Okafor N. Modern Industrial Microbiology and Biotechnology
Подождите немного. Документ загружается.


# Modern Industrial Microbiology and Biotechnology
those exhibited in the small study populations of phase I and II studies, and identify the
best way of administering and using the drug for the purpose intended. If the drug is
approved, this information forms the basis for deciding the content of the product label.
Phase III studies can involve several hundred to several thousand subjects (1,000 – 3,000).
28.4.2.3 Process development for manufacturing and
quality control
The firm’s manufacturing capability is assessed.
Engineering and manufacturing design activities to establish a company’s capacity to
produce a product in large volume and development of procedures to ensure chemical
stability, batch-to-batch uniformity, and overall product quality.
28.4.2.4 Bioavailability studies
The use of healthy volunteers to document the rate of absorption and excretion from the
body of a compound’s active ingredients.
Companies conduct bioavailability studies both at the beginning of human testing
and just prior to marketing to show that the formulation used to demonstrate safety and
efficacy in clinical trials is equivalent to the product that will be distributed for sale.
Companies also conduct bioavailability studies on marketed products whenever they
change the method used to administer the drug (e.g., from injection or oral dose form), the
composition of the drug, the concentration of the active ingredient, or the manufacturing
process used to produce the drug.
28.4.2.5 Regulatory review: New drug application (NDA)
The firm puts in an application for a new drug, New Drug Application (NDA)
An NDA is an application to the FDA for approval to market a new drug. All
information about the drug gathered during the drug discovery and development
process is assembled in the NDA Following the completion of all three phases of clinical
trials, the company analyzes all of the data and files an NDA with FDA if the data
successfully demonstrate safety and effectiveness. The NDA must contain all of the
scientific information that the company has gathered. NDAs typically run 100,000 pages
or more. By law, FDA is allowed six months to review an NDA. In almost all cases, the
period between the first submission of an NDA and final FDA approval exceeds that
limit; the average NDA review time for new molecular entities approved in 1992 was 29.9
months.
28.4.3 Approval
Once FDA approves the NDA, the new medicine becomes available for physicians to
prescribe. The company must continue to submit periodic reports to FDA, including any
cases of adverse reactions and appropriate quality-control records. For some medicines,
FDA requires additional studies (Phase IV) to evaluate long-term effects.

Drug Discovery in Microbial Metabolites #
28.4.4 Post Approval Research
Experimental studies and surveillance activities undertaken after a drug is approved for
marketing.
Clinical trials conducted after a drug is marketed (referred to as phase IV studies in the
United States) are an important source of information on as yet undetected adverse
outcomes, especially in populations that may not have been involved the premarketing
trials (e.g., children, the elderly, pregnant women) and the drug’s long-term morbidity
and mortality profile. Regulatory authorities can require companies to conduct Phase IV
studies as a condition of market approval. Companies often conduct post-marketing
studies even in the absence of a necessity to do so.
SUGGESTED READINGS
Allsop, A., Illingworth, R. 2002. The impact of genomics and related technologies on the search
for new antibiotics. Journal of Applied Microbiology, 92, 7-12.
Anon, 1993. Congress of the United States, Office of Technology Assessment. Pharmaceutical
R&D: Costs, Risks and Rewards: 1993; Washington, DC, USA. pp. 4-5.
Anon, 1999. From Test Tube to Patient: Improving Health Through Human Drugs Special
Report, Center Drug Evaluation and Research. Food and Drug Administration. Rockville,
MD, USA.
Austin, C. 2004. The Impact of the Completed Human Genome Sequence on the Development of
Novel Therapeutics for Human Disease. Annual Review of Medicine, 55, 1–13.
Bansal, A.K. 2005. Bioinformatics in the microbial biotechnology – a mini review Microbial Cell
Factories, 4, 4–19.
Beamer, L. 2002. Human BPI: One protein’s journey from laboratory to clinical trials. ASM News.
68, 543-548.
