Okafor N. Modern Industrial Microbiology and Biotechnology
Подождите немного. Документ загружается.


!% Modern Industrial Microbiology and Biotechnology
deficiency therapy, and as an anti caries agent. Its biodegradable polymer has medical
applications as sutures, orthopedic implants, controlled drug release, etc. Polymers of
lactic acids are biodegradable thermoplastics. These polymers are transparent and their
degradation can be controlled by adjusting the composition, and the molecular weight.
Their properties approach those of petroleum derived plastics. Lactic acid esters like
ethyl/butyl lactate can be used as green solvents. They are high boiling, non-toxic and
degradable components. Poly L-lactic acid with low degree of polymerization can help in
controlled release or degradable mulch films for large-scale agricultural applications.
Lactic acid was among the earliest materials to be produced commercially by
fermentation and the first organic acid to be produced by fermentation.
Table 20.1 Physical properties of ethyl alcohol
Boiling point 78.2
Explosive limit in air, vol % 43-19.0
Freezing point 114.1
o
C
Specific gravity at 20/20
o
C 0.7905
Surface tension at 20
o
C dynes/cm 22.3
Vapor pressure at 20
o
C mg/HG 44
Chemical processing has offered and continues to offer stiff competition to
fermentation lactic acid. Very few firms around the world produce it fermentatively, but
this could change when the hydrocarbon-based raw material, lactonitrile, used in the
chemical preparation becomes too expensive because of the increase in petroleum prices.
Lactic acid exists in two forms, the D-form and the L-form. When the symbols (+) or
(-) are used, they refer to the optical rotation of the acid in a refractometer. However optical
rotation in lactic acid is difficult to determine because the pure acid has low optical
properties. The acid also spontaneously polymerizes in aqueous solutions; furthermore,
salts, esters, and polymers have rotational properties opposite to that of the pure acid
from which they are derived. All this makes it difficult to use optical rotation for
characterizing lactic acid.
Many organisms produce either the D-or the L-form of the acid. However, a few
organisms such as Lactobacillus plantarum produce both. When both the D- and L- form of
lactic acid are mixed it is a racemic mixture. The DL form which is optically inactive is the
form in which lactic acid is commercially marketed.
20.1.6.2 Uses of lactic acid
(i) It is used in the baking industry. Originally fermentation lactic acid was produced
to replace tartarates in baking powder with calcium lactate. Later it was used to
produce calcium stearyl 2- lactylate, a bread additive.
(ii) In medicine it is sometimes used to introduce calcium in to the body in the form of
calcium lactate, in diseases of calcium deficiency.
(iii) Esters of lactic acid are also used in the food industry as emulsifiers.
(iv) Lactic acid is used in the manufacture of rye bread.
(v) It is used in the manufacture of plastics.

Production of Organic Acids and Industrial Alcohol !%
(vi) Lactic acid is used as acidulant/ flavoring/ pH buffering agent or inhibitor of
bacterial spoilage in a wide variety of processed foods. It has the advantage, in
contrast to other food acids in having a mild acidic taste.
(vii) It is non-volatile odorless and is classified as GRAS (generally regarded as safe) by
the FDA.
(viii) It is a very good preservative and pickling agent. Addition of lactic acid aqueous
solution to the packaging of poultry and fish increases their shelf life.
(ix) The esters of lactic acid are used as emulsifying agents in baking foods (stearoyl-2-
lactylate, glyceryl lactostearate, glyceryl lactopalmitate). The manufacture of these
emulsifiers requires heat stable lactic acid, hence only the synthetic or the heat
stable fermentation grades can be used for this application.
(x) Lactic acid has many pharmaceutical and cosmetic applications and formulations
in topical ointments, lotions, anti acne solutions, humectants, parenteral solutions
and dialysis applications, for anti carries agent.
(xi) Calcium lactate can be used for calcium deficiency therapy and as anti caries
agent.
(xii) Its biodegradable polymer has medical applications as sutures, orthopaedic
implants, controlled drug release, etc.
(xiii) Polymers of lactic acids are biodegradable thermoplastics. These polymers are
transparent and their degradation can be controlled by adjusting the composition,
and the molecular weight. Their properties approach those of petroleum derived
plastics.
(xiv) Lactic acid esters like ethyl/butyl lactate can be used as environment-friendly
solvents. They are high boiling, non-toxic and degradable components.
