Polaina J., MacCabe A.P. (ed.). Industrial Enzymes. Structure, Function and Applications
Подождите немного. Документ загружается.

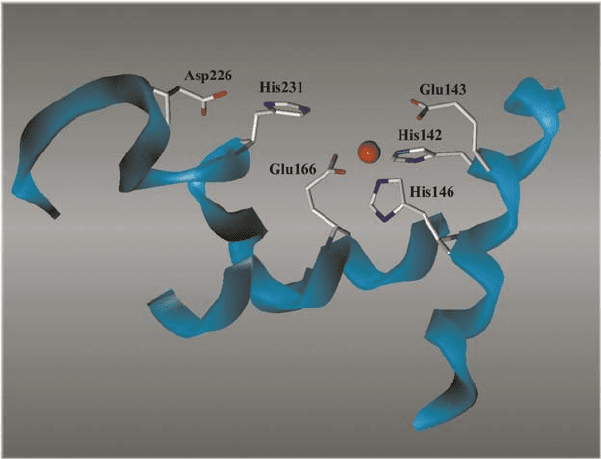
METALLOPROTEASES 227
Figure 3. The active site of thermolysin. Amino acids involved in catalysis and mentioned in the text
are shown in detail
and its subsequent nucleophilic attack on the carbonyl carbon atom of the scissile
bond. A protonated His residue (His231 in thermolysin), which is bound via a
hydrogen bond to an Asp residue in the active site cleft (Asp226 in thermolysin), is
claimed to stabilise the transition state by forming a hydrogen bond to the carbonyl
oxygen atom. The breakdown of the tetrahedral intermediate in the transition state is
accomplished by protonation of the amide nitrogen of the scissile bond via Glu143.
An alternative proposal, the ‘reverse protonation’ mechanism, derives from the
observed dependence of the catalytic constants on pH in the hydrolysis of arazo-
formyl peptides which are poor substrates for thermolysin. This alternative questions
the role of Glu143 as a general base and instead proposes the assignment of this
function to a His residue (His231 in thermolysin) in the active site (Mock and
Aksamawati, 1994; Mock and Stanford, 1996). In this case, the scissile bond is
activated for hydrolysis by direct coordination of the carbonyl oxygen atom to
the zinc ion as a potent Lewis acid with simultaneous displacement of the water
molecule otherwise bound to the zinc ion. By analogy to serine proteases, His231
along with Asp226 is supposed to enable proton transfer by deprotonation of an
incoming water molecule which is not bound to the zinc ion.
A recent quantum chemical study (Pelmenschikov et al., 2002) has confirmed
the key role of Glu143 in the thermolysin-catalysed hydrolysis of peptides, thus
supporting the initially proposed mechanism of Matthews and co-workers. In the
context of these data, the reverse protonation mechanism seems to be less favoured.
228 MANSFELD
Glu143 is absolutely conserved in all metalloproteases having the HEXXH
motif, whereas His231 is only partly conserved in metalloproteases employing the
same catalytic mechanism. Additional arguments strengthening the essential role of
Glu143 are provided by site-directed mutagenesis studies. The charge-conserving
replacement of Glu143 by Asp in NprM from B. stearothermophilus MK232, an
enzyme identical to thermolysin at the primary structure level, led to complete
loss of activity (Kubo et al., 1992). This makes it rather unlikely that Glu143
acts solely as a negatively charged counter-ion providing electrostatic stabilisation
of the transition state. Replacement of Glu143 in TLP from B. subtilis caused
nearly complete loss of secreted enzyme, whereas His231 (thermolysin numbering)
mutants were secreted and retained a certain degree of activity (Toma et al., 1989).
Replacement of His231 by Ala or Phe in the TLP from B. stearothermophilus
reduced the k
cat
/K
m
value 430 and 500-fold, respectively (Beaumont et al., 1995).
