Polaina J., MacCabe A.P. (ed.). Industrial Enzymes. Structure, Function and Applications
Подождите немного. Документ загружается.

216 CLAVERIE-MARTÍN AND VEGA-HERNÁNDEZ
5.2. Endothiapepsin
The chestnut blight fungus C. parasitica produces an aspartic protease, endoth-
iapepsin, with milk-clotting properties similar to those of calf rennet (Awad
et al., 1999). Due to its very high thermolability this enzyme is particularly suited for
use in the production of Emmental and Italian style cheeses. Extensive production
of a wide variety of cheeses including Cheddar, Swiss, Colby and Italian varieties
manufactured with partially purified preparations of C. parasitica enzyme are
considered to be equal or superior to control cheeses made with animal rennet.
DSM markets Endothiapepsin under the trade name Suparen®.
C. parasitica protease has greater proteolytic activity than MAP or chymosin.
Proteolysis of both
s1
-casein and -casein occurs during storage of Mozzarella
and Cheddar cheeses made with C. parasitica protease (Yun et al., 1993). The
change in cheese properties during storage is related to the combined effects of
hydrolysis of
s1
-casein and -casein. Kim et al. (2004) have combined the use of
chymosin and the C. parasitica enzyme to control the hydrolysis of
s1
-casein and
-casein during aging of Cheddar cheese to independently control its firmness and
meltability while regulating undesirable levels of bitterness associated with high
levels of C. parasitica protease.
5.3. Irpex Lacteus Protease
The wood-decaying basidiomycete Irpex lacteus produces an AP (ILAP) that has
high milk-clotting activity in relation to its proteolytic activity. This enzyme may
become a good chymosin alternative (Kobayashi et al., 1985). ILAP contains 340
amino acid residues with a molecular mass of 35 kDa. It is most active at pH 3.0
and is inhibited by pepstatin. A feature of ILAP is its high content of serine and
threonine residues (48 and 54, respectively), accounting for 30% of the residues
which is double the average for other proteins. It lacks the three-disulfide bridges
that are generally present in most pepsin-type APs.
REFERENCES
Aguilar, C.F., Cronin, N.B., Badasso, M., Dreyer, T., Newman, M.P., Cooper, J.B., Hoover, D.J., Wood,
S.P., Johnson, M.,S. and Blundell, T. (1997) The three-dimensional structure at 2.4Å resolution of
glycosylated proteinase A from the lysosome-like vacuole of Saccharomyces cerevisiae J. Mol. Biol.
267, 899–915.
Aikawa, J., Park, Y.N., Sugiyama, M., Nishiyama, M., Horinouchi, S. and Beppu, T. (2001) Replacement
of amino acid residues at subsites and their effects on the catalytic properties of Rhizomucor pusillus
pepsin, an aspartic proteinase from Rhizomucor pusillus. J. Biochem. 129, 791–794.
Aikawa, J., Yamashita, T., Nishiyama, M., Horinouchi, S. and Beppu, T. (1990) Effects of glycosylation
on the secretion and enzyme activity of Mucor rennin, an aspartic proteinase of Mucor pusillus,
produced by recombinant yeast. J. Biol. Chem. 265, 13955–13959.
Andreeva, N. and Rumsh, L.D. (2001) Analysis of crystal structures of aspartic proteinases: On the
role of amino acid residues adjacent to the catalytic site of pepsin-like enzymes. Protein Sci. 10,
2439–2450.
ASPARTIC PROTEASES USED IN CHEESE MAKING 217
Awad, S., Luthi-Peng, Q.Q. and Puhan, Z. (1999) Proteolytic activities of Suparen and Rennilase on
buffalo, cow, and goat whole casein and beta-casein. J. Agric. Food Chem. 47, 3632–3639.
Blundell, T.L., Jenkins J.A., Sewell, B.T., Pearl, L.H., Cooper, J. B., Tickle, I.J., Veerapandian, B. and
Wood, S.P. (1990) X-ray analyses of aspartic proteinases. The three-dimensional structure at 2.1 Å
resolution of endothiapepsin. J. Mol. Bio941.
Branner-Jorgensen, S., Inventor; Branner-Jorgensen, S., assignee. (1981 March 10) Thermal destabi-
lization of microbial rennet. U.S. Patent 4,255,454.
Branner-Jorgensen, S., Schneider, P. and Eigtved, P., inventors; Novo Industri A/S, assignee. (1982
November 2) Thermal destabilization of microbial rennet. U.S. Patent 4, 357, 357.
