Parinov I.A. Microstructure and Properties of High-Temperature Superconductors
Подождите немного. Документ загружается.


188 4 Carbon Problem
400
300
200
100
0
900 1200
Sintering Temperature (°C)
J
c
(A/cm
2
)
5000 ppm CO
2
500 ppm CO
2
50 ppm CO
2
100% O
2
1000980960940920
Fig. 4.5. The effect of sintering temperature on J
c
[958]
We do not know test evidence of secondary phases at grain boundaries in
samples sintered in a 100% O
2
atmosphere. At the same time, two distinct
types of grain boundaries can be observed in the samples sintered in 0.5%
CO
2
+99.5%O
2
[958]. Approximately 10% of the grain boundaries are found
to be wet by a thin layer of a second phase, as shown in Fig. 4.6, and the
remaining boundaries are sharp grain boundaries and free from the presence
of secondary phases. The local change of YBCO stoichiometry leads to the
eutectic reaction, in accordance with the phase diagram, and formation of
BaCuO
2
and CuO at the grain boundaries. The decomposition of YBCO phase
at the grain boundaries weakens the path for the current, thereby decreasing
the overall J
c
value. The wetting of the boundaries with non-superconducting
phases decreases the effective contact area between the superconducting grains
and decreases J
c
. From Fig. 4.6, the width of the secondary phase region along
the grain boundaries is determined to be approximately 50 nm, which is much
larger than the coherence length in this material. Therefore, these secondary
phases block the passage of currents.
There are two potential sources of CO
2
, when YBCO are processed by
solid-state sintering, namely (i) the CO
2
, contained in the oxygen gas, used
during sintering and/or annealing and (ii) the CO
2
, derived from the decom-
position of BaCO
3
during the calcining step. Each source affects the quality of
the final product in different ways and can lead to a drop in the critical current
density. An additional potential source is the organic binders that are used
in the YBCO processing. The CO
2
, formed by the interaction of the organic
binders and the oxygen from the gas atmosphere, could have a deleterious
effect on the performance capabilities of the final product. By using thermo-
dynamics, it may by shown [958] that for pressure values, p
CO
2
< 1atm,a
sintering temperature must increase as p
CO
2
increases. Therefore, increasing
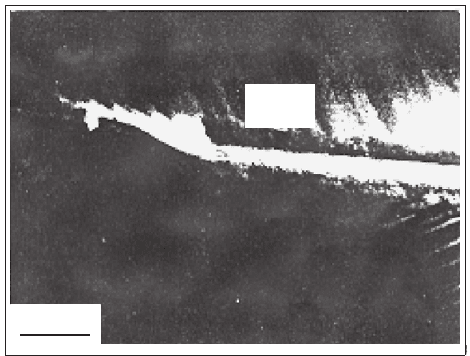
4.1 YBCO System 189
GB
200 nm
Fig. 4.6. The sample sintered at 970
◦
Cin0.5%CO
2
+99.5% O
2
. TEM micrograph
for the grain boundary (GB) which is wet by a layer of BaCuO
2
second phase [958]
the temperature results in a higher p
CO
2
. The sintering temperatures can be
lowered if p
CO
2
can be lowered, and one expedient means of doing this is to
remove the CO
2
contained in the O
2
gas.
Carbon also affects negatively the temperature and broadness of super-
conducting transition [1031]. It is known that YBCO samples, sintered to
closed porosity, exhibit broad and suppressed superconducting transitions
(Fig. 4.7). The observations suggested that during sintering of the closed-
porosity material, “impurities” that suppress the superconducting transition
become trapped in the center of the sample, while in the open-porosity sam-
ple and near the surface of the closed-porosity material, volatilization of the
impurity can occur during sintering.
The highest carbon content of ≈1.2 wt% is detected in the grains of the cen-
tral part from the closed-porosity material. Significantly lower carbon contents
are detected in specimens, cut from both the exterior of the closed-porosity
material and the central part of the open-porosity material (≈0.6 wt%). Car-
bon removal occurs more readily in the open-porosity material and near
the surface of the denser material, consistent with the suggestion that car-
bon removal is limited by the slow diffusion of carbon out of the YBCO
grains.
Under appropriate conditions, carbon has an appreciable solubility in
YBCO bulk. The depression of the transition temperature, detected by mag-
netic susceptibility measurements, correlates well with carbon content. This
correlation suggests that incorporation of carbon in the structure is the dom-
inant cause of the lowering of the transition temperature in the high-dense
superconductors.