These mutants showed reduced pH dependence in the alkaline range. Bearing all
this in mind, the essential role of His231 is not supported. Attempts performed
by our group to produce completely inactive TLPs from B. stearothermophilus
showed that the least active enzyme was the Glu143Gln mutant having less than
0.1% residual activity, whereas the His231Ala mutant enzyme restored 1.6% of
wild-type activity. A combination of both mutations was not able to decrease the
activity further (Mansfeld, unpublished results). The enzymes were expressed in
E. coli and renatured as described in Mansfeld et al. (2005).
Based on structural comparisons, a hinge-bending motion leading to the closure
of the active site cleft upon substrate or inhibitor binding has been proposed
(Holland et al., 1992, 1995; Hausrath and Matthews, 2002). The involvement
of conserved glycine residues at positions 78, 135 and 136 has been assumed
and experimentally proven for thermolysin (Holland et al., 1992), a TLP from
B. cereus (Stark et al., 1992) and a TLP from B. stearothermophilus (Veltman
et al., 1998). Recent comparisons of the ‘open’ and the ‘closed’ structures (Hausrath
and Matthews, 2002) have however shown that these two regions cannot account
completely for the observed movement of the domains at the active site cleft. The
concerted movement of a group of side chains is proposed instead.
Thermolysin and related proteases preferably cleave substrates having bulky
hydrophobic residues (Leu, Phe) in the P
1
' position and smaller amino acids
in position P
1
(nomenclature according to Schechter and Berger, 1967). Four
major substrate binding pockets S
2
S
1
S
1
' S
2
' on the enzyme have been
identified (Hangauer et al., 1984). The hydrophobic substrate binding pocket S
1
'of
thermolysin, mainly formed by Phe130, Leu133, Val139 and Leu202, is considered
to be the main determinant of substrate specificity and preferably binds hydrophobic
residues such as Leu (e.g. Hangauer et al., 1984; Matthews, 1988). Mutagenesis
studies on the TLP of B. stearothermophilus have shown that the preference of
this protease for Phe at P
1
' can be altered toward that of thermolysin by changing
Phe133 to Leu, the latter residue being present in thermolysin at that position (de
Kreij et al., 2000, 2001). Enlargement of the binding pocket by replacement of
Leu202 by smaller amino acids (Val, Ala, Gly) resulted in higher efficiency toward
METALLOPROTEASES 229
substrates with Phe at P
1
'. Unexpectedly, reduction of its size by substitution of
Leu202 by Phe or Tyr also caused a large increase in activity toward substrates
with Phe at P
1
'.
The role of other substrate binding pockets has recently been highlighted by the
adaptation of vimelysin (Vibrio sp.) substrate specificity in the P
3
' position to that
of thermolysin upon exchange of Arg215 in the S
3
' binding pocket for Asp215
present in thermolysin (Oda et al., 2005). Vimelysin has 35% sequence identity
with thermolysin and is characterised by high stability in organic solvents. It shows
a strong preference for Phe over Leu at position P
1
' and also a preference for neutral
or acidic amino acids in the P
3
' position in contrast to thermolysin in which basic
amino acids are preferred at position C.
Enhancements of the activity of thermolysin have been achieved by mutation
of Tyr110 and Phe114 in the S
2
subsite, as well as Tyr211 (Kubo et al., 1992),
Gln119 (Kidokoro et al., 1995) and Leu155 (Matsumiya et al., 2004). Some of
the effects were additive (Kidokoro, 1998; de Kreij et al., 2002). However, the
effects of mutations on activity were in most cases smaller for large proteinaceous
substrates compared to those observed for short peptides probably as a consequence
of the different possible productive binding modes for the former. The contribution
of binding to catalysis is expected to be much less in the case of short peptides.
Thermolysin activity is considerably enhanced by the addition of neutral salts
(Inouye, 1992; Inouye et al., 1998a; Bedell et al., 1998). A further increase in
activity is observed when the catalytic zinc ion is substituted by cobalt ions (Kuzuya
and Inouye, 2001). Both effects are independent of each other. Activation by NaCl
has been shown to be caused by an increase in k
cat
values (Inouye et al., 1996).
The addition of neutral salts also has beneficial effects on thermolysin solubility
and thermal stability (Inouye et al., 1998a and b). Preliminary studies of crystals
soaked in 4 M NaCl did not show significant changes in the space group (Kamo
et al., 2005). Positive effects on thermolysin activity have also been described for
sugars (e.g. sucrose, trehalose) and other polyols (Mejri et al., 1998), and pressure
up to 2.5 kbar (Kudryashova et al., 1998).
