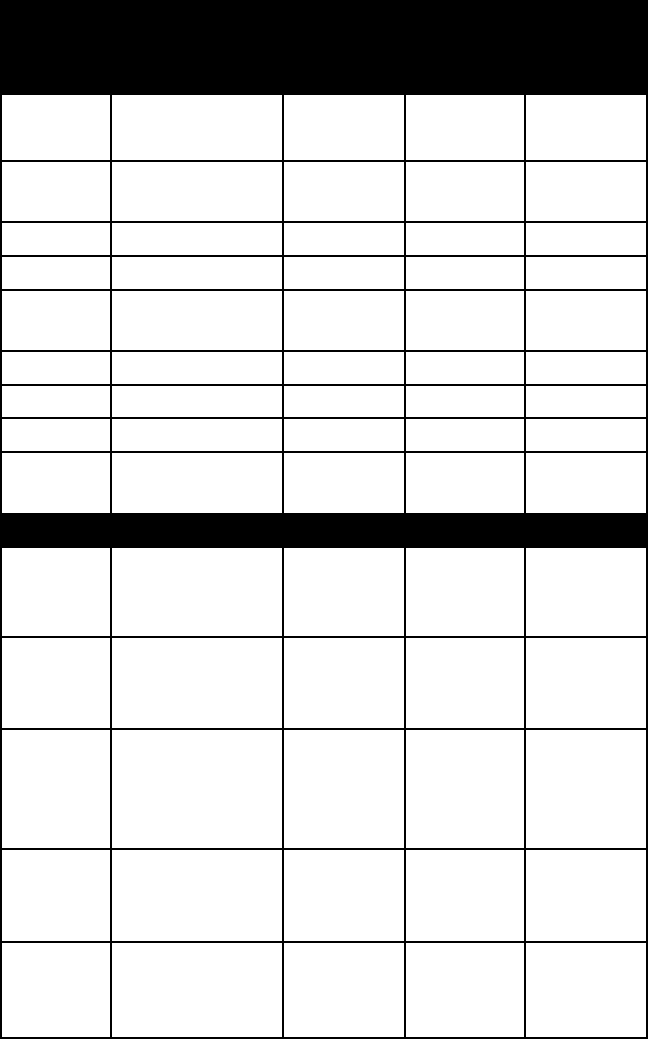
Nesosilicates (independent tetrahedral structures)
andalusite pink, white, or
rose-red; also
variable
vitreous 6½–7½ 3.1–3.2
chryso-
colla
green, bluish
green
vitreous 2–4 2.0–2.8
datolite colourless or
white; also vari-
ous pale tints
vitreous 5–5½ 2.9–3.0
7 the Silicates 7
7 Minerals 7
116
name colour lustre Mohs
hard-
ness
specific
gravity
epidote yellowish green
to dark green
vitreous 6–7 3.3–3.5
garnet variable vitreous to
resinous
6–7½
almandine 4.3
andradite 3.9
grossula-
rite
3.6
pyrope 3.6
spessartite 4.2
uvarovite 3.9
kyanite blue; white; also
variable
vitreous to
pearly
4–7
(variable)
3.5–3.7
olivine (for other examples, see olivines)
forsterite-
fayalite
series
various greens
and yellows
vitreous 6½–7 3.2 (forster-
ite) to 4.4
(fayalite)
phenacite colourless; also
wine-yellow, pale
rose, brown
vitreous 7½–8 3.0
sillimanite colourless or
white; also vari-
ous browns and
greens
vitreous
to subada-
mantine
6½–7½ 3.2–3.3
sphene colourless,
yellow, green,
brown, black
adaman-
tine to
resinous
5 3.4–3.6
staurolite dark red-brown;
yellow-brown;
brown-black
subvitreous
to resinous
7–7½ 3.7–3.8
117
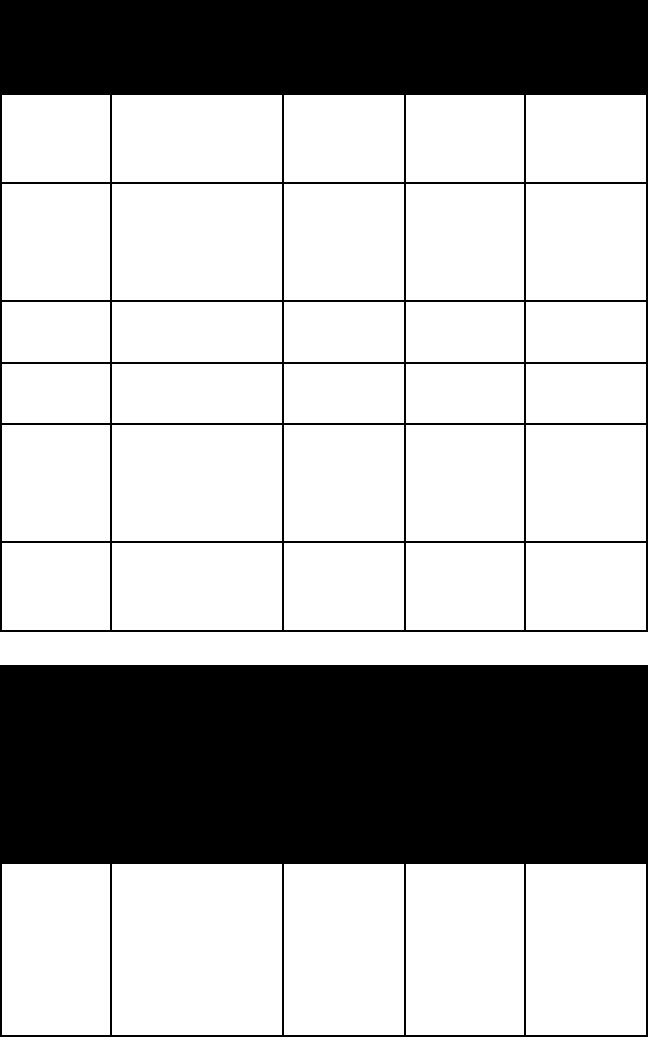
name colour lustre Mohs
hard-
ness
specific
gravity
thorite black; also
orange-yellow
(orangite)
4½–5 4.5–5.0;
5.2–5.4
(orangite)
topaz straw- or wine-
yellow; white;
grayish, greenish,
bluish, reddish
vitreous 8 (a hard-
ness
standard)
3.5–3.6
vesuvianite yellow, green,
brown
