Okafor N. Modern Industrial Microbiology and Biotechnology
Подождите немного. Документ загружается.


470 Modern Industrial Microbiology and Biotechnology
A major transformation in which interest has grown sharply in recent times is the
cleavage of the C
17
side chain of sterols. An important source of steroids for the synthesis
and production of pharmacologically active steroids used in contraceptives,
corticosteroids, geriatic drugs etc. is diosgenin (Fig. 26.1) from Dioscorea spp. Due to the
shortage of diosgenin, interest has shifted to more abundant sterols from phytosterols (i.e.
sterols from plants) and cholesterols from animals. The phytosterols include soy bean
sterols mainly >-sitosterol and stigmasterol and tall oil sterols mainly sitosterol and
campesterol. For these to be used as starting materials for the production of progesterone
and other drugs, the C
17
side chain must be cleaved hence the interest. The microbial
removal of the side chain offers more promise than chemical means. Unfortunately micro-
organisms which cleave off the side-chain will also attack the D ring to which the chain
is attached. Three methods have therefore been evolved to solve the problem of inhibiting
ring degradation, while cleaving the chain.
(i) The substrate may be modified structurally by chemical means so that the ring is
stable while the side-chain is cleaved. Thus while cholesterol rings are degraded
when the side-chain is cleaved by Nocardia sp. 3-Acetoxy-9- hydroxy-5-cholestene
is not. This later compound can be prepared from cholesterol by three chemical
steps. The cleavage of the side-chain of cholesterol yields esterone which can then
be used for further transformations.
(ii) The enzymes which open the D nucleus may be selectively inhibited. The key stage
in the opening of the ring is at the ninth position and since the enzyme for this
hydroxylation contains metals, the enzyme and its process may be inhibiting by
using chelating agents which remove metals from them.
(iii) Finally, mutants have been developed which will degrade only the side chain. One
of the best known is a mutant of Mycobacterium sp.
26.3.2 Fermentation Conditions Used in Steroid Transformation
The media used are highly variable, but in the main are not very complex. They are
basically mineral salts media containing some carbon source such as glucose, dextrin or
glycerol. Nitrogen sources may be ammonium salts, corn steep liquor, soybean, or a
protein digest. In some cases yeast extract is added.
Steroid and sterols are lipids; they are not water soluble and therefore must be
dissolved in a water-miscible lipid-solvent. Acetone, ethanol, propylene glycol, and
methanol are suitable because they dissolve a reasonable amount of the steroid while
being relatively non-inhibitory to the enzymes; dimethyl formamide dissolves a
reasonable amount of the steroids but has only a minimum of toxicity. Sometimes the
steroid is added in small amounts at a time. In this way, any toxic effect of the solvent is
minimized.
The level of steroid added is variable and depends both on the transforming ability of
the organisms as well as its susceptibility to the toxic effects of the steroid. Normally 200-
800 mg/litre are added but much higher amounts are sometimes used. To solve the
problem of the insolubility of steroids in water, non-ionic surface-acting agents which
reduce surface tension e.g. Tween 80 are often added to the medium. Some poly-
saccharides in the medium e.g. yeast cell wall mannan, bind to the steroids and cause
them to be more available to the organism.

Microbial Transformation of Steroids and Sterols 471
A wide range of microorganisms, mainly fungi and bacteria, are used in the
transformation of steroids. Some of these include the fungi Rhizopus nigricans, Curvularia
lunata, Fusarium spp. Cylindrocarpon radicicola as well as the bacteria Mycobacterium spp.,
Corynedbacterium simplex, and Streptomyces spp. As has been mentioned, there are
organisms to perform just about any conceivable transformation of the steroid molecule.
The transformation may occur at different stages of the growth and the steroid may be
added to the growing cultures either simultaneously with the inoculation of the culture
or the resting or stationary stage of the organism. Fungal spores may sometimes be
inoculated as the steroid is introduced into the medium. In recent times immobilized cells
have been employed in the transformations of steroids.
Steroid transformations require vigorous aeration and a temperature of about 28°C is
usually employed. The fermentation is usually complete in four to five days.