Thermolysin is inhibited by zinc-chelating agents (e.g. 1,10-phenanthroline,
EDTA) (Holmquist and Vallee, 1974). Other more specific inhibitors for
thermolysin have been developed over the years, and their number is still increasing
(reviewed in van den Burg and Eijsink, 2004).
3.2. Stability
Apart from high activity, the stability of an enzyme at higher temperatures and
toward other denaturing influences is one of the most important criteria for the
application of enzymes in biocatalysis. To meet the demands of industry, high
operational stability of enzymes is required as this significantly contributes to cost
reduction.
In order to obtain highly stable enzymes several strategies are used. One is
focused on the isolation of new enzymes from extremophilic organisms (reviewed
230 MANSFELD
by Vieille and Zeikus, 2001). Other strategies are based on: the stabilisation of
available enzymes by rational design of enzyme variants (Eijsink et al., 2004), high-
throughput screening of randomly generated mutant libraries (Eijsink et al., 2005)
in combination with recently described ‘semi-rational’ approaches for a guided
design of these libraries (Patrick and Firth, 2005), chemical modification including
immobilisation (Ulbrich-Hofmann et al., 1999) and de novo design of catalysts
(Kaplan and DeGrado, 2004).
Since thermolysin is produced by a highly thermophilic Bacillus strain it
is relatively thermostable compared to other metalloproteases produced by less
thermophilic strains. As a result of a series of mutational studies a surface-
exposed region between amino acid residues 56 – 69 in the N-terminal part
of the thermolysin-like protease from B. stearothermophilus (TLP-ste) (Takagi
et al., 1985; 85% sequence identity to thermolysin) has been identified that is
extremely sensitive to mutation, whereas the C-terminal part of the protease is only
slightly affected by even dramatic amino acid changes (Vriend and Eijsink, 1993;
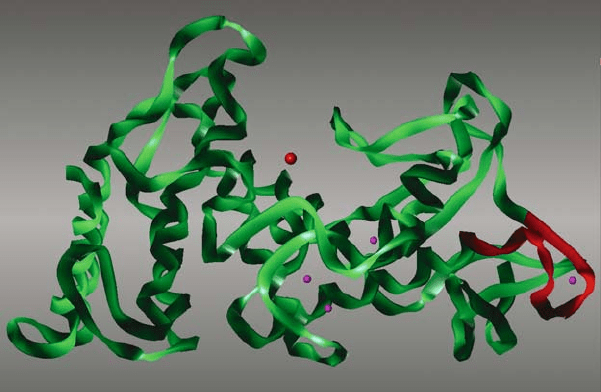
Eijsink et al., 1995). Later this region was recognised as being the most labile region
of the protein where local unfolding processes start, resulting in rapid autoprote-
olytic degradation of the protein (Eijsink et al., 1995; Vriend et al., 1998; Fig. 4).
In light of the concept of protein stabilisation developed by Schellenberger and
Ulbrich (1989), this region was consequently called the unfolding region (Mansfeld
et al., 1999). Coherently, stabilisation of this region enabled the construction of
variants displaying considerably enhanced thermostability. The amino acid residues
Figure 4. Homology model of the 3D structure of TLP from B. stearothermophilus (Vriend and
Eijsink, 1993). The large sphere represents the Zn
2+
ion in the active site. The four smaller spheres
represent the Ca
2+
ions bound to the molecule
METALLOPROTEASES 231
selected for replacement were chosen on the one hand on the basis of rational
design strategies, and on the other because they corresponded to residues naturally
occurring in the more thermostable enzyme thermolysin (reviewed in Eijsink
et al., 1995). The introduction of a disulfide bridge between residues 8 and 60
(Mansfeld et al., 1997) was found to strongly stabilise this region against local
unfolding. Detailed studies of this mutant have shown that this effect is mainly
attributable to stabilisation against autoproteolysis rather than global unfolding
(Dürrschmidt et al., 2001). The disulfide bridge was shown to be able to mimic
the stabilising effect of calcium ions in local unfolding processes (Dürrschmidt
et al., 2005). Calcium ions are major determinants of protease stability (Dahlquist
et al., 1976).
These studies culminated in the successful conversion of the moderately stable
TLP from B. stearothermophilus into an extremely stable enzyme (named boilysin)
via a limited number of mutations (van den Burg et al., 1998). The half-life of
the mutant enzyme was 170 min at 100
C in contrast to 1 min for thermolysin.