vitreous 6–7 3.3–3.4
willemite white or green-
ish yellow
vitreous to
resinous
5½ 3.9–4.2
zircon reddish brown,
yellow, gray,
green, or
colourless
adamantine 7½ 4.6–4.7
zoisite white; gray;
green-brown;
pink (thulite)
vitreous 6–6½ 3.2–3.4
7 the Silicates 7
SIlICATe MINerAlS
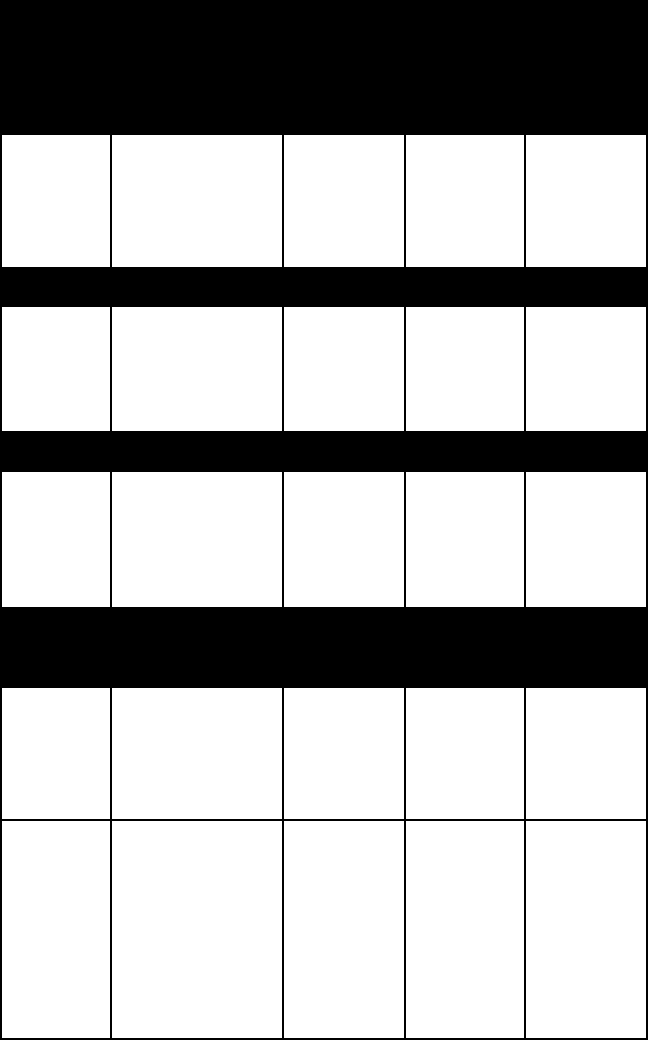
name habit frac
-
ture or
cleavage
refrac-
tive
indices
crystal
system
Tectosilicates (three-dimensional networks)
feldspar (for other examples, see feldspar)
orthoclase twinned crystals two good
cleav-
ages of 90
degrees
alpha =
1.518–1.529
beta =
1.522–1.533
gamma =
1.522–1.539
monoclinic
7 Minerals 7
118
name habit frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
feldspathoid (for other examples, see feldspathoid)
nepheline small glassy crys-
tals or grains
poor
cleavage
omega =
1.529–1.546
epsilon =
1.526–1.542
hexagonal
silica (for other examples, see silica mineral)
quartz prismatic and
rhombohedral
crystals; massive
conchoidal
fracture
omega =
1.544
epsilon =
1.553
hexagonal
zeolite (for other examples, see zeolite)
chabazite single, cubelike
rhombohedrons
poor
cleavage
omega =
1.470–1.494
epsilon =
1.470–1.494
hexagonal
Phyllosilicates (sheet structures)
clay (for other examples, see clay mineral)
chlorite large crystalline
blocks; fine-
grained, flaky
aggregates
platy
cleavage
alpha =
1.57–1.64
gamma =
1.575–1.645
monoclinic
or triclinic
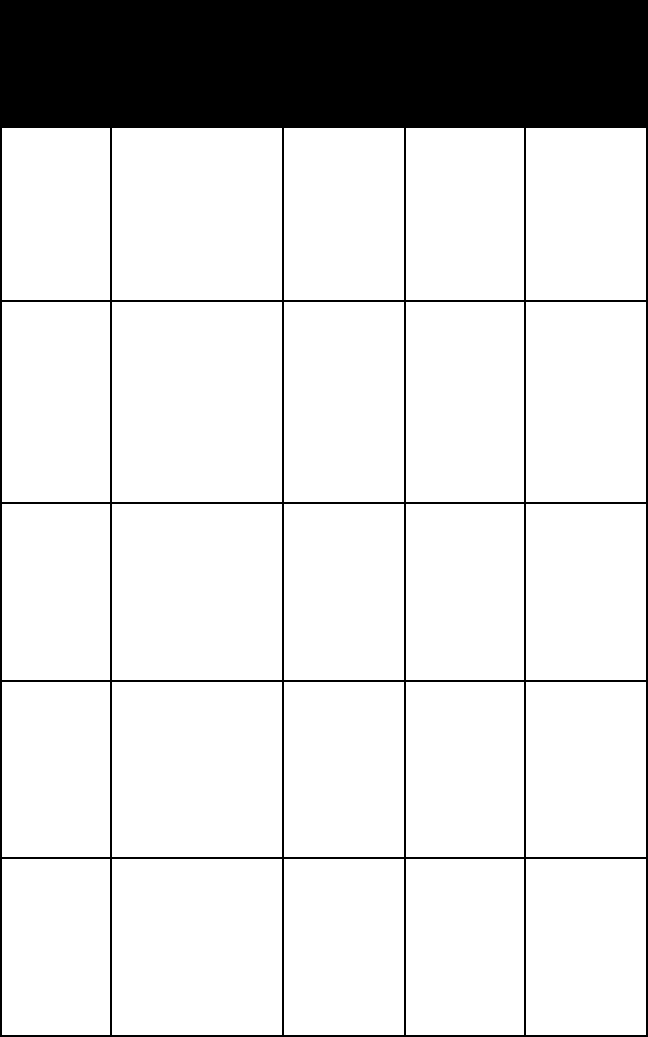
smectite broad undu-
lating mosaic
sheets that
break into