26.4 SCREENING FOR MICROORGANISMS
The screening for microorganisms capable of transforming steroids to yield products of
useful pharmacological properties is a continuing one. The processes which are followed
in the screening are as follows:
(i) The microorganism is isolated from soil or some suitable source and grown in a
suitable medium for 24-28 hours.
(ii) The steroid in a suitable carrier is added to the fermentation and the growth
continues for a further period which could be as long as one week.
(iii) The transformation products are extracted with solvents such as methyl acetate
and purified by chromatography etc.
(iv) The product is tested for pharmacological properties.
(v) Finally, the structure is elucidated by classical methods of organic chemistry.
SUGGESTED READINGS
Flickinger, Michael C., Drew and Stephen W. 1999. Encyclopedia of Bioprocess Technology -
Fermentation, Biocatalysis, and Bioseparation Wiley. Electronic ISBN: 1-59124-457-9.
Martin, C.K.A. 1984. Sterols. In: Biotechnology. Kiesich (ed) Vol 6A Biotransformations Verlag
Chemie. Weinheim: Germany. pp. 79–96.
Morgan, B.P., Moynihan, M.S. 1997. Steroids. Kirk-Othmer Encyclopedia of Chemical
Technology, 2, 71-113.
Smith, L.L. 1984. Steroids. In: Biotechnology. Kiesich (ed) Vol 6A Biotransformations Verlag
Chemie. Weinheim Germany: pp. 31-78.

"% Modern Industrial Microbiology and Biotechnology
27.1 NATURE AND IMPORTANCE OF VACCINES
Vaccines are materials which when introduced into the human body help protect the
vaccinated person against specified communicable diseases. Communicable diseases
are diseases caused by microorganisms, including viruses. Vaccines are preparations of
dead or weakened pathogens, or their products, that when introduced into the body,
stimulate the production of protective antibodies or T cells without causing the disease.
Vaccination is also called active immunization because the immune system of the
body is stimulated to actively develop its own immunity against the pathogen. Passive
immunity, in contrast, results from the injection of antibodies formed by another animal
(e.g., horse, human) which provide immediate, but temporary, protection for the recipient.
The name ‘vaccine’ comes from the Latin vacca (for cow). This is because the earliest
vaccination was done using the cow pox virus (which causes the disease in cow) as a
vaccine against small pox in humans. The English physician, Edward Jenner carried out
the above vaccination in the late 18th century and published his paper in 1798.
Over the past 200 or so years vaccination has contributed greatly to reducing
morbidity and mortality from communicable diseases. The greatest triumph of
vaccination is the eradication of smallpox from the earth; no naturally-occurring cases
has been reported since 1977. A program to try to eliminate another virus disease,
poliomyelitis (polio for short), from the world has been on for some time and the
indications are that the number of cases has drastically dropped. Except for the few cases
caused by oral polio vaccine (OPV) (see below), in which the live virus reverts, the disease
has now been eliminated from the Western hemisphere. Outbreaks of polio still occur in
Africa, the Indian subcontinent, and parts of the Near East. Due to the success of
vaccination near 100% reduction has been obtained in the cases of many diseases which
were previously sources of great mortality and morbidity. These include diphtheria,
measles, mumps, pertusis, rubella and tetanus. Table 27.1 gives a list of the most
commonly used vaccines today.
27.2 BODY DEFENSES AGAINST
COMMUNICABLE DISEASES
In order to better understand the nature of vaccines and their design and production, it is
important that the defenses of the human body against communicable diseases be
Vaccines
27
+0)26-4

Vaccines "%!