Compared to naturally occurring enzymes from thermophilic organisms, boilysin
is characterised by wild-type like activity under the usually employed operating
temperatures making this enzyme interesting for industrial application. As temper-
ature is increased, activity is further enhanced. This enzyme has been tested under
extreme conditions for the hydrolysis of substrates that are difficult to digest (van
den Burg et al., 1999; de Kreij et al., 2000), the hydrolysis of prion proteins and
protein removal in nucleic acid purification (van den Burg, personal communi-
cation), and for the synthesis of an aspartame precursor (Kühn et al., 2002).
Taking advantage of knowledge on the enzyme’s unfolding process and the
concept of stabilisation by strengthening the most labile region in a protein (Schel-
lenberger and Ulbrich, 1989; Ulbrich-Hofmann et al., 1999), strong stabilisation
of TLP-ste was achieved by immobilisation in a site-specific manner (Mansfeld
et al., 1999). This was most effective when the protein was fixed to the carrier via
cysteine residues in the unfolding region. Very strong stabilisation has also been
obtained by immobilisation via multiple bonds to a carrier having a high density
of functional groups (Mansfeld and Ulbrich-Hofmann, 2000), suggesting that more
than one labile region might be present in the molecule as has been argued by Vriend
et al. (1998). Rigidification of the enzyme due to the formation of multiple bonds
with the carrier material was found to occur at the expense of activity (Mansfeld
and Ulbrich-Hofmann, 2000).
Another strategy to protect TLPs against autoproteolytic degradation and to
stabilise against thermal inactivation is the identification of primary cleavage sites.
The removal of these autodegradation sites has been described for TLP from
B. subtilis (van den Burg et al., 1990) and thermolysin (Matsumiya et al., 2004).
4. APPLICATIONS
Thermolysin is commercially available at industrial scale as Thermoase PC10F from
Amano Enzyme Inc., formerly Daiwa Kasei Co. Ltd., Japan. Other bacterial metal-
loproteases produced commercially are the TLPs from B. subtilis (Neutrase
®
from
232 MANSFELD
Novo Nordisk, Denmark and Protin PC10F from Amano Enzyme Inc., Japan). The
highly stable TLP-ste variant Boilysin can be requested from IMEnz (Groningen,
The Netherlands).
At laboratory scale, thermolysin can be purchased from different suppliers
(e.g. Sigma, MERCK Biosciences). Thermolysin and TLPs from Bacillus species
are traditionally produced in protease-deficient B. subtilis strains such as DB104
(Kawamura and Doi, 1984) and DB117 (Eijsink et al., 1990). These enzymes
are synthesized as inactive preproenzymes (Takagi et al., 1985; Kubo and
Imanaka, 1988; van den Burg et al., 1991) and processed to the active mature
enzymes via autocatalytic removal of the large propeptides (about 200 amino acids)
(Wetmore et al., 1992; Marie-Claire et al., 1998). The thermolysin propeptide has
been shown to act as a mixed, non-competitive inhibitor of the protease and to facil-
itate the recovery of active enzyme from denatured thermolysin in a stoichiometric
manner (O’Donohue and Beaumont, 1996; Marie-Claire et al., 1999). Recently,
several strategies have been described for the expression of these enzymes in E. coli
with the prosequence in cis or trans (Marie-Claire et al., 1999; Inouye et al., 2005)
and even in the absence of the prosequence (Mansfeld et al., 2005).
The enzymes secreted into the culture broths of B. subtilis and E. coli or renatured
from inclusion bodies formed in E. coli (Mansfeld et al., 2005) can be purified
by affinity chromatography on Bacitracin-silica (van den Burg et al., 1989) or
Gly-D-Phe columns (Walsh et al., 1974).
4.1. Synthesis of Peptides
Large scale synthesis of peptides has become increasingly important for the food
and pharmaceutical industries over the last few decades. The main application of
peptides is their use as low-calorie sweeteners. In addition, several biologically
active peptides have found interest as drugs in the treatment of diseases. Apart
from conventional peptide synthesis, new strategies for production have been tested,
one of which is enzymatic synthesis. The advantages of using enzymes are the
stereospecificity they confer on the reaction, the necessity for only minimal side
chain protection, the mild reaction conditions, and the avoidance of racemisation.