irregular fluffy
masses of min-
ute particles
alpha =
1.480–1.590
gamma =
1.515–1.630
119
7
the Silicates 7
name habit frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
mica (for other examples, see mica)
apophyllite tabular, pris-
matic, or
granular crys-
tals; prisms and
bipyramids when
well-formed
one per-
fect, one
poor
cleavage
omega =
1.534–1.535
epsilon =
1.535–1.537
tetragonal
muscovite large tabular
blocks (called
books); pseu-
dohexagonal
crystals; fine-
grained
aggregates
one per-
fect, platy
cleavage
alpha =
1.552–1.574
beta =
1.582–1.610
gamma =
1.587–1.616
prehnite rosettes of small
radiating crystals;
tabular or pris-
matic crystals;
lamellar or botry-
oidal massive
one good
cleavage
alpha =
1.611–1.632
beta =
1.615–1.642
gamma =
1.632–1.665
ortho-
rhombic
pyrophyl-
lite
lamellar massive;
granular to com-
pact massive
one perfect
cleavage
alpha =
1.534–1.556
beta =
1.586–1.589
gamma =
1.596–1.601
monoclinic
talc compact foliated
masses
one perfect
cleavage
alpha =
1.539–1.553
beta =
1.589–1.594
gamma =
1.589–1.600
monoclinic
7 Minerals 7
120
name habit frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
Inosilicates (chain structures)
amphibole (for other examples, see amphibole)
common
horn-
blende
massive one good
cleavage of
56 degrees
alpha =
1.615–1.705
beta =
1.618–1.714
gamma =
1.632–1.730
monoclinic
mullite elongated pris-
matic crystals;
melts
one dis-
tinct
cleavage
alpha =
1.642–1.653
beta = 1.644
gamma =
1.654–1.679
ortho-