Table 27.1 Vaccines most commonly used in the world
Disease Preparation Notes
Diphtheria Toxoid Often given to children in a
single preparation (DTP; the
Tetanus Toxoid ‘triple vaccine’) or the now-
preferred DTaP using acellular
pertussis
Pertussis Killed bacteria (‘P’) or their
purified components
(acellular pertussis = ‘aP’)
Inactivated virus previously Inactivated polio vaccine: IPV
grown on monkey or (Salk)
human diploid cells
Polio Attenuated virus Oral polio vaccine; OPV (Sabin)
inactivated virus Both vaccines trivalent (types 1,
previously grown on 2, and 3)
monkey or human
diploid cells
Hepatitis B Protein (HBsAg) from Made by genetic engineering
the surface of the virus
Diphtheria, tetanus, uses acellular pertussis and Pediarix®; combination
pertussis, polio, and IPV (Salk) vaccine given in 3 doses to
hepatitis B infants
Measles Attenuated virus
Mumps 1 Attenuated virus Often given as a mixture
2 Vaccine: Live in duck cells (MMR) Does not increase the
risk of autism. (Nor do any
vaccines containing thimerosal
as a preservative.)
Rubella Attenuated virus
Pig, chick embryo or
canine tissue-culture grown
Chickenpox Attenuated virus Caused by the varicella-zoster
(Varicella) virus (VZV)
Influenza Egg-grown virus, formalin Contains hemagglutinins
inactivated, highly purified from the type A and type B
by zonal ultracentrifugation viruses recently in circulation
Hemagglutinins
Pneumococcal Capsular polysaccharides A mixture of the capsular
infections polysaccharides of 23 common
types. Works poorly in infants.
7 capsular polysaccharides Mobilizes helper T cells; works
conjugated to protein well in infants.
Staphylococcal 2 capsular polysaccharides To prevent infection by Staph.
infections conjugated to protein aureus in patients hospitalized
and/or receiving dialysis
Contd.

"%" Modern Industrial Microbiology and Biotechnology
discussed briefly. Ordinarily the human body is surrounded by microorganisms: in the
air it breathes, the water it drinks, in the soil around it and on the clothes he wears. Most
of these are not normally pathogenic. But even the pathogenic ones do not always cause
disease when they come in contact with the human body because the body has evolved
ways of dealing with microorganisms and preventing them from causing disease,
collectively known as the immune system. The immune system is a complex network of
cells and organs which work together to protect the body from communicable diseases. It
has two components: the innate or non-specific immunity and the acquired or specific
methods. While the innate immunity eliminates the organism no matter the type,
acquired or specific immunity specifically recognizes and selectively eliminates the
microorganism or foreign molecule.
Meningococcal disease Polysaccharides Used chiefly to prevent
outbreaks among the military
Hemophilus influenzae, Capsular polysaccharide Prevents ear infections in
type b (Hib) conjugated to protein children
Hepatitis A Inactivated virus Available in single shot with
HBsAg (Twinrix®)
Rabies Inactivated virus Vaccine prepared from human
Active: diploid cell cultures (HDCV)
(1) b-propiolactone- has replaced the duck vaccine
inactivated virus grown in (DEV)
embryonated duck eggs
(2) phenol-inactivated virus
grown in rabbit brain
Passive: equine hyper-
immune serum
Smallpox Attenuated live virus: Despite the global eradication
attenuated by passing of smallpox, is used to protect
through calves against a possible bioterrorist
attack
Anthrax Extract of attenuated bacteria Primarily for veterinarians and
military personnel
Typhoid Three available:
1. killed bacteria
2. live, attenuated
bacteria (oral)
3. polysaccharide
conjugated to protein
Yellow fever Live attenuated virus
Prepared in chick embryo:
Dakar strain or 17D strain
Tuberculosis Live attenuated mycobacte- Rarely used in the US
rium BCG (Bacille calmette
Guerin) strain (BCG)
Table 27.1 Contd.
Disease Preparation Notes

Vaccines "%#
27.2.1 Innate or Non-specific Immunity
The innate or non-specific defense mechanisms are the first line defense against invading
microorganisms. They will act irrespective of the type of microorganism. Briefly they
consist of the following:
(a) Anatomic barriers: these include mechanical barriers such as skin, which physically
keeps out microorganisms and mucous membranes of the alimentary canal
respiratory and urinogenital tracts which entrap microorganisms. In addition the
mucous membranes harbor a normal set or flora of microorganisms which keep out
foreign organisms.