190 4 Carbon Problem
Open-Porosity Material
Whole Pellet
Half Pellet
Temperature(K)
0
−1
Normalized ac-Susceptibility
(a)
1009080706050
Normalized ac-Susceptibility
Closed-Porosity Material
Whole Pellet
Half Pellet
0
–1
(b)
Re-oxygenated
half (600°C)
Temperature (K)
100
9080706050
Fig. 4.7. Change in ac-susceptibility with temperature for (a) an open-porosity
material (ρ = 87%) and (b) a closed-porosity material (ρ = 92%). In the open-
porosity material both whole and halved samples exhibit sharp transitions, whereas
in the closed-porosity material a stepped transition is seen in the halved sample even
after an additional oxygenation treatment [1031]
4.1 YBCO System 191
In the interior of the closed-porosity material, CO
2
or CO gas may be
trapped in the pores. As a result, a pressure of CO
2
or CO gas (in the range
0.01–0.1atm) can develop in the pores that is enough to slow down sintering
processes. Then, several steps may be taken to lower the amount of carbon,
trapped in a dense material, namely:
(1) The external partial pressure of CO
2
should be as low as possible in order
to favor decomposition of carbonate in the material.
(2) If the densification process can be delayed (to allow a longer period, in
which the sample contains interconnected porosity), more decomposition
and gas evolution may occur before the gas becomes trapped.
(3) A successful approach to carbon removal is to heat more slowly to the
sintering temperature.
Carbon in the crystal structure of YBCO interacts with Ba and O, reducing
the Ba–O coordination [1031]. The carbon connected with Ba ion could be
incorporated in the structure either substitutionally or interstitially. In the
first case, a direct substitution of oxygen by carbon ions is possible (it is
not supported in tests). An alternative possibility is that the carbon lies in
an interstitial site adjacent to the barium ion. In the absence of a detailed
theoretical understanding of the origin of the superconducting transition in
YBCO, the origin of the depression of T
c
, produced by the incorporation of
carbon, may be speculated. It is known that there is a strong correlation
between T
c
and the concentration of holes in the material [966]. The presence
of the carbon causes localization of some of the holes and thus reduces the
mobile holes’ concentration in the sample. This suggestion is consistent with
the location of the carbon in an interstitial site adjacent to the CuO
2
planes.
Because the carbon concentration can approach one atom per unit cell, the
location of the carbon in such a site could be expected to cause appreciable
local distortions of the CuO
2
planes and thereby lower T
c
.
It may be shown [651] that carbon is released from the matrix at temper-
atures higher than that of the peritectic decomposition. Nevertheless, there
is no doubt that even in a melt-processed sample, depression of T
c
may still
occur. Consider proper technological procedures, leading to decreasing of car-
bon content in the melt-processed YBCO samples by using as example the
results of [1127]. The melt-texturing was performed by a melt-texture growth
(MTG) process, using a temperature gradient furnace (temperature gradient
about 25 K/cm). As a result, two samples were synthesized, namely, (i) the
sample Sr5a, containing large grains (100 μm) with a very dense, closed poros-
ity structure, and (ii) the sample Sr5b, containing small grains (< 10 μm) with
open porosity. Their superconducting transition curves are shown in Fig. 4.8.
The T
c,onset
of sample Sr5a is depressed to 80 K, with a broad transition.
Sample Sr5b has T
c,onset
at 92 K, which is the optimum value for the Y-123
system. The analysis shows rather a high degree of carbon retention in the
two samples: 2600 ppm for sample Sr5a and 1200 ppm for sample Sr5b. Thus,

192 4 Carbon Problem
20 40 60 100
Temperature (K)
Magnetization,
M (emu/g
.
Oe)
0.005
0.000
–0.005
–0.010
–0.015
–0.020
−0.025
Sr5a
Sr5b
080
Fig. 4.8. The temperature dependence of magnetization of Y-123 ceramics sintered
at different temperatures: Sr5a (sintered at 950
◦
Cinoxygenflow)andSr5b(sintered
at 920
◦
C in oxygen flow). Average particle size of precursor (2.5 μm) is the same for
both ceramics [1127]
almost all carbon of the initial powder (3000 ppm) is retained in sample Sr5a,
and its low T
c,onset
is in accordance with the correlation stated above.
In sample Sr5a, three experimental features can explain the carbon content
in this sample as follows:
(1) The obtained lattice parameters (a =3.8288
˚
A,b =3.889
˚
Aandc =
11.667
˚
A) showed that the decrease of the c parameter, which was sensi-
tive to the carbon content, was considerably less than that observed for
samples prepared under a CO
2
atmosphere (c =11.585
˚
A) [76].
(2) The characteristic features of the YBCO carbonated phases is the exis-
tence of oriented domains, where the c-axes are perpendicular; the for-
mation of these domains is correlated not only to the decrease of the a/b
and a/c ratios, but also to the presence of carbonate groups, which are
preferentially located at the level of the domain boundaries.
(3) In carbon-rich materials, the existence of distributions of local superstruc-
tures, leading to the formation of streaks along a-direction and to short-
range aligned areas, is clearly visible on the images.
The above two sintered samples were further processed by MTG. The re-
sults presented in Fig. 4.9 show that this procedure allowed to further decrease
the carbon content. However, an excessive amount of carbon can still be re-
tained, if the carbon content of the initial precursor is too high, as shown for
sample Sr5amt. Therefore, the absolute necessity of controlling the quality of
the precursor material before melt texturing is obvious.
Then, in order to examine the influence of the 211 fine-disperse additives
and Pt doping on carbon retention, two sets of samples were prepared, one
without Pt addition (5avmt) and another with the addition of 0.5 wt% PtO
2
(5avpmt). Both sets had nominal composition Y
1.4
Ba
2.2
Cu
3.2
O
x
. Addition of