Budtz, P. and Heldt-Hansen, H. P., inventors; Gist-brocades, B. V., assignee. (1998 September 1) Cheese
making with recombinant aspartic protease. U.S. Patent 5, 800, 849.
Castanheira, P., Samyn, B., Sergeant, K., Clemente, J.C., Dunn, B. M., Pires, E. Van Beeumen, J. and
Faro., C. (2005) Activation, proteolytic processing, and peptide specificity of recombinant cardosin
A. J. Biol. Chem. 280, 13047–13054
Chitpinitoyl, S. and Crabbe, M.J.C. (1998) Chymosin and aspartic proteinases. Food Chem. 61, 395–418.
Coates, L., Erskine, P.T., Crump, M.P., Wood, S.P. and Cooper, J.B. (2002) Five atomic resolution
structures of endothiapepsin inhibitor complexes: Implications for the aspartic proteinase mechanism.
J. Mol. Biol. 318, 1405–415.
Cooper, J.B., Khan, G., Taylor, G., Tickle, I.J. and Blundell, T. (1990) X-ray analysis of aspartic
proteinases. II. Three-dimensional structure of the hexagonal crystal form of porcine pepsin at 2.3 Å
resolution. J. Mol. Biol. 214, 199–222.
Davies, D.R. (1990) The structure and function of the aspartic proteinases. Annu. Rev. Biophys. Biophys.
Chem. 19, 189–215.
Domingos, A., Cardoso, P.C., Xue, Z.T., Clemente, A., Brodelius, P.E. and Pais, M.S. (2000) Purification,
cloning and autoproteolytic processing of an aspartic proteinase from Centaurea calcitrapa. Eur. J.
Biochem. 267, 6824–6831.
Dunn-Coleman, N.S., Bloebaum, P., Berka, R.M., Bodie, E., Robinson, N., Armstrong, G., Ward, M.,
Przetak, M., Carter, G.L., LaCost, R., Wilson, L.J., Kodama, H.K., Baliu, E.F., Bower, B., Lamsa, M.
and Heinsohn, H. (1991) Commercial levels of chymosin production by Aspergillus. Biotechnol. 9,
976–891.
Elagamy, E.I. (2000) Physicochemical, molecular and immunological characterization of camel calf
rennet: A comparison with buffalo rennet. J. Dairy Res. 67, 73–81.
Egas, C., Lavoura, N., Resende, R., Brito, R.M.M., Pires, E., Pedroso de Lima, M.C. and Faro, C.
(2000) The saposin-like domain of the plant aspartic proteinase precursor is a potent inducer of vesicle
leakage. J. Biol. Chem. 49, 38190–38196.
Faro, C., Verissimo, P., Lin, Y., Tang, J. and Pires, E. (1995) Cardosin A and B, aspartic proteases from
the flowers of cardoon. Adv. Exp. Med. Biol. 362, 373–377.
Foltmann, B. (1992) Chymosin: a short review on foetal and neonatal gastric proteases. Scand. J. Clin.
Lab. Invest. Suppl. 210, 65–79.
Foltmann, B., Barkholt, P., Kauffman, D. and Wybrandt, G. (1979) The primary structure of calf
chymosin. J. Biol. Chem. 254, 8447–8451.
Foltmann, B., Pedersen, V.B., Jacobsen, H., Kauffman, D. and Wybrandt, G. (1977) The complete amino
acid sequence of prochymosin. Proc. Natl. Acad. Sci.USA.74, 2321–2324.
Fox, P.F. and McSweeney, P.L.H. (1999) Rennets: their role in milk coagulation and cheese ripening.
In Microbiology and Biochemistry of Cheese and Fermented Milk. Law, B.A. ed. Blackie Academic
and Proffesional, London, pp.1–49.
Francky, A., Francky, B.M., Strukelj, B., Gruden, K., Ritonja, A., Krizaj, I., Kregar, I., Pain, R. H. and
Pungercar, J. (2001) A basic residue at position 36p of the propeptide is not essential for the correct
folding and subsequent autocatalytic activation of prochymosin. Eur. J. Biochem. 268, 2362–2368.
Frazao, C. Bento, I., Costa J., Soares, C.M., Veríssimo, P., Faro, C., Pires, E., Cooper, J. and
Larrondo, M.A. (1999) Crystal structure of cardosin A, a glycosilated and Arg-Gly-Asp-containing
aspartic proteinase from the flowers of Cynara cardunculus L. J. Biol. Chem. 274, 27694–27701.