(xv) Poly L-lactic acid with low degree of polymerization can help in controlled release
or degradable mulch films for large-scale agricultural applications.
Table 20.2 Physical properties of lactic acid
Appearance Yellow to colorless crystals or syrupy 50% liquid
Melting point 16.8°C
Relative density 1.249 at 15°C
Boiling point 122° @ 15 millimeter
Flash point 110°C
Solubility Soluble in water, alcohol, furfurol
Slightly soluble in ether
Insoluble in chloroform, petroleum ether, and carbon
disulfide
20.1.6.3 Fermentation for lactic acid
Although many organisms can produce lactic acid, the amount so produced is small: the
organisms which produce adequate amounts and are therefore used in industry are the
homofermentative lactic acid bacteria, Lactobacillus spp., especially L. delbruckii. In recent
times Rhizopus oryzae has been used. Both organisms produce the L- form of the acid, but

!% Modern Industrial Microbiology and Biotechnology
Rhizopus fermentation has the advantage of being much shorter in duration; further, the
isolation of the acid is much easier when the fungus is used.
Lactic acid is very corrosive and the fermentor, which is usually between 25,000 and
110,000 liters in capacity is made of wood. Alternatively special stainless steel (type 316)
may be used. They are sterilized by steaming before the introduction of the broth as
contamination with thermophilic clostridia yielding butanol and butyric acid is
common. Such contamination drastically reduces the value of the product.
During the step-wise preparation of the inoculum, which forms about 5% of the total
beer, calcium carbonate is added to the medium to maintain the pH at around 5.5-6.5. The
carbon source used in the broth has varied widely and have included whey, sugars in
potato and corn hydrolysates, sulfite liquour, and molasses. However, because of the
problems of recovery for high quality lactic acid, purified sugar and a minimum of other
nutrients are used.
Lactobacillus requires the addition of vitamins and growth factors for growth. These
requirements along with that of nitrogen are often met with ground vegetable materials
such as ground malt sprouts or malt rootlets. To aid recovery the initial sugar content of
the broth is not more than 12% to enable its exhaustion at the end of 72 hours.
Fermentation with Lactobacillus delbruckii is usually for 5 to 10 days whereas with
Rhizopus oryzae, it is about two days.
Although lactic fermentation is anaerobic, the organisms involved are facultative and
while air is excluded as much as possible, complete anaerobiosis is not necessary.
The temperature of the fermentation is high in comparison with other fermentation,
and is around 45°C. Contamination is therefore not a problem, except by thermophilic
clostridia.
20.1.6.4 Extraction
The main problem in lactic acid production is not fermentation but the recovery of the
acid. Lactic acid is crystallized with great difficulty and in low yield. The purest forms are
usually colorless syrups which readily absorb water.
At the end of the fermentation when the sugar content is about 0.1%, the beer is
pumped into settling tanks. Calcium hydroxide at pH 10 is mixed in and the mixture is
allowed to settle. The clear calcium lactate is decanted off and combined with the filtrate
from the slurry. It is then treated with sodium sulfide, decolorized by adsorption with
activated charcoal, acidified to pH 6.2 with lactic acid and filtered. The calcium lactate
liquor may then be spray-dried.
For technical grade lactic acid the calcium is precipitated as CaSO
4
.2H
2
O which is
filtered off. It is 44-45% total acidity. Food grade acid has a total acidity of about 50%. It is
made from the fermentation of higher grade sugar and bleached with activated carbon.
Metals especially iron and copper are removed by treatment with ferrocyanide. It is then
filtered. Plastic grade is obtained by esterification with methanol after concentration.
High-grade lactic acid is made by various methods: steam distillation under high
vacuum, solvent extraction etc.
Production of Organic Acids and Industrial Alcohol !%!
20.2 INDUSTRIAL ALCOHOL PRODUCTION
Ethyl alcohol, CH
3
CH
2
OH (synonyms: ethanol, methyl carbinol, grain alcohol, molasses
alcohol, grain neutral spirits, cologne spirit, wine spirit), is a colorless, neutral, mobile
flammable liquid with a molecular weight of 46.47, a boiling point of 78.3 and a sharp
burning taste. Although known from antiquity as the intoxicating component of
alcoholic beverages, its formula was worked out in 1808. It is rarely found in nature,
being found only in the unripe seeds of Heracleum giganteun and H. spondylium.