4.1 YBCO System 193
0
Temperature(K)
0.25
–0.31
–0.88
–1.44
–2.00
Sr5amt
Sr5bmt
Susceptibility(a.u.)
10080604020
Fig. 4.9. The dependence of magnetic susceptibility on temperature of melt-
processed samples. Sr5amt (or Sr5bmt) is an MTG sample, using Sr5a (or Sr5b)
as a pre-sintered precursor [1127]
Y-211 does not significantly decrease carbon retention in the Y-123. In con-
trast, as it follows from Fig. 4.10, for sample 5avpmt with added Pt, T
c,onset
is enhanced up to the optimum value of 92 K, and a remarkably sharp transi-
tion (ΔT
c
= 2 K) is obtained. Analysis shows that the carbon content in this
sample is as low as 200 ppm. At the same time, the sample 5avmt contains
100
Temperature (K)
0.3
−0.3
−0.9
−1.4
−2.0
5avmt
5avpmt
Susceptibility (a. u.)
0 20406080
Fig. 4.10. The temperature dependence of the susceptibility curve for the samples
processed by an MTG method with starting composition Y
1.4
Ba
2.2
Cu
3.2
O
x
,with
(5avpmt) and without (5avmt) addition of Pt [1127]

194 4 Carbon Problem
about 870 ppm of carbon. Although the exact mechanism by which the plat-
inum acts to decrease the carbon content is unknown, it may be suggested
that Pt acts as a catalyst for de-carbonation.
4.2 BSCCO Systems
The behavior of BSCCO samples due to carbon effect somewhat differs from
the behavior of YBCO superconductors. The dependencies of sintering tem-
perature and density of Bi-2223 precursor samples on carbon content are
shown in Fig. 4.11. Obviously, the higher sintering temperature and increas-
ing density of the Bi-2223 samples cause the lower carbon content.
740 750 760 770 780 790 800
400
300
200
100
Sintering Temperature (°C)
Carbon Content (ppm)
(a)
Densit
y
(
g
/cm
3
)
Carbon Content (ppm)
400
300
200
100
234
(b)
Fig. 4.11. The dependencies of (a) sintering temperature and (b) density of Bi-2223
precursor samples on carbon content [1164]