218 CLAVERIE-MARTÍN AND VEGA-HERNÁNDEZ
Fujimoto, Z., Fujii, Y., Kaneko, S., Kobayashi, H. and Mizuno, H. (2004) Crystal structure of aspartic
proteinase from Irpex lacteus in complex with inhibitor pepstatin. J. Mol. Biol. 341, 1227–1235.
Gilliland, G.L., Winborne, E.L., Nachman, J. and Wlodawer, A. (1990) The three-dimensional structure
of recombinant bovine chymosin at 2.3 Å resolution. Proteins. 8, 82–101.
Groves, M.R., Dhanaraj, V., Badasso, M., Nugent, P., Pitts, J.E., Hoover, D.J. and Blundell, T.L. (1998)
A 2.3 Å resolution structure of chymosin complexed with a reduced bond inhibitor shows that the
active site beta-hairpin flap is rearranged when compared with the native crystal structure. Protein
Eng. 11, 833–840.
Gustchina, E., Rumsh, L., Ginodman, L., Majer, P. and Andreeva, N. (1996) Post X-ray crystallographic
studies of chymosin: the existence of two structural forms and the regulation of activity by the
interaction with the histidine-proline cluster of kappa-casein. FEBS Lett. 379, 60–62.
Harboe, M.K. and Budtz, P. (1999) The production, action and application of rennet and coagulants. In
Tecnology of cheese making. Law, B. A. ed. Sheffield Academic Press, Sheffield,pp. 33–65.
Harboe, M.K. and Kristensen, P.B., inventors; Chr. Hansen A/S, assignee. (2000 October 3) Microbially
derived enzymes having enhanced milk clotting activity and method producing the same. US Patent
6, 127, 142.
Hiramatsu, R., Aikawa, J., Horinouchi, S. and Beppu, T. (1989) Secretion by yeast of the zymogen form
of Mucor rennin, an aspartic proteinase of Mucor pusillus, and its conversion to the mature form. J.
Biol. Chem. 264, 16862–16866.
Houen, G., Madsen, M.T., Harlow, K.W., Lonblad, P. and Foltmann, B. (1996) The primary structure
and enzymic properties of porcine prochymosin and chymosin. Int. J. Biochem. Cell Biol. 28,
667–675.
Hong, L. and Tang, J. (2004) Flap position of free memapsin 2 (beta-secretase), a model for flap opening
in aspartic protease catalysis. Biochem. 43, 4689–4695.
James, M.N.G. (2004) Catalytic pathway of aspartic peptidases. In Handbook of Proteolytic Enzymes.
Barrett, A.J., Rawlings, N.D. and Woessner, J.F. eds. Elsevier, London, pp. 12–19.
Jia, Z., Vandonselaar, M., Schneider, P. and Quail, J.W. (1995) Crystallization and preliminary X-ray
structure solution of Rhizomucor miehei aspartic proteinase. Acta Crystallogr. D. 51, 243–244.
Kageyama, T. (2002) Pepsinogens, progastricsins, and prochymosins: structure, function, evolution, and
development. Cell Mol. Life Sci. 59, 288–306.
Kappeler, S., Farah, Z., van den Brink, J.M., Rahbeck-Nielsen, H., Budtz, P., inventors; (2004 September
16) Method of producing non-bovine chymosin and use hereof. United States Patent Application
20040180410.
Kim, S.Y., Gunasekaran, S. and Olson, N.F. (2004) Combined use of chymosin and protease from
Cryphonectria parasitica for control of meltability and firmness of cheddar cheese. J. Dairy Sci. 87,
274–283.
Kobayashi, H., Kusakabe, I. and Murakami, K. (1985) Milk-clotting enzyme from Irpex lacteus as a
calf rennet substitute for Cheddar cheese manufacture. Agric. Biol. Chem. 49, 1605–1609.
Marciniszyn, J. Jr., Hartsuck, J.A. and Tang, J. (1976) Mode of inhibition of acid proteases by pepstatin.
J. Biol. Chem. 251, 7088–7094.
Mohanty, A.K., Mukhopadhyay, U.K., Grover, S. and Batish, V.K. (1999) Bovine chymosin: Production
by rDNA technology and application in cheese manufacture. Biotechnol. Adv. 17, 205–217.
Mohanty, A.K., Mukhopadhyay, U.K., Kaushik, J.K., Grover, S. and Batish, V.K. (2003) Isolation,
purification and characterization of chymosin from riverine buffalo (Bubalos bubalis). J. Dairy Res.
70, 37–43.
Newman, M., Safro, M., Frazao, C., Khan, G., Zdanov, A., Tickle, I.J., Blundell, T.L. and Andreeva, sN.