Behal, V. 2000. Bioactive Products from Streptomyces. Advances in Applied Microbiology. 47, 113–
156.
Bull, A.T., Ward, A.C., Goodfellow, M. 2000. Search and Discovery Strategies For Biotechnology:.
The Paradigm Shift. Microbiology and Molecular Biology Reviews, 64, 573-548.
Dale, E., Wierenga, D.E., Eaton, C.R. 2001. Processes of Product Develpoment. http://
www.allpcom/drug-dev.htm. Accessed on September 28, 2005 at 12.05 pm GMT.
Debouck, C., Metcalf, B. 2000. The Impact of Genomics on Drug Discovery. Annual Review of
Pharmacology and Toxicology, 40, 193–208.
Erlanson, D.A., Wells, J.A., Braisted, A.C. 2004. Tethering: Fragment-Based Drug Discovery.
Annual Reviews of Biophysical and Biomolecular Structure, 33, 199–223.
Fan, F., McDevitt, D. 2002. Microbial Genomics for Antibiotic Target Discovery. In : Methods in
Microbiology. Vol 33, Academic Press. Amsterdam the Netherlands, pp. 272–288.
Feling, R.H., Buchanan, G.O., Mincer, T.J., Kauffman, C.A., Jensen, P.R., Fenical, W. 2003.
Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a
marine bacterium of the new genus Salinospora. Angewandte Chemie International Edition 42,
355-357.
Manyak, D.M., Carlson, P.S. 1999. Combinatorial Genomics
TM
: New tools to access microbial
chemical diversity In : Microbial Biosystems: New Frontiers. C.R., Bell, M. Brylinsky, P.
Johnson-Green, (eds). Proceedings of the 8th International Symposium on Microbial Ecology
Atlantic Canada Society for Microbial Ecology, Halifax, Canada, 1999.
Modern Industrial
Microbiology and Biotechnology
Waste Disposal
Section 0
Modern Industrial
Microbiology and Biotechnology

Wastes, unwanted materials, result inevitably from industrial activities in the same way
as they also do in domestic ones. If allowed to accumulate on the ground, or if dumped
indiscriminately into rivers and other bodies of water, unacceptable environmental
problems would result. Governments the world over usually institute legislation which
regulates the handling of wastes, including those resulting from industry. In the US the
Environmental Protection Agency (EPA) is the regulating agency. The EPA works to
develop and enforce regulations that implement environmental laws enacted by
Congress. EPA is responsible for researching and setting national standards for a variety
of environmental programs, and delegates to states the responsibility for issuing permits
and for monitoring and enforcing compliance.
The activities of industrial microorganisms usually occur in large volumes of water;
the resulting wastes are therefore transported in aqueous medium. This chapter will
examine briefly the treatment of waste water. The subject is of interest, not only from the
intrinsic need to dispose of wastes in industry, but especially because the basis for
ultimate waste disposal is microbial.
Waste carried in water, whether from industry or from domestic activity is known as
sewage. Waste water disposal constitutes a peculiar branch of industrial microbiology.
The methods to be discussed were evolved originally to handle domestic sewage, but they
have been extended for use in those industries, such as the food and fermentation indus-
tries, which yield wastes degradable by microorganisms. Sewage emanating from some
chemical industries especially those dealing with manmade chemicals are not only less
degradable but are sometimes toxic to microorganisms and man. The processes of bio-
logical waste-water treatment to be discussed here are really an aspect of industrial
microbiology within the definition of the subject adopted in this book, because they in-
volve micro-organisms on a large scale, although there is no direct expectation of profit.