Thermolysin is one of the enzymes that has been studied for its potential in
enzymatic peptide synthesis. Since its first use for this purpose (Isowa et al., 1979) it
has been extensively studied in the laboratory and is used for large-scale production
of N-carbobenzoxy-L-aspartyl-L-phenylalanine methyl ester (Z-Asp-Phe-OMe), the
precursor of the widely used artificial sweetener aspartame (Ooshima et al., 1985;
Murakami et al., 1996). Thermolysin proved to be advantageous in the synthesis of
aspartame due to its low esterolytic activity that results in the preservation of the
methyl ester group which is essential for the sweet taste of the peptide.
As no acyl enzyme is formed in thermolysin catalysis, the reactions cannot be
run in kinetically controlled mode. Therefore, several strategies have been tested
to shift the equilibrium of the thermodynamically controlled enzymatic synthesis
of Z-Asp-Phe-OMe to the desired product. These are based on aqueous systems
METALLOPROTEASES 233
(Inouye, 1992; Murakami et al., 1996), systems with water-miscible organic solvents
(Lee et al., 1992; Kühn et al., 2002), biphasic systems (Hirata et al., 1997; Murakami
and Hirata, 1997; Murakami et al., 1998; Miyanaga et al., 2000b), solid-to-solid
synthesis (Erbeldinger et al., 1998a, b; Erbeldinger et al., 2001) and low-water
solvent systems (Nakanishi et al., 1985). For syntheses in aqueous/organic biphasic
systems (Murakami and Hirata, 1997; Hirata et al., 1997), in low-water solvent
systems with immobilized thermolysin (Nakanishi et al., 1985, 1990) or in
membrane systems (Iacobucci et al., 1994), continuous operation has been used
successfully.
Yields in pure aqueous systems are usually very low. The activity of thermolysin
and, accordingly, the reaction rates in aqueous systems have been found to be
enhanced by the addition of sodium and potassium salts (Inouye, 1992). However,
the pH increase due to salt addition may result in non-enzymatic hydrolysis of the
reactants, a problem which is avoided in reactions at low pH. High yields (95%)
have been achieved by insoluble salt formation between the product and excess Phe-
OMe or unreacted enantiomer D-Phe-OMe followed by subsequent removal of the
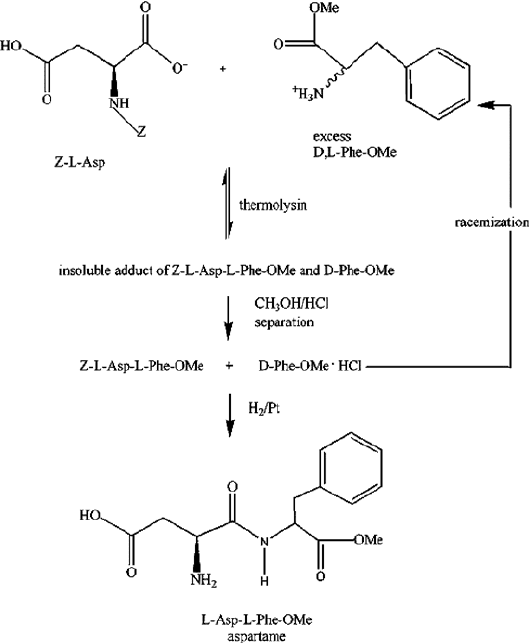
precipitate from the aqueous solution (Ager et al., 1998). This method is used in the
commercial aspartame precursor production process of TOSOH (Japan) performed
at Holland Sweetener (The Netherlands) (Fig. 5). Addition of a water-immiscible
solvent like toluene or 4-methylpentan-2-one after the start of formation of the
precipitate was found to permit the process to be run continuously. In the presence
of water-miscible organic solvents (e.g. dimethylsulfoxide) reaction rates were also
enhanced by the addition of salts (Kühn et al., 2002), though yields decreased with
increasing salt concentrations. Yields could be markedly improved by the addition of
alcohols (methanol, 2-propanol) to aqueous systems even though reaction rates were
reduced due to inhibitory effects on thermolysin (Kühn et al., 2002). In biphasic
organic solvent systems, ethyl acetate, tert.-amyl alcohol (Miyanaga et al., 2000b),
n-butyl acetate (Murakami and Hirata, 1997), tributylphosphate and 1-butanol (in
the synthesis of N-formyl-Asp-Phe-OMe – Murakami et al., 2000a) and ionic liquids
(e.g. 1-butyl-3-methylimidazolium hexafluorophosphate – Erbeldinger et al., 2000)
have all been used as solvents. In the solid-to-solid system the pH adjusted by basic