(1991) X-ray analyses of aspartic proteinases. IV. Structure and refinement at 2.2 Å resolution of
bovine chymosin. J. Mol. Biol. 221, 1295–1309.
Newman, M., Watson, F., Roychowdhury, P., Jones, H., Badasso, M., Cleasby, A., Wood, S.P.,
Tickle, I.J. and Blundell, T.L. (1993) X-ray analyses of aspartic proteinases. V. Structure and
refinement at 2.0 A resolution of the aspartic proteinase from Mucor pusillus. J. Mol. Biol. 230,
260–283.
ASPARTIC PROTEASES USED IN CHEESE MAKING 219
Park, Y.N., Aikawa, J., Nishiyama, M., Horinouchi, S. and Beppu, T. (1996) Involvement of a residue
at position 75 in the catalytic mechanism of a fungal aspartic proteinase, Rhizomucor pusillus pepsin.
Replacement of tyrosine 75 on the flap by asparagines enhances catalytic efficiency. Protein Eng. 9,
869–875.
Pedersen, V.B., Christensen, K.A. and Foltmann, B. (1979) Investigations on the activation of bovine
prochymosin. Eur. J. Biochem. 94, 573–80.
Pungercar, J., Strukelj, B., Gubensek, F., Turk, V. and Kregar, I. (1990) Complete primary structure of
lamb preprochymosin deduced from cDNA. Nucleic Acids Res. 18, 4602.
Ramalho-Santos, M., Pissarra, J.,Veríssimo, P., Pereira, S., Salema, R., Pires, E. and Faro, C.J. (1997)
Cardosin A, an abundant aspartic proteinase, accumulates in protein storage vacuoles in the stigmatic
papillae of Cynara cardunculus L. Planta. 203, 204–212.
Ramalho-Santos, M., Veríssimo, P., Cortes, L., Samyn, B. Van Beeumen, J., Pires, E. and Faro, C.
(1998) Identification and proteolytic processing of procardosin A. Eur. J. Biochem. 255, 133–138.
Rawlings, N.D., Tolle, D.P. and Barret, A.J. (2004) MEROPS: The peptidase database. Nucleic Acid
Res. 32 Database issue, D160-D164. http://www.merops.sanger.ac.uk/
Rickert, W.S. and McBride-Warren, P.A. (1974) Structural and functional determinants of Mucor miehei
protease. IV. Nitration and spectrophotometric titration of tyrosine residues. Biochim. Biophys. Acta.
371, 368–378.
Rogelj, I., Perko, B., Francky, A., Penca, V. and Pungercar, J. (2001) Recombinant lamb chymosin as
an alternative coagulating enzyme in cheese production. J. Dairy Sci. 84, 1020–1026.
Roserio, L.B., Barbosa, M., Ames, J.M. and Wilbey R.A. (2003) Cheesemaking with vegetable
coagulants-the use of Cynara L. for the production of ovine milk cheeses. Int. J. Dairy Tech. 56,
76–85.
Sidrach, L., García-Cánovas, F., Tudela, J. and Rodríguez-López, J. N. (2004) Purification of cynarases
from artichoke (Cynara scolymus L.): enzymatic properties of cynarase A. Phytochem. 66, 41–49.
Simöes, I. and Faro, C. (2004) Structure and function of plant aspartic proteinases. Eur. J. Biochem.
271, 2067–2075.
Soares Pais, M.S., Calixto, F.C. and Planta, R.J. inventors; Instituto de Ciencia Aplicada e Technologia,
assignee. (2000 December 14) Production by yeast of aspartic proteinases from plant origin.
International Patent WO 00/75283 A1.
Tang, J. (2004) Pepsin A. In Handbook of Proteolytic Enzymes. Barrett, A.J., Rawlings, N.D. and
Woessner, J.F. eds. Elsevier, London, pp. 19–28.
Tonouchi, N., Shoun, H., Uozumi, T. and Beppu, T. (1986) Cloning and sequencing of a gene for Mucor
rennin, an aspartate protease from Mucor pusillus. Nucleic Acids Res. 14, 7557–7568.
Vega-Hernández, M.C., Gómez-Coello, A., Villar, J. and Claverie-Martín, F. (2004) Molecular cloning
and expression in yeast of caprine prochymosin. J. Biotechnol. 114, 69–79
Vieira, M., Pissarr, J., Veríssimo, P., Castanheira, P., Costa, Y., Pires, E. and Faro, C. (2001) Molecular
cloning and characterization of cDNA encoding cardosin B, an aspartic proteinase accumulating
extracellularly in the transmitting tissue of Cynara cardunculus L. Plant Mol. Biol. 45, 529–539.