20.2.1 Properties of Ethanol
Some of the physical properties of ethanol are given in Table 20.1
Ethyl alcohol undergoes a wide range of reactions, which makes it useful as a raw
material in the chemical industry. Some of the reactions are as follow:
(i) Oxidation: Ethanol may be oxidized to acetaldehyde by oxidation with copper or
silver as a catalyst:
Cu, Ag
CH
3
CH
2
OH CH
3
CHO + H
2
(ii) Halogenation: Halides of hydrogen, phosphorous and other compounds react with
ethanol to replace the – OH group with a halogen:
3CH
3
CH
2
OH + PCl
3
3CH
3
CH
2
Cl + P (OH)
3
(iii) Reaction with metals: Ethanol reacts with sodium, potassium and calcium to give the
alcoholates (alkoxides) of these metals:
2CH
3
CH
2
OH + 2Na 2CH
3
ONa + H
2
(iv) Haloform Reaction: Hypohalides will react with ethanol to yield first acetaldehyde
and finally the haloform reaction:
CH
3
CH
2
OH + NaOCl CH
3
CHO + NaCl + H
2
O
CH
3
CHO + 2NaOCl CCl
3
CHO + 3NaOH
CCl
3
CHO + NaOH CHCl
3
+ HCOONa
Chloroform
(v) Esters: Ethanol reacts with organic and inorganic acids to give esters:
CH
3
CH
2
OH + HCl CH
3
CH
2
Cl + H20
Ethylchoride
(vi) Ethers: Ethanol may be dehydrated to give ethers:
Catalyst
2CH
3
CH
2
OH CH
3
CN
2
OCH
2
CH
3
+ H
2
O
(vii) Alkylation: Ethanol alkylates (adds alkyl-group to) a large number of compounds:
H
3
SO
4
:CH
3
CH
2
HSO
4
(ethyl hydrogen sulfate)
NH
3
:CH
3
CH
2
NH
2
(ethyl amine)

!%" Modern Industrial Microbiology and Biotechnology
20.2.2 Uses of Ethanol
(i) Use as a chemical feed stock: In the chemical industry, ethanol is an intermediate in
many chemical processes because of its great reactivity as shown above. It is thus a
very important chemical feed stock.
(ii) Solvent use: Ethanol is widely used in industry as a solvent for dyes, oils, waxes,
explosives, cosmetics etc.
(iii) General utility: Alcohol is used as a disinfectant in hospitals, for cleaning and
lighting in the home, and in the laboratory second only to water as a solvent.
(iv) Fuel: Ethanol is mixed with petrol or gasoline up to 10% and known as gasohol and
used in automobiles.
20.2.3 Denatured Alcohol
All over the world and even in ancient times, governments have derived revenue from
potable alcohol. For this reason when alcohol is used in large quantities it is denatured or
rendered unpleasant to drink. The base of denatured alcohol is usually 95% alcohol with
5% water; for domestic burning or hospital use denatured alcohol is dispened as
methylated spirit, which contains a 10% solution of methanol, pyridine and coloring
material. For industrial purpose methanol is used as the denaturant. In the United States
alcohol may be completely denatured (C.D.A. – completely denatured alcohol) when it
cannot be used orally because of a foul taste or four smelling additives. It may be specially
denatured (S.D.A. – specially denatured alcohol) when it can still be used for special
purposes such as vinegar manufacture without being suitable for consumption.
20.2.4 Manufacture of Ethanol
Ethanol may be produced by either synthetic chemical method or by fermentation.
Fermentation was until about 1930 the main means of alcohol production. In 1939, for
example 75% of the ethanol produced in the US was by fermentation, in 1968 over 90%
was made by synthesis from ethylene. The production of alcohol from ethylene is
discussed in Chapter 13.
Due to the increase in price of crude petroleum, the source of ethylene used for alcohol
production, attention has turned worldwide to the production of alcohol by
fermentation. Fermentation alcohol has the potential to replace two important needs
currently satisfied by petroleum, namely the provision of fuel and that of feedstock in the
chemical industry.