29.1 METHODS FOR THE DETERMINATION OF ORGANIC
MATTER CONTENT IN WASTE WATERS
Waste waters are sampled and analyzed in order to determine the efficiency of the treat-
ment system in use. This is particularly important at the point of the discharge of the
Treatment of
Wastes in Industry
29
+0)26-4

#$ Modern Industrial Microbiology and Biotechnology
treated waste water into rivers, streams and other natural bodies of water. If waste water
discharged into a natural water is rich in degradable organic matter, large numbers of
aerobic microorganisms will develop to break down the organic matter. They will use up
the available oxygen and as a consequence fish and other aquatic life will die. Further-
more, anaerobic bacteria will develop following the exhaustion of oxygen; the activities of
the latter will result in foul odors. Some of the methods for analyzing the organic matter
content of waste waters are given below.
29.1.1 Dissolved Oxygen
Dissolved oxygen is one of the most important, though indirect, means of determining the
organic matter content of waters. The heavier the amount of degradable material present
in water, the greater the growth of aerobic organisms and hence the less the oxygen
content. The Winkler method is widely used for determining the oxygen in water. In this
method, dissolved oxygen reacts with manganous oxide to form manganic oxide. On
acidification in an iodide solution, iodine is released in an amount equivalent to the
oxygen reacting to form the manganic oxide. The iodine may then be titrated using
thiosulphate. Membrane electrodes are now available for the same purpose. In these
electrodes oxygen diffuses through the electrode and reacts with a metal to produce a
current proportional to the amount of oxygen reacting with the metal.
29.1.2 The Biological or Biochemical Oxygen
Demand (BOD) Tests
Due to the complexity of the organic materials introduced into water and the key role
played by oxygen in supporting the aerobic bacteria which break down this organic
matter, the method of the Biochemical Oxygen Demand (BOD) was developed. It is a
measure of the oxygen required to stabilize or decompose the organic matter in a body of
water over a five-day period at 20°C. In carrying out the test, two 250-300 ml bottles are
filled with water whose BOD is to be determined. The oxygen content of one is determined
immediately by the Winkler method and in the other at the end of five days incubation at
20°C. The difference between the two is the BOD.
Although it has been severely criticized, the BOD test is still widely used. Some of the
criticisms are that it takes too long to obtain results and that it may infact relate only
loosely to the actual organic matter content of water since it represents the overall value of
the respiration of the organisms present therein. Furthermore, many industrial wastes
contain materials which are either difficult to degrade or which may even be toxic to the
organisms. In such cases an inoculum capable of degrading the materials must be
developed by enrichment and introduced into the bottles.
29.1.3 Permanganate Value (PV) Test
This PV method determines the amount of oxygen used up by a sample in four hours from
a solution of potassium permanganate in dilute H
2
SO
4
in a stoppered bottle at 27°C. It
gives an idea of the oxidizable materials present in water, although the actual oxidation
is only 30-50% of the theoretical value. The method records the oxidation of organic

Treatment of Wastes in Industry #%
materials such as phenol and aniline as well as those of sulfide, thiosulfate, and
thiocyanate and would be useful in some industries. However because oxidation is
incomplete it is not favored by some workers.
29.1.4 Chemical Oxygen Demand (COD)
The chemical oxygen demand is the total oxygen consumed by the chemical oxidation of
that portion of organic materials in water which can be oxidized by a strong chemical
oxidant. The oxidant used is a mixture of potassium dichromate and sulfuric acid and is
refluxed with the sample of water being studied. The excess dichromate is titrated with
ferrous ammonium sulfate. The amount of oxidizable material measured in oxygen
equivalent is proportional to the dichromate used up. It is a more rapid test than BOD and
since the oxidizing agents are stronger than those used in the PV test, the method can be
used for a wider variety of wastes. Furthermore, when materials toxic to bacteria are
present it is perhaps the best method available. Its major disadvantage is that bulky
equipment and hot concentrated sulfuric acid are used.
29.1.5 Total Organic Carbon (TOC)
Total organic carbon provides a speedy and convenient way of determining the degree of
organic contamination. A carbon analyzer using an infrared detection system is used to
measure total organic carbon. Organic carbon is oxidized to carbon dioxide.