(b) Physiologic barriers: The physiology of the human body keeps out some pathogens.
Thus the high temperature of the human body, including the fever response keeps
out some microorganisms as does the acidic nature of the stomach. Chemical
mediators such as lysozyme found in tears breakdown bacterial cell walls.
(c) Phagocysis and endocytosis: white blood cells kill and digest whole micro-
organisms, while specialized cells engulf and breakdown foreign particles.
(d) Inflammatory responses: Tissue damage and infection induce leakage of vascular
fluid serum protein with antibacterial fluid and influx of white blood cells leading
to pus formation.
27.2.1.1 Acquired or Specific immunity
Acquired or specific immunity has three important properties among others, which are
crucial in understanding vaccines and how they function.
(i) Antigenic specificity: An antigen is a material usually a protein which binds
specifically to an antibody or to T-cell receptor (see below). Great specifity occurs in
the anatigen-antibody or antigen-T-cell receptor relations. Often a small difference
of a single amino acid can decide whether or not binding to antibody or T-cell will
take place.
(ii) Immunologic memory: Once the acquired immune system has recognized and
responded to an antigen, it exhibits immunologic memory: a second encounter
with the same antigen induces an increased response.
(iii) Self/non-self recognition: Specific immunity recognizes foreign bodies in
contrast to those of the body and seeks to destroy the intruders. In rare cases the
system of recognition breaks down and the system fails to recognize body cells and
proceeds to destroy them, giving rise to auto-immune diseases.
Specific immunity has two components, humoral and cell-mediated immunity which
are mediated by white blood cells known as lymphocytes, and as will be seen below both
sectors are linked. Humoral immunity is mediated by B Lymphocytes, while cell-
mediated immunity is brought about by T Lymphocytes. White blood cells including
lymphocytes and like red blood cells are produced from stem cells in the bone marrow.
The B lymphocytes, remain in the bone marrow to mature, while T lymphocytes mature in
the thymus, a small organ located above the heart.
27.2.2.2 Specific immunity: humoral immunity
Humoral immunity is also known as antibody immunity. Antibodies are soluble proteins
in the blood which bind to foreign agents and mark them for destruction, or neutralize
"%$ Modern Industrial Microbiology and Biotechnology
toxins produced by microorganisms. Also known as immunoglobulins, antibodies are
glycoproteins by nature (i.e. proteins to which carbohydrates are conjugated). (see Fig.
27.1)
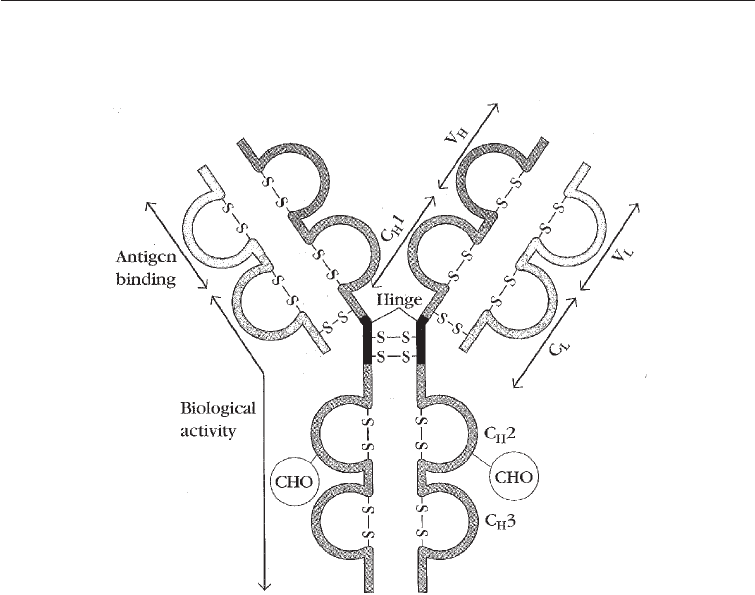
Fig. 27.1 General Structure of an Antibody Molecule
-S-S-, Disulphide bonds; CHO, carbohydrate molecule attached to the constant region of the heavy chain;
C
H
2, C
H
3, Constant regions of the heavy chain in the biological activity end of the antigen molecule; C
L
,
Constant region of the light chain; V
L
, Variable region of the light chain; C
H
1, Constant region of the heavy
chain in the constant part antigen binding end; V
H
, Variable region of the light chain (see text)
When B lymphocytes mature each has one unique antigen-binding molecule, an
antibody attached to its membrane; up to 10 different antibody molecules may be carried
on the B lymphocytes. When such a mature B lymphocyte which has not encountered any
antigen, known as a naïve lymphocyte, encounters an antigen for which its membrane
bound antibody is specific, it begins to divide rapidly and differentiates into two types of
cells: memory cells and plasma cells. The memory cells have a longer span of life and
continue to express membrane-bound antibody just like the original parent cell. The
plasma cells, on the other hand live for four to five days and do not have cell-membrane
bound antibodies; instead they produce antibody in a form in which it can be secreted,
often in huge amounts, sometimes reaching 2,000 molecules per second. The memory
cells are the source of the long-term protection which vaccines confer.