The production of gasohol (gasoline – alcohol blend) appears to have received more
attention than alcohol use as a feed stock. Nevertheless, the latter will also surely assume
more importance if petroleum price continues to ride. Governments the world over have
set up programs designed to conserve petroleum and to seek other energy sources. One of
the most widely publicized programs designed to utilize a new source of energy is the
Brazilian National Ethanol Program. Set-up in 1975, the first phase of this program aims
at extending gasoline by blending it with ethanol to the extent of 20% by volume. The
United States government also introduced the gasoline programme based on corn
fermentation in 1980 following the embargo on grain sales to the then Soviet Union.

Production of Organic Acids and Industrial Alcohol !%#
20.2.4.1 Substrates
The various substrates which may be used for ethanol production have been discussed in
Chapter 2. It is clear that the substrate used will vary among countries. Thus, in Brazil
sugar cane, already widely grown in the country, is the major source of fermentation
alcohol, while it is planned to use cassava and sweet sorghum. In the United States
enormous quantities of corn and other cereals are grown and these are the obvious
substrates. Cassava grows in many tropical countries and since it is high yielding it is an
important source in tropical countries where sugar cane is not grown. It is recognized
that two important conditions must be met before fermentation alcohol can play a major
role in the economy either as gasohol or as a chemical feedstock. First, the production of
the crop to be used must be available to produce the crop without extensive and excessive
deforestation. Secondly, the substrate should not compete with human food.
20.2.4.2 Fermentation
The conditions of fermentation for alcohol production are similar to those already
described for whisky or rum production. Alcohol-resistant yeasts, strains of
Saccharomyces cerevisiae are used, and nutrients such as nitrogen and phosphate lacking
in the broth are added.
20.2.4.3 Distillation
After fermentation the fermented liquor or ‘beer’ contains alcohol as well as low boiling
point volatile compounds such as acetaldeydes, esters and the higher boiling, fusel oils.
The alcohol is obtained by several operations. First, steam is passed through the beer
which is said to be steam-stripped. The result is a dilute alcohol solution which still
contains part of the undesirable volatile compounds. Secondly, the dilute alcohol
solution is passed into the center of a multi-plate aldehyde column in which the
following fractions are separated: esters and aldehydes, fusel oil, water, and an ethanol
solution containing about 25% ethanol. Thirdly, the dilute alcohol solution is passed into
a rectifying column where a constant boiling mixture, an azeotrope, distils off at 95.6%
alcohol concentration.
To obtain 200° proof alcohol, such as is used in gasohol blending, the 96.58% alcohol
is obtained by azeotropic distillation. The principle of this method is to add an organic
solvent which will form a ternary (three-membered) azeotrope with most of the water, but
with only a small proportion of the alcohol. Benzene, carbon tetrachloride, chloroform,
and cyclohezane may be used, but in practice, benzene is used. Azeotropes usually have