White, P.C., Cordeiro, M.C., Arnold, D., Brodelius, P. and Kay, J. (1999) Processing, activity, and
inhibition of recombinant cyprosin, an aspartic proteinase from cardoon (Cynara cardunculus). J.
Biol. Chem. 274, 16685–16693.
Yamashita, T., Higashi, S., Higashi, T., Machida, H., Iwasaki, S., Nishiyama, M. and Beppu, T. (1994)
Mutation of a fungal aspartic proteinase, Mucor pusillus rennin, to decrease thermostability for use
as a milk coagulant. J Biotechnol. 32, 17–28.
Yang, J. and Quail, J.W. (1999) Structure of the Rhizomucor miehei aspartic proteinase complexed with
the inhibitor pepstatin A at 2.7 Å resolution. Acta Crystallogr. D. Biol. Crystallogr. 55, 625–630.
Yang, J., Teplyakov, A. and Quail, J.W. (1997) Crystal structure of the aspartic proteinase from
Rhizomucor miehei at 2.15- Å resolution. J. Mol. Biol. 268, 449–459.
Yun, J.J., Kiely, L.J., Kindstedt, P.S. and Barbano, D.M. (1993) Mozzarella cheese: impact of coagulant
type on functional properties. J. Dairy Sci. 76, 3657–3663.
CHAPTER 14
METALLOPROTEASES
JOHANNA MANSFELD
∗
Department of Biochemistry, Institute of Biotechnology, Martin-Luther University Halle-Wittenberg,
Halle, Germany
∗
mansfeld@biochemtech.uni-halle.de
1. INTRODUCTION: CLASSIFICATION
OF METALLOPROTEASES
Metalloproteases (metallopeptidases or metalloproteinases) represent an extensive
class of hydrolases which cleave peptide bonds by the action of a water molecule
which is activated by complexing to bivalent metal ions. Most metalloproteases
are characterised by a catalytic zinc ion. However, in some enzymes this function
is undertaken by manganese, cobalt, nickel or even copper ions. In some metallo-
proteases two metal ions act co-catalytically. The metal ion is complexed by three
conserved amino acid residues that can be His, Asp, Glu or Lys.
According to the classification of proteases based on protein structure and
homology implemented in the MEROPS database (http://merops.sanger.ac.uk) (see
Rawlings et al., 2004; Barrett et al., 2004; Rawlings et al., Chapter 10, this volume),
metalloproteases are found in 14 different clans. In addition, clan M- contains
metalloprotease families not yet assigned to a clan. Proteases from clans MA, MC,
MD, ME, MJ, MK, MM, MO and MP require only one catalytic metal ion, in most
cases zinc ions, whereas clans MF, MG, MH, MN and MQ contain two metal ions
acting co-catalytically on the substrate (M stands for metalloprotease).
Clan MA, comprising subclans MA(E) (gluzincins) and MA(M) (metzincins), is
one of the most comprehensive clans and contains some of the most prominent
and industrially relevant members of metalloproteases which will be discussed
in detail in the following sections. All members of this clan are characterised
by a zinc ion at the active site and the highly conserved HEXXH motif which
is integrated into the central -helix. This motif is also found in proteins other
than metalloproteases. The two His residues of this motif are involved in zinc
binding, whereas Glu is considered to be the catalytically important amino acid
221
J. Polaina and A.P. MacCabe (eds.), Industrial Enzymes, 221–242.
© 2007 Springer.