Antibodies (immunoglobulins) are Y- shaped and consist of two identical light chains
and two identical heavy chains (Fig. 27.1). The upper end of the Y of the light and heavy
chains of the antibody molecule is the variable region. The amino acids in this region vary
greatly among different antibodies and this variability confers on antibodies the vast
specificity for which they are known. The lower ends of the light and heavy chains are the
‘constant’ regions and do not show the variability found at the tips of the Y. The
variability in protein composition in the ‘constant’ region of the heavy chains (Fig. 27. 1)
Vaccines "%%
leads to antibodies being divided into five major classes, each with a different and
distinct property: IgG, IgA, IgD, IgM, and IgE. IgG is the most abundant (80%) of all Igs,
and it is the only one able to cross the placenta, helping to confer maternal immunity on
the newborn.
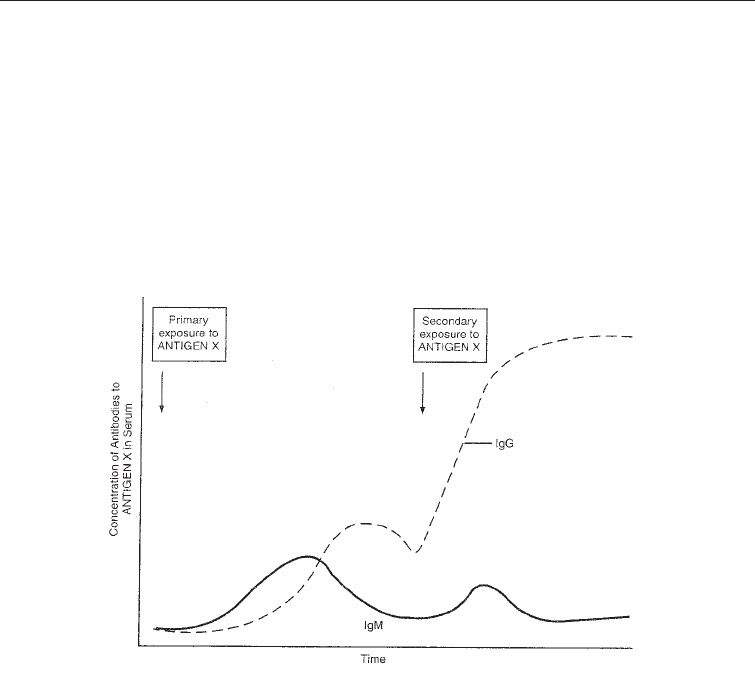
An antibody recognizes an antigen in a specific manner and the immune system
acquires memory towards it. The first encounter with an antigen is known as the primary
response. Re-encounter with the same antigen causes a secondary response that is more
rapid and powerful. This is the basis on which vaccines function; they induce the
memory lymphocytes to proliferate and the resulting plasma cells to produce soluble
antibodies (Fig. 27.2).