rhombic
pyroxene (for other examples, see pyroxene)
augite short, thick,
tabular crystals
one good
cleavage of
87 degrees
alpha =
1.671–1.735
beta =
1.672–1.741
gamma =
1.703–1.761
monoclinic
rhodonite rounded tabular
crystals; cleav-
able to compact
massive; embed-
ded grains
two perfect
cleavages
alpha =
1.711–1.738
beta =
1.715–1.741
gamma =
1.724–1.751
triclinic
wollas-
tonite
cleavable,
fibrous, or com-
pact massive;
tabular crystals
one per-
fect, two
good
cleavages
alpha =
1.616–1.640
beta =
1.628–1.650
gamma =
1.631–1.653
triclinic
121
7
the Silicates 7
name habit frac-
ture or
cleavage
refrac-
tive
indices
crystal
system
Cyclosilicates (ring structures)
axinite broad,
sharp-edged,
wedge-shaped
crystals; lamellar
massive
one good
cleavage
alpha =
1.674–1.693
beta =
1.681–1.701
gamma =
1.684–1.704
triclinic
beryl long hexagonal
crystals
conchoidal
to uneven
fracture
omega =
1.569–1.598
epsilon =
1.565–1.590
hexagonal
cordierite short prismatic
crystals; embed-
ded grains;
compact massive
one dis-
tinct
cleavage
alpha =
1.522–1.558
beta =
1.524–1.574
gamma =
1.527–1.578
ortho-
rhombic
tourmaline parallel or radi-
ating groups of
striated, elon-
gated hexagonal
prisms, often
rounded or
barrel-shaped;
massive
subcon-
choidal
to uneven
fracture
omega =
1.635–1.675
epsilon =
1.610–1.650
hexagonal
Sorosilicates (double tetrahedral structures)
hemimor-
phite
sheaflike crystal
aggregates
one perfect
cleavage
alpha =
1.614
beta = 1.617
gamma =
1.636
ortho-
rhombic