222 MANSFELD
residue. Subclans MA(E) and MA(M) differ in the third zinc liganding amino
acid residue. In the case of the gluzincins this ligand is a Glu residue that is
18–72 amino acid residues distant from the HEXXH motif. The following families
belong to the gluzincins (a representative member of the family and its origin are
given in parentheses): M1 (aminopeptidase N, Homo sapiens), M2 (angiotensin-
converting enzyme peptidase unit 1, Homo sapiens), M3 (thimet oligopep-
tidase, Rattus norvegicus), M4 (thermolysin, Bacillus thermoproteolyticus), M5
(mycolysin, Streptomyces cacaoi), M9 (microbial collagenase, Vibrio alginolyticus),
M13 (neprilysin, Homo sapiens), M26 (IgA1-specific metalloendopeptidase, Strep-
tococcus sanguis), M27 (tentoxilysin, Clostridium tetani), M30 (hyicolysin,
Staphylococcus hyicus), M32 (carboxypeptidase Taq, Thermus aquaticus), M34
(anthrax lethal factor, Bacillus anthracis), M36 (fungalysin, Aspergillus fumigatus),
M41 (FtsH peptidase, Escherichia coli), M48 (Ste24 peptidase, Saccharomyces
cerevisiae), M56 (BlaR1 peptidase, Staphylococcus aureus), M60 (enhancin,
Lymantria dispar nucleopolyhedrovirus), M61 (glycyl aminopeptidase, Sphin-
gomonas capsulata) (Barrett et al., 2004). The metzincins contain either an Asp or a
His residue in an extended HEXXHXXGXXH/D motif as the third zinc ligand and in
addition are characterised by a conserved Met-turn (Stöcker et al., 1995) underlying
the active site. The conserved glycine in this motif is important for the formation
of the -turn that brings the three zinc ligands into the required position (Bode
et al., 1992). The catalytic mechanism of the metzincins is not as well known as
that of the gluzincins. Some metzincins such as meprin, astacin and serralysin
are able to hydrolyse peptide nitroanilides. The following families belong to the
MA(M) metzincin subclan: M6 (immune inhibitor A, Bacillus thuringiensis), M7
(snapalysin, Streptomyces lividans), M8 (leishmanolysin, Leishmania major ), M10
(matrix metallopeptidase-1, Homo sapiens), M11 (gametolysin, Chlamydomonas
reinhardtii), M12 (astacin, Astacus astacus), M35 (deuterolysin, Aspergillus flavus),
M43 (cytophagalysin, Cytophaga sp.), M57 (prtB gene product, Myxococcus
xanthus), M64 (IgA protease, Clostridium ramosum), M66 (StcE peptidase
Escherichia coli), and M72 (peptidyl-Asp metalloendopeptidase, Pseudomonas
aeruginosa).
Clan MC (family M14) contains a number of carboxypeptidases, e.g. the
important animal enzymes carboxypeptidases A, B, D and E, as well as carboxypep-
tidase T from actinomycetes having the conserved motif HXXE.
Clan MD contains families M15 and M74. Family M15 includes the zinc
D-Ala-D-Ala carboxypeptidase from Streptomyces albus which releases the
D-amino acid-containing cross-linking peptide (required for bacterial cell wall
biosynthesis) from its precursor, the VanX D-Ala-D-Ala dipeptidase and the
VanY D-Ala-D-Ala carboxypeptidase from Enterococcus (involved in vancomycin
resistance), and endolysins from bacteriophages A118 and A500. Family M74
(containing the murein endopeptidase (MepA) from Escherichia coli
) hydrolyses
the murein crosslinks in bacterial cell walls.
Members of clan ME (formed by families M16 which contains the mitochondrial-
processing peptidase MPP and M44, containing the vaccinia virus polyprotein
METALLOPROTEASES 223
processing endopeptidase, called G1L protein) are characterised by a conserved
HXXEH motif. MPPs have an active site similar to that of thermolysin and catalyse
the removal of N-terminal targeting signals from mitochondrial proteins synthesized
in the cytoplasm.
Clan MF, comprising family M17, only contains eukaryotic and bacterial leucyl
aminopeptidases both of which require two metal ions for catalytic activity.
A number of very dissimilar exopeptidases belong to clan MG (family M24).
Most of these enzymes require two cobalt or manganese ions. The most important
members of this family are the bacterial methionyl aminopeptidase and X-Pro
aminopeptidase, as well as the type I (mitochondrion) and type II (cytoplasmic)
eukaryotic methionyl aminopeptidases that cleave the initial methionine co-
translationally from newly synthesized proteins. These enzymes have been shown
to comprise a group of proteases occurring ubiquitously in all genomes.
Members of clan MH, which is further divided into families M20, M28 (mainly
carboxy- and aminopeptidases), M18 and M42 (mainly aminopeptidases), require
co-catalytic zinc ions.
Members of clan MJ (families M19 and M38) are dipeptidases, one of which
cleaves rather exotic substrates having isoaspartyl residues.
The only peptidase of clan MK (family M22) is the O-sialoglycoprotein endopep-
tidase. The other members of this clan are ubiquitously present in all genomes and
are characterised by a fold that seems to be similar to that of the non-metalloproteins
actin, Hsp70 and DnaK.
The most important feature of the M50 family members of clan MM is the
presence of an HEXXH motif like that of clan MA. These enzymes are bound
to membranes, contain one zinc ion and have been shown to be involved in the
regulation of gene expression by proteolytic processing of transcription regulators.
Members of clan MN (family M55), represented by D-aminopeptidase DppA,
contain co-catalytic zinc ions.