Antibodies are proteins known as immumoglogulins (Ig). The are five different kinds of immunoglobulins
IgA, IgD, IgE, IgG, and IgM. The animal body produces antibodies when challemged with materials to which
the body can react by producing antibodies (known as antigens). When the animal body is challenged with
the same antigen a second time the production of antibodies is not only produced in a shorter time, but the
antibody production is more pronounced as shown in the figure above. In the figure above IgM is produced
in the first challenge and IgM in the second. (see text).
Fig. 27.2 Antibody Response of the Animal Body to a Second Challenge of an Antigen
27.2.2.3 Specific immunity: cell-mediated immunity
While B lymphocytes mediate antibody or humoral immunity, T lymphocytes are
responsible for cell-mediated immunity. T lymphocytes do not have cell membrane
bound antibodies, nor do they secrete antibodies. Instead they have T-cell receptors
(TCRs). Unlike antibodies which can recognize antigens directly, T-cell receptors can
recognize an antigen only if the antigen is associated with cell membrane proteins
known as major histocompatibility compatibility (MHC) molecules, of which two classes
exist: MHC I and MHC II.
When a naïve T cell encounters an antigen associated with an MHC on a cell, the T cell
proliferates and differentiates into memory T cells, T helper cells (TH), and T cytotoxic
cells (TC). T
H
and T
C
cellscarry on their membranes different glycoproteins. T
H
cells carry
glycoprotein CD4, while T
C
cells carry CD8.
"%& Modern Industrial Microbiology and Biotechnology
When a T
H
cells interacts with an antigen linked to an MHC II compound, it is
activated to produce cytokins which also activate B cells to produce antibodies. Cytokins
also activate T
C
cells when they interact with an antigen linked to an MHC I compound,
to differentiate into cytoxic T lymphocytes (CTLs). CTLs do not secrete cytokins; instead
they monitor the body cells and eliminate any cells which are foreign or contain foreign
bodies such as cancer cells or cells containing viruses. To ensure that self cells are not
attacked by CTLs, they attack only cells displaying foreign foreigns complexed to an
MHC molecule on the surface of cells called antigen presenting cells (APCs). Antigen
presenting cells adsorb foreign antigens such as viruses, digest them and display the
peptides from them on their surfaces. CTLs identify such cells and destroy them. APCS
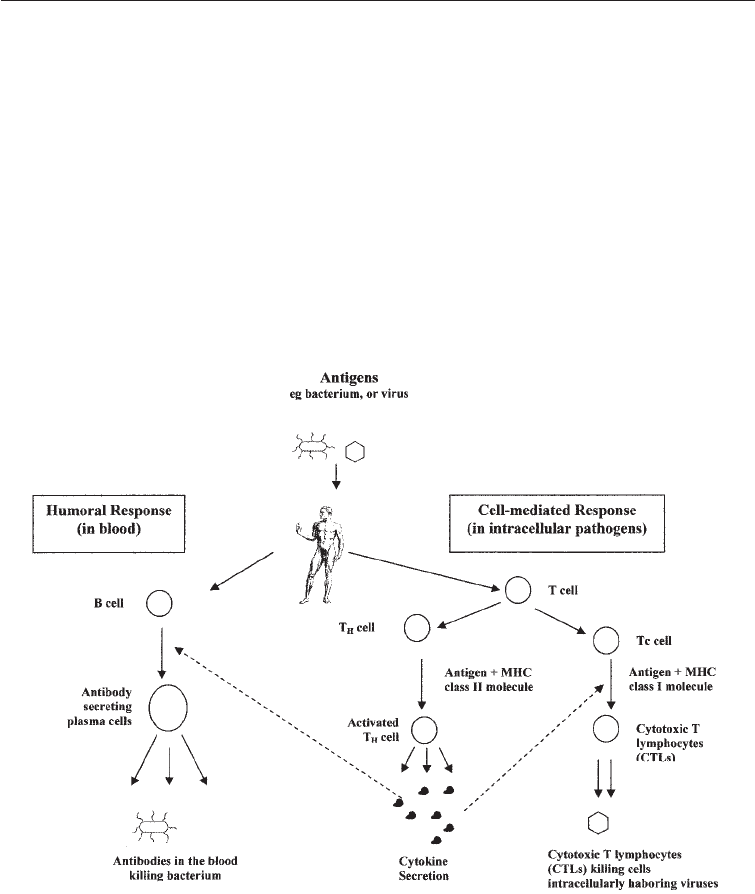
are specialized white blood cells. The relationships between B lymphocytes, T
lymphocytes, cytokins, Tc cells and CTLs are depicted in Fig. 27.3. The cell-mediated
immune response is important in cases where the pathogen is intracellular as in viruses
Fig. 27.3 Scheme Showing Immune System in Man
27.2.2.3 Antigens and Epitopes
Antigens are macromolecules that elicit an immune response in the body. Antigens can
be proteins, polysaccharides or conjugates of lipids with proteins (lipoproteins) and
polysaccharides (glycolipids). Antigens are generally very large and complex and the
lymphocytes may not recognize all the sites of a particular antigen. Rather both B and T
lymphocytes recognize discreet sites on an antigen known as epitopes or antigenic