inorganic salt addition played an important role (Erbeldinger et al., 2001). In low-
water solvent systems the water is usually provided by the carrier materials that are
used for adsorptive binding of the enzyme. Polyacrylic ester resins such as XAD-7
(ICN Biomedicals Inc., USA) in ethyl acetate and tert.-amyl alcohol (Miyanaga
et al., 2000a, b), Celite R-640 (FLUKA) in combination with toluene as solvent
(de Martin et al., 2001) or molecularly imprinted polymers (methacrylate-ethylene
glycol dimethacrylate-copolymers) in ethyl acetate (Ye et al., 1999) have all been
used as carrier materials. A considerable increase in thermolysin activity in non-
aqueous media has been achieved by lyophilisation in the presence of KCl or other
inorganic salts (Bedell et al., 1998). Activity could be further improved by the use
of molecular imprinting in combination with activation by salts (Rich et al., 2002).
Cross-linked enzyme crystals (CLECs) of thermolysin which have been used
234 MANSFELD
Figure 5. Principle of commercial aspartame synthesis of TOSOH at Holland Sweetener (The
Netherlands)
successfully for the synthesis of the aspartame precursor (Persichetti et al., 1995)
represent an interesting tool for organic chemists due to their high specific activity
and increased resistance to inactivation by organic solvents, elevated temperatures
and proteolysis.
In all these systems the reaction velocities and yields obtained result from the
interplay between the reaction medium (i.e. buffer, organic solvent, salt concen-
tration), the types and ratios of reactants, and the type of product, as well as
the activity and stability of thermolysin in the corresponding reaction systems.
A compromise always has to be found between initial reaction rates and final yields.
To broaden the scope for enzymatic peptide synthesis new enzymes have been
searched for. A metalloprotease called vimelysin from Vibrio sp. proved to be
superior to thermolysin for the synthesis of aspartame at lower temperatures
and higher solvent concentrations. Like vibriolysin from the Antarctic bacterium
METALLOPROTEASES 235
strain 643 (Adekoya et al., 2006), it might be an interesting alternative for
thermolabile substrates (Kunugi et al., 1997). Due to its broader substrate speci-
ficity, pseudolysin from Pseudomonas aeruginosa might also be interesting for
synthetic purposes (Rival et al., 2000). At laboratory scale, free or immobilized
thermolysin has also been used for the synthesis of other peptides: Z-Gln-Leu-
NH
2
, Z-Phe-Leu-NH
2
, various dipeptide fragments of cholecystokinin, and peptides
containing non-proteogenic amino acids (Wayne and Fruton, 1983; Erbeldinger
et al., 1998a, b, 1999; Calvet et al., 1996; Krix et al., 1997). Neutrase
®
, a TLP
from B. subtilis, with a slightly different substrate specificity to that of thermolysin,
was also applied in peptide synthesis (either as free or immobilized enzyme (on
Celite-545 (Fluka, Germany) or Polyamide-PA6 (Akzo)) (Clapes et al., 1995, 1997).
A scale-up of the suspension-to-suspension approach using mainly undissolved
substrates was performed by Eichhorn et al. (1997). The possibility of using the
extractive reaction in aqueous/organic biphasic systems for the continuous synthesis
of Z-Gly-Phe-OMe at larger scale and in high yield was reported by Murakami
et al. (2000b). Unexpectedly, thermolysin was also shown to catalyse the acylation
of paclitaxel with divinyl adipate (Khmelnitsky et al., 1997) and an acylation of
sucrose with vinyl laurate (Pedersen et al., 2002).