Members of clan MO (family M23), such as -lytic metallopeptidase from Achro-
mobacter lyticus, are endopeptidases that lyse bacterial cell wall peptidoglycans.
Clan MP, family M67 comprises isopeptidases (e.g. Poh1 peptidase from
Saccharomyces cerevisiae) that release ubiquitin from ubiquitinated proteins.
Clan MQ, family M29 includes aminopeptidases from thermophilic bacteria such
as aminopeptidase T from Thermus aquaticus and PepS aminopeptidase from Strep-
tococcus thermophilus.
Clan M- contains metallopeptidase families that have not yet been assigned
to a well-defined clan. It comprises families M49 (represented by dipeptidyl-
peptidase III from Rattus norvegicus which releases N-terminal dipeptides sequen-
tially from peptides like angiotensins II and III, Leu-enkephalin, prolactin and
alpha-melanocyte-stimulating hormone), M73 (camelysin, a cell-surface endopep-
tidase from Bacillus cereus) and M75 (imelysin or ‘insulin-cleaving membrane
protease’ from Pseudomonas aeruginosa).
224 MANSFELD
Thermolysin and thermolysin-like proteases, which are the only metalloproteases
that have achieved industrial application, will be discussed in detail in the following
sections of this chapter.
2. THERMOLYSIN - THE PROTOTYPE
OF METALLOPROTEASES
Thermolysin (EC 3.4.24.27; MEROPS classification: M04.001, family M4 of
subclan MA(E)) is an extracellular metalloendoproteinase produced by the gram-
positive bacterium Bacillus thermoproteolyticus (Endo, 1962). Its three-dimensional
structure has been resolved (Matthews et al., 1972). Family M4 contains several
other extracellular metalloproteases that are produced by various gram-positive
bacilli. Phylogenetic analysis (Barrett et al., 2004) reveals the occurrence of a group
of very closely related enzymes and the existence of others which are less related
both in sequence and properties. In the following, only thermolysin and some of
the most closely related members of family M4, the thermolysin-like proteases
(TLPs), are discussed. The enzymes from B. brevis, B. polymyxa, B. megaterium,
B. amyloliquefaciens, B. amylosacchariticus and Lactobacilli are less well charac-
terised and therefore not considered.
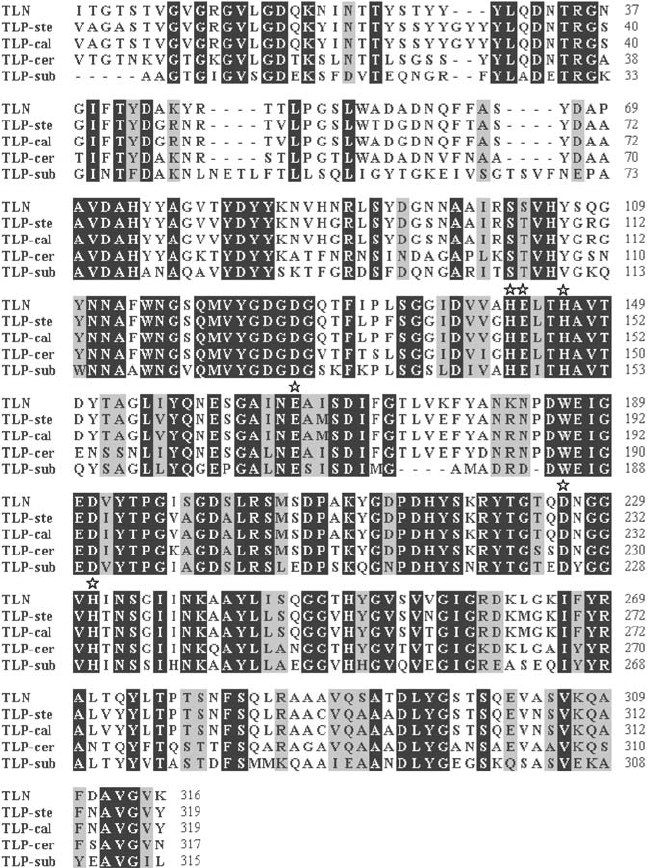
As the alignment in Fig. 1 shows, TLPs from B. stearothermophilus (SwissProt
P06874; Fujii et al., 1983) and B. caldolyticus (SwissProt P23384; van den Burg
et al., 1991) are very similar to thermolysin (SwissProt P00800) whereas the
enzymes from B. subtilis (SwissProt P39899; Tran et al., 1991) and B. cereus
(UniProt P05806; Wetmore et al., 1992) are more distantly related.