determinants. The aim in vaccine production is to ensure that epitopes exist on the vaccine
which will elicit humoral or cell-mediated response.

Vaccines "%'
27.3 TRADITIONAL AND MODERN METHODS OF
VACCINE PRODUCTION
Traditionally three types of vaccines have been used: attenuated live vaccines, killed
vaccines and bacterial toxoids. Recent advances in molecular biology and genomic
science have spilled over into vaccine production. This chapter discusses the traditional
vaccines, but will also discuss the newer approaches which have been influenced by
advances in molecular biology and genomic science.
27.3.1 Traditional Vaccines
27.3.1.1 Live attenuated organisms
In live attenuated vaccines, the organism has been cultured so as to reduce its pathogenicity,
but still retains some of the antigens of the virulent form. They consist of the living patho-
gens whose virulence has been reduced (attenuated) by passaging them through hosts
different from the usual. Alternatively, non-virulent strains of the pathogen may be used.
Live agents may be used for one or more of the following reasons:
(i) When the protection-inducing substance is produced as a diffusible product of
metabolizing organisms e.g. Bacillus anthracis.
(ii) When it is not feasible to produce sufficient amounts of nonviable agents and a
small concentration of the living agent can propagate within the vaccinated
subject to overcome the deficiency.
(iii) When immunity is induced by the modification of parasitized cells.
Live vaccines in use include those against polio (Sabin oral polio vaccine - OPV), foot
and mouth disease of farm animals, mumps, measles, rubella (German measles),
tuberculosis, rabies and yellow fever. For tuberculosis the vaccine is derived the Bacillus
Calmette-Guérin (BCG) strain of Mycobacterium tuberculosis, a weakened version of the
bacterium that causes tuberculosis in cows. BCG is used as a vaccine against
tuberculosis in many European countries; it is however not commonly used in the U. S.
The OPV has advantages and disadvantages when compared with the (inactivated)
Salk polio vaccine (IPV). OPV can be given by mouth rather than by injection, and it can
spread to the other members of the vaccinee’s family thus immunizing them as well. Its
disadvantage is that on rare occasions, the virus regains full virulence and cause the
disease. On account of this, the Salk vaccine has gained prominence over the Sabin
vaccine in some countries.
27.3.1.2 Killed vaccines
These consist of suspensions of fully virulent organisms (bacteria or viruses) killed as
mildly as possible in order not to destroy the antigenic determinants on the organism.
Killing can be achieved by heat, (usually about 60°C for 1 hour) chemicals (phenol,
alcohol, formalin, b-propiolactone) or ultraviolet irradiation. Killed vaccines do not
provide as prolonged antigenic stimuli as living vaccines and two, three or more sub-
cutaneous injections are required to give adequate protection. Examples of killed
vaccines include TAB vaccine against typhoid fever and which consists of heat-killed
phenol-preserved suspension of Salmonella typhii and Salm. Paratyphii A & B, whooping
cough, cholera, and the Salk IPV.