4.2. Production of Protein Hydrolysates
Another very important industrial application of metalloproteases (mostly in combi-
nation with other proteases) is the production of hydrolysed food proteins and
flavour-enhancing peptides to replace the chemical methods of synthesis. Soy and
wheat hydrolysates are used in flavour-enhancement of soups and sauces, and milk
protein hydrolysates are preferred for the refinement of cheese products. Meat
hydrolysates find application in the enhancement of the flavour of meat products,
soups, sauces and other instant products. An advantage of enzymatic processes
is the minor formation of unwanted by-products of negative impact on health;
the disadvantageous formation of bitter tasting peptides in enzymatically produced
protein hydrolysates can be overcome by simultaneous treatment with exopeptidases
(reviews in Saha and Hayashi, 2001; Raksakulthai and Haard, 2003). Bitter peptides
are characterised by a high content of hydrophobic amino acids. Hydrolysates of
meat and fish proteins or gelatin develop less bitter taste than hydrolysates of maize
protein, casein or haemoglobin. Flavour development by a cocktail of proteases,
including Neutrase
®
has been used to accelerate the ripening of dry fermented
sausages (e.g. Fernandez et al., 2000). The development of high-value functional
foods and nutraceuticals has made a major impact on dairy protein hydrolysate
production because of the latter’s probiotic, antimicrobial and digestive effects.
Another beneficial effect of protein hydrolysates on health might be their antiox-
idant effects (Hernandez-Ledesma et al., 2005) and their inhibition of angiotensin-
converting enzyme (Vercruysse et al., 2005). Low-molecular weight hydrolysis
products of protamine have been tested successfully as a delivery system for DNA
in gene therapy (Park et al., 2003).
236 MANSFELD
4.3. Other Applications
Further commercial applications of metalloproteases can be found in the brewing
industry (improved filtration of beer, reduced calorie content), in the leather industry
(bating and dehairing), the processing of slaughter waste, improvement of the
baking characteristics of flour, and in the film industry (recovery of waste silver).
An interesting application of immobilized thermolysin is the removal of protein
coatings from the surface of old documents and art work (Moeschel et al., 2003).
In protein science, thermolysin is an important tool for limited proteolysis to
determine primary structures and gain first insights into the conformation of proteins
whose crystal structures are not yet known (reviewed in Fontana et al., 2004).
It is also used to analyse confined local fluctuations and global unfolding events
in proteins and to determine their stabilities (Arnold et al., 2005; Park and
Marqusee, 2005), or to isolate protein fragments that can fold autonomously and
therefore be considered as domains which might be useful in crystallisation and
high-throughput applications (Gao et al., 2005).
Inhibitors of metalloproteases involved in diseases are of potential therapeutic
use. In this respect, thermolysin has been used as a template for the creation
of homology models of the active sites of medically relevant mammalian metal-
loproteases. Details of these important classes of metalloproteases can be found
in Barrett et al. (2004). Interesting targets are: neprilysin (Roques et al., 1993);
angiotensin-converting enzyme, being responsible for degradation of biologically
active peptides such as enkephalins; endothelin-converting enzymes, which liberate
endothelin (a potent vasoconstrictor) from its precursor; highly potent neuro-
toxins like bontoxilysin and tentoxilysin from Clostridium species which block the
release of acetylcholine at neuromuscular junctions and cause motor paralysis in
tetanus and botulism; anthrax lethal factor, which acts by disrupting intracellular
signalling by cleaving mitogen-activated protein kinase kinases and causes multiple
haemorrhagic lesions; matrix metalloproteases or matrixins (like collagenase,
elastase, stromelysin, matrilysin, gelatinase) which are involved in the degradation
of extracellular matrix proteins including collagen and are required for tissue
repair and remodelling but are also involved in pathological processes (arthritis,
atherosclerosis, tumour growth and metastasis); ADAM17 (tumour necrosis factor
-converting enzyme) and ADAM10 (myelin-associated metalloendopeptidase),
pappalysins 1 and 2 which cleave insulin-like growth factor 1 binding protein-4
and liberate the growth factor.
5. ACKNOWLEDGEMENT
I would like to thank Prof. Dr. R. Ulbrich-Hofmann for critical reading of the
manuscript, Dr. S. Gebauer’s group of Molecular Modelling at our department for
help in preparing the Figures, Prof. G. Vriend, Dr. B. van den Burg, Prof. V.
Eijsink, and Prof. G. Venema for getting me started with the metalloprotease work
and fruitful cooperation.