4.2 BSCCO Systems 195
Oxide superconducting powder can absorb atmospheric moisture and then
react with CO
2
, worsening superconducting properties. Therefore, a prepara-
tion process, proposed for Bi-2223 superconductor [928], in the first place
restricts absorption of moisture in order to suppress the deterioration of the
calcined powder. The preparation process includes the processes of dissolving,
coprecipitation, filtering, primary calcining (T = 800
◦
C), wet pulverizing to
obtain a fine powder, drying and secondary calcining (T = 800–815
◦
C). Then,
in order to fabricate bulk sample the calcined powder is molded by using a
coaxial pressing technique. The sample is sintered at 850
◦
C sometimes by us-
ing intermediate cold isostatic pressing (CIP). The results obtained for three
various samples are presented in Table 4.1. The intermediate pressing leads
to increasing of the sample density (Fig. 4.12a) and the corresponding rise of
critical current density (Fig. 4.12b). The saturation of the results occurs after
third intermediate pressing. The results obtained confirm that superconduct-
ing properties of the bulk superconductor are found by content of moisture
and carbon in the calcined powder. Carbon and admixture phases segregate
at the grain boundaries, forming weak links and decreasing J
c
.
The cause of the porosity formation and broken uniformity of Bi-2212/Ag
tapes may be as a result of the gas release, such as CO
2
, when Bi-2212 phase
reforms from the liquid phase [1196]. Carbon exists in the sample in the car-
bonate form and can transfer into CO
2
gas in proper condition during the
heat treatment, such as at high temperature by the reaction:
SrCO
3
→ SrO+CO
2
. (4.7)
CO
2
gas may be released and causes severe bubble formation and porosity,
depending on the carbon content. It may also dissolve into the liquid phase
and define its behavior. The carbon content effects on the melting behavior
of the Bi-2212 phase [1195] state that increasing the carbon content of the
powder can greatly decrease the melting and reformation temperatures of the
Bi-2212 phase in the OPIT tapes.
During cooling, when Bi-2212 phase re-forms from liquid, [C] will be re-
leased in accordance with the reaction:
[C] + O
2
→ CO
2
, (4.8)
where [C] is the carbon, which is dissolved in the liquid phase.
Table 4.1. Moisture content and carbon content of calcined powders [928]
Sample Process Moisture content
(wt %)
Carbon content
(wt %)
A After drying in air 1.01 0.610
2nd-calcined powder at 800
◦
C 0.13 0.041
B After drying in vacuum 0.65 0.486
2nd-calcined powder at 800
◦
C 0.11 0.028
C After drying in vacuum 0.64 0.482
2nd-calcined powder at 815
◦
C 0.12 0.025

196 4 Carbon Problem
12
10
8
6
4
2
0
C
B
A
(b)
J
c
(kA/cm
2
)
01234
CIP Number
5.5
5
4.5
4
01234
3.5
°
CIP Number
C
B
A
Density (g/cm
3
)
(a)
Fig. 4.12. Increase of (a) sample density and (b) J
c
(77 K, 0 T) due to intermediate
pressing (CIP) and sintering [928]
4.3 Carbon Embrittlement and Fracture of YBCO Superconductor 197
The volume change, caused by the formation of CO
2
gas from the solid-
state carbon, can be tremendous. A rough calculation indicates that 200 ppm
of carbon can cause about 36% porosity in the core, if all carbon form CO
2
at high temperature [1196].
The effect of the introduction of carbon into YBCO and Bi-2212 bulks,
obtained by using a partial melting (both samples were heated some above
the peritectic temperature), was studied in [962]. The presence of carbon has
strong effect on the YBCO melting, but is not essential for Bi-2212. In this
case, the exposed carbon excess can cause an increasing of the magnetic flux
pinning. At slow cooling of the YBCO and Bi-2212 from the temperature of
partial melting, it is necessary to strictly control temperature and gas atmo-
sphere in order to obtain high superconducting properties. Most important
factors are the oxygen gas release [143, 408, 742, 961] and the presence of
carbon and CO
2
in the sample volume [1101, 1195]. Thus, due to the re-
action of YBCO and BSCCO with CO
2
atmosphere, the carbon trapped in
the sample can render unfavorable effect on the sample density [1196], in-
tergranular boundaries [307, 958], critical currents [969], and critical current
density [1101, 1158]. Besides atmospheric sources, carbon may be introduced
due to carbonized precursors and organic binders during sample processing
[212] and also by solvents [304]. Finally, the carbon can be precipitated into
superconductor due to carbonized gases and liquids. Existing techniques can
form carbon particles with size of some nanometers, which can be the centers
of magnetic flux pinning into superconducting grains. Then, on one hand,
carbon can render useful effect, in particular, using Pb and some rare-earth
elements [742], and, on the other hand, it can deteriorate a structure of the
intergranular boundaries and structure-sensitive properties of HTSC.
4.3 Carbon Embrittlement
and Fracture of YBCO Superconductor
Carbonates, forming in result of the chemical reactions (4.3)–(4.6), are the
brittle phase, leading to embrittlement of superconductor. It may lead to
delayed carbonate cracking, a subcritical crack growth mechanism with the
next formation and fracture carbonate. The carbon-induced embrittlement is
a complicated mechanism, which results from the simultaneous operation of
several coupled processes, namely (i) carbon diffusion, (ii) carbonate precipi-
tation, (iii) non-mechanical energy flow and (iv) material deformation. In this
case, carbon diffusion occurs due to gradients of chemical potential and tem-
perature [189, 974]. Similar to hydride formation in metals [69, 620, 839, 872],
it may be proposed that the carbon chemical potential depends on stress,
and therefore the carbon diffusion is coupled with material deformationand
non-mechanical energy flow. The final carbon dissolution into material de-
pends on the thermal stresses due to carbonate expansion at precipitation
defining coupling between carbonate precipitation, material deformation and