3. MOLECULAR STRUCTURE AND FUNCTION
In addition to thermolysin, the crystal structures of other members of clan MA
have been resolved (e.g. TLP from B. cereus - Stark et al., 1992, and pseudolysin,
the elastase from Pseudomonas aeruginosa - Thayer et al., 1991). Based on these
structures, a homology model of the enzyme from B. stearothermophilus has been
proposed (Vriend and Eijsink, 1993).
As detected later (Holland et al., 1992), the first thermolysin structure published
(Matthews et al., 1972) contained a dipeptide at the active site. Since then the
structure has been refined several times and has now also been resolved both in
the presence of inhibitors (Matthews, 1988; Hausrath and Matthews, 2002) and in
free form (Hausrath and Matthews, 2002). The mature enzyme consisting of 316
amino acid residues comprises two domains – the N-terminal domain which mainly
consists of -sheets and minor -helical parts, and the C-terminal domain which
is predominantly -helical in character. The active site cleft with the catalytically
essential zinc ion is located between the two domains, and the HEXXH motif
(amino acid residues 142 - 146) is integrated into the central -helix (Fig. 2). The
spacing of the zinc ligands follows a short-long pattern as in all members of this
clan, i.e. the first two ligands are arranged close together (H142 and H146) and the
METALLOPROTEASES 225
Figure 1. Alignment of the amino acid sequences of selected thermolysin-like proteases. The alignment
was created by CLUSTAL W (Thompson et al., 1994) in BioEdit 6.0.7. Stars indicate the amino acids
of the HEXXH motif and other residues assumed to be involved in catalysis. TLN – thermolysin,
TLP-ste – TLP from B. stearothermophilus, TLP-cal – TLP from B. caldolyticus, TLP-cer – TLP from
B. cereus, TLP-sub – TLP from B. subtilis. Identical amino acids are highlighted by white letters on a
black background; similar amino acids are shown in dark grey letters on a light grey background
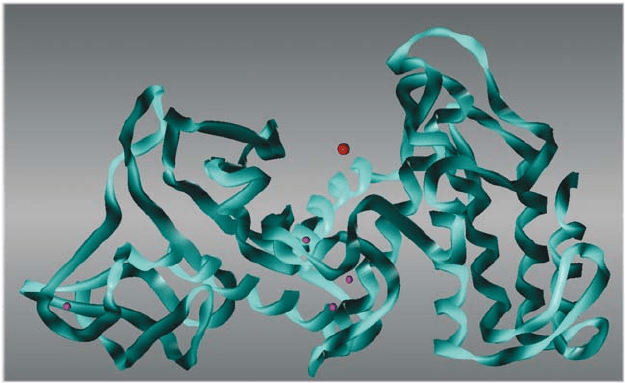
226 MANSFELD
Figure 2. Structure of thermolysin from B. thermoproteolyticus. The larger sphere in the centre repre-
sents the Zn
2+
ion in the active site. The four smaller spheres represent the Ca
2+
ions bound to the
molecule
third one (E166) is more distant in the C-terminal direction (Figs. 1 and 3). The
fourth ligand represents a water molecule. Removal of the tetrahedrally coordinated
zinc ion leads to an inactive enzyme (Holmquist and Vallee, 1974) the activity of
which towards furylacryloyl-glycyl-L-leucine amide (FAGLA) can be restored by
addition of stoichiometric amounts of Zn
2+
,Co
2+
and Mn
2+
ions. Excess of Zn
2+
ions inhibits the enzyme (Holmquist and Vallee, 1974). In the crystal structures of
metal-substituted thermolysin derivatives, conformational changes in the active site
cleft have been observed (Holland et al., 1995).
In addition to the catalytic zinc ion, thermolysin and the other more thermostable
TLPs possess four calcium ions which are principle determinants of the stability of
these enzymes. The less thermostable TLPs have only two calcium binding sites.
Calcium ions 1 and 2 are bound at a double binding site near the active site cleft,
whereas calcium ions 3 and 4 are bound to exposed loops in the N-terminal or
C-terminal domains, respectively (Matthews et al., 1972).
3.1. Catalytic Mechanism
Despite numerous crystallographic, kinetic, inhibition, quantum chemical and site-
directed mutagenesis studies, the mechanism of peptide hydrolysis by thermolysin
remains controversial. Nevertheless, the zinc ion plays the main role in all the
mechanistic proposals. The first mechanism to be proposed (Hangauer et al., 1984;
Matthews, 1988) was based on the polarisation of the zinc-bound water molecule
by the glutamate residue in the HEXXH motif (Glu143 in thermolysin, Fig. 3)