The CO
2
produced is carried by a ‘carrier gas’ into an infrared analyzer that measures
the absorption wavelength of CO
2
. The instrument utilizes a microprocessor that will
calculate the concentration of carbon based on the absorption of light in the CO
2
. The
amount of carbon will be expressed in mg/L. TOC provides a more direct expression of
the organic chemical content of water than BOD or COD.
29.1.6 Total Suspended Solids (TSS)
The term ‘total solids’ refers to matter suspended or dissolved in water or wastewater,
and is related to both specific conductance and turbidity. Total solids (also referred to as
total residue) is the term used for material left in a container after evaporation and drying
of a water sample. Total Solids include both total suspended solids, the portion of total
solids retained by a filter and total dissolved solids, the portion that passes through a
filter. Total solids can be measured by evaporating a water sample in a weighed dish, and
then drying the residue in an oven at 103 to 105°C. The increase in weight of the dish
represents the total solids. Instead of total solids, laboratories often measure total
suspended solids and/or total dissolved solids. To measure total suspended solids
(TSS), the water sample is filtered through a preweighed filter. The residue retained on the
filter is dried in an oven at 103 to 105°C until the weight of the filter no longer changes.
The increase in weight of the filter represents the total suspended solids. TSS can also be
measured by analyzing for total solids and subtracting total dissolved solids.
29.1.7 Volatile Suspended Solids (VSS)
Volatile suspended solids (VSS) are those solids (mg/liter) which can be oxidized to gas
at 550°C. Most organic compounds are oxidized to CO
2
and
H
2
O at that temperature;
inorganic compounds remain as ash.
#& Modern Industrial Microbiology and Biotechnology
29.2 WASTES FROM MAJOR INDUSTRIES
The composition of industrial wastes depend on the industry. Wastes from three key
industries in the US are given in Table 29.1 for illustration: the oil, the pulp and paper and
the food industries.
Table 29.1 Typical wastes from three industries
A
Typical Components of an Oil Refinery Waste Water
Handling of oil crude Oil, sludge oil emulsions, sulfur- and nitrogen
corrosion inhibitors
Crude oil distillation Hydrocarbons, organic and inorganic acids,
phenols and sulfur
Thermal cracking Phenols, triphenols, cyanides, hydrogen sulfide
Alkylation, polymerization, cid sludge, spent acid, mineral acids (sulfuric,
isomerization processes hydrochloric), catalyst support
Refining Hydrogen sulfide, ammonium sulfide, gums.
catalyst support
Purification and extraction Phenols, glycols, amines, spent caustic
Sweetening, stripping, fltration Sulfur and nitrogen compounds, copper chloride,
suspended matter
B
Typical Effluent Loads from Pulp and Paper Manufacture
Effluent Kg/1,000 kg of product
Suspended solids 5-day BOD
Pulps: unbleached sulfite 10 - 20 200 - 300
Pulps: bleached sulfite 12 - 30 220 - 400
Fine paper 25 - 30 7 -20
Tissue paper 15 – 20 10 - 15
C
Typical Effluent Loads from Food industries
Effluent Kg/1,000 kg of product
Suspended solids 5-day BOD
Cannery wastes
Apple canning 300 - 600 1680 - 5530
Cherries canning 200 - 600 700 - 2100
Mushrooms 50 - 240 76 - 850
Meat Packing Industry
Slaughter house 3000 - 930 2200 - 650
Parking house 2000 - 230 3000 - 400
Processing plant 800 - 200 800 - 200
Poultry
Plant waste 100 - 1500 150 - 2400
Treatment of Wastes in Industry #'
29.3 SYSTEMS FOR THE TREATMENT OF WASTES
The basic microbiological phenomenon in the treatment of wastes in aqueous
environments is as follows:
(i) The degradable organic compounds in the waste water (carbohydrates, proteins,
fats, etc.) are broken down by aerobic micro-organisms mainly bacteria and to some
extent, fungi. The result is an effluent with a drastically reduced organic matter
content.
(ii) The materials difficult to digest form a sludge which must be removed from time to
time and which is also treated separately.
The discussion will therefore be under two headings: aerobic breakdown of raw
waste-water and anaerobic breakdown of sludge.
29.3.1 Aerobic Breakdown of Raw Waste Waters
The two methods which are usually employed include the activated sludge and the
trickling filter.
29.3.1.1 The activated sludge system
The activated sludge method is the most widely used method for treating waste waters.
Its main features are as follows:
(a) It uses a complex population of microorganisms of bacteria and protozoa;
(b) This community of microorganisms has to cope with an uncontrollably diverse
range of organic and inorganic compounds some of which may be toxic to the
organisms.
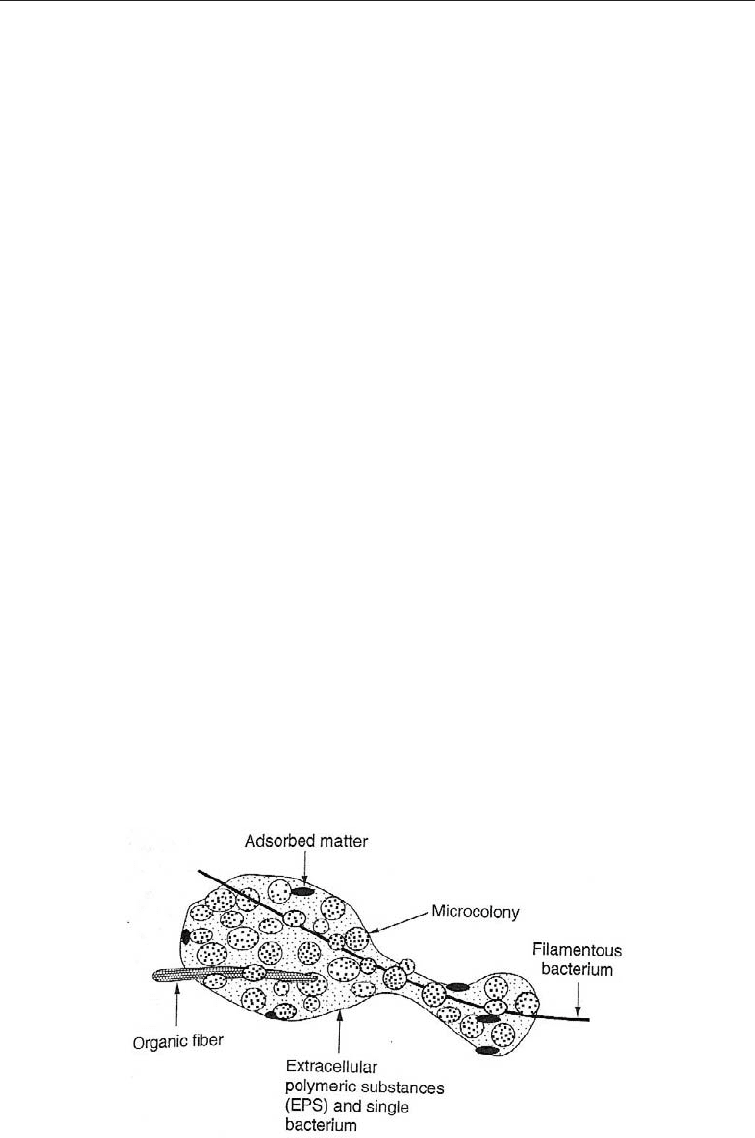
(c) The microorganisms occur in discreet aggregates known as flocs which are
maintained in suspension in the aeration tank by mechanical agitation or during
aeration or by the mixing action of bubbles from submerged aeration systems. Flocs
consist of bacterial cells, extracellular polymeric substances, adsorbed organic
matter, and inorganic matter. Flocs are highly variable in morphology, typically 40
to 400 mm and not easy to break apart (Fig 29.1).
Fig. 29.1 Diagram of a Floc