lower boiling point than their individual components and that of benzene-ethanol-water
is 64.6°C. On condensation, it separates into two layers. The upper layer, which has
about 84% of the condensate, has the following percentage composition: benzene 85%,
ethanol 18%, water 1%. The heavier, lower portion, constituting 16% of the condensate,
has the following composition: benzene 11%, ethanol 53%, and water 36%.
In practice, the condensate is not allowed to separate out, but the arrangement of plates
within the columns enable separation of the alcohol. Four columns are usually used. The
first and second columns remove aldehydes and fusel oils, respectively, while the last
two towers are for the concentration of the alcohol. A flow diagram of conventional
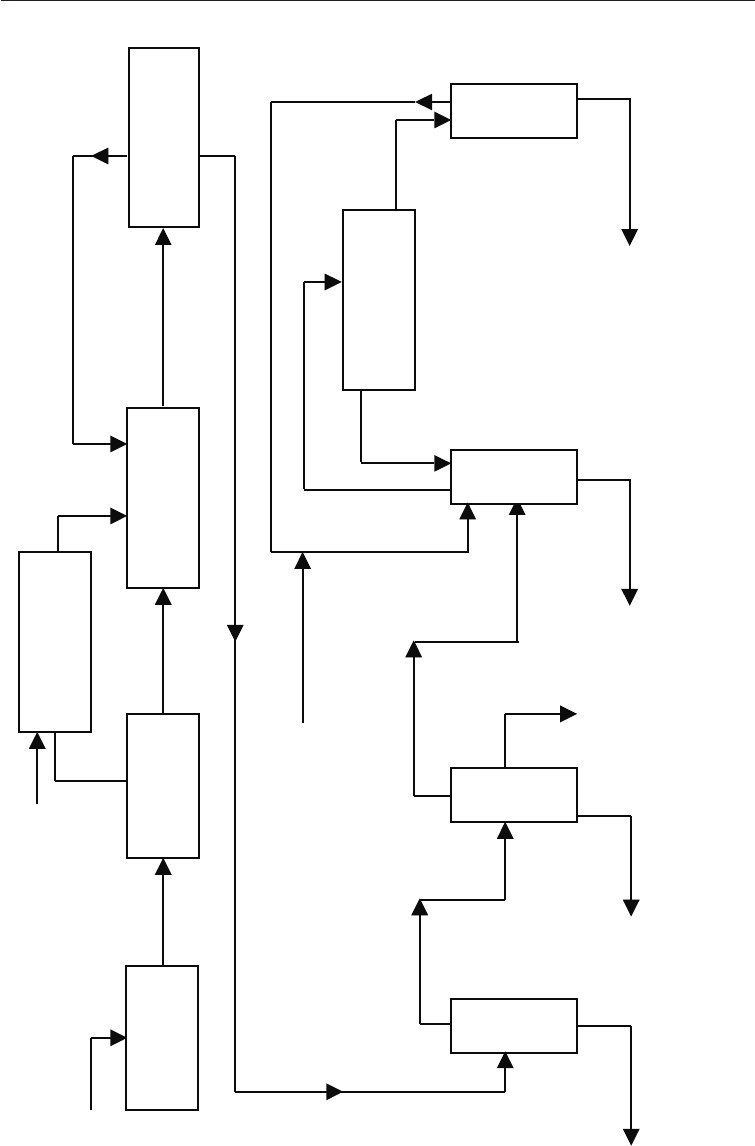
absolute alcohol production from molasses is given in Fig. 20.4
!%$ Modern Industrial Microbiology and Biotechnology
Fig. 20.4 Flow Diagram of Conventional Alcohol Production
YEAST
CULTURE
MOLASSES OR
CANE JUICE
PREPARATION
OF INOCULUM
WEIGHING
BRIX
ADJUSTMENT
14
o
TO 22
o
WORT
FERMENTATION
RECUPERATION OF YEAST
CENTRIFUGATION
FERMENTED
WORT
(7–8%V/V)
CENTRIFUGED MEDIUM
BENZENE
DECANTATION
RECYCLE
(TO RECTIFICATION)
(RECUPERATION
OF BENZENE)
ABSOLUTE
ALCOHOL
(DEHYDRATION)
(RECTIFICATION)(DISTILLATION)
STILLAGE
ALCOHOL
(40% - 50% V/V)
ALCOHOL
(96% V/V)
WATER
FUSEL
OIL

Production of Organic Acids and Industrial Alcohol !%%
20.2.5 Some Developments in Alcohol Production
Due to the current interest in the potential of ethanol as a fuel and a chemical feedstock,
research aimed at improving the conventional method of production has been
undertaken, and more will, most certainly, be undertaken. Some of the techniques aimed
at improving productivity are the following:
(i) Developments of new strains of yeast of Saccharomyces uvarum able to ferment sugar
rapidly, to tolerate high alcohol concentrations, flocculate rapidly, and whose
regulatory system permits it to produce alcohol during growth.
(ii) The use of continuous fermentation with recycle using the rapidly flocculating yeasts.
(iii) Continuous vacuum fermentation in which alcohol is continuously evaporated
under low pressure from the fermentation broth.
(iv) The use of immobilized Saccharomyces cerevisiae in a packed column, instead of in a
conventional stirred tank fermentor. Higher productivity consequent on a higher
cell concentration was said to be the advantage.
(v) In the ‘Ex-ferm’ process sugar cane chips are fermented directly with a yeast
without first expressing the cane juice. The chips may be dried and used in the off-
season period of cane production. It is claimed that there is no need to add
nutrients as would be the case with molasses, since these are derived from the cane
itself. A more complete extraction of the sugar, resulting in a 10% increase in
alcohol yield, is also claimed.
(vi) The use of Zymomonas mobilis, a Gram-negative bacterium which is found in some
tropical alcoholic beverages, rather than yeast is advocated. The advantages
claimed for the use of Zymomonas are the following:
(a) Higher specific rates of glucose uptake and ethanol production than reported
for yeasts. Up to 300% more ethanol is claimed for Zymomonas than for yeasts
in continuous fermentation with all recycle.
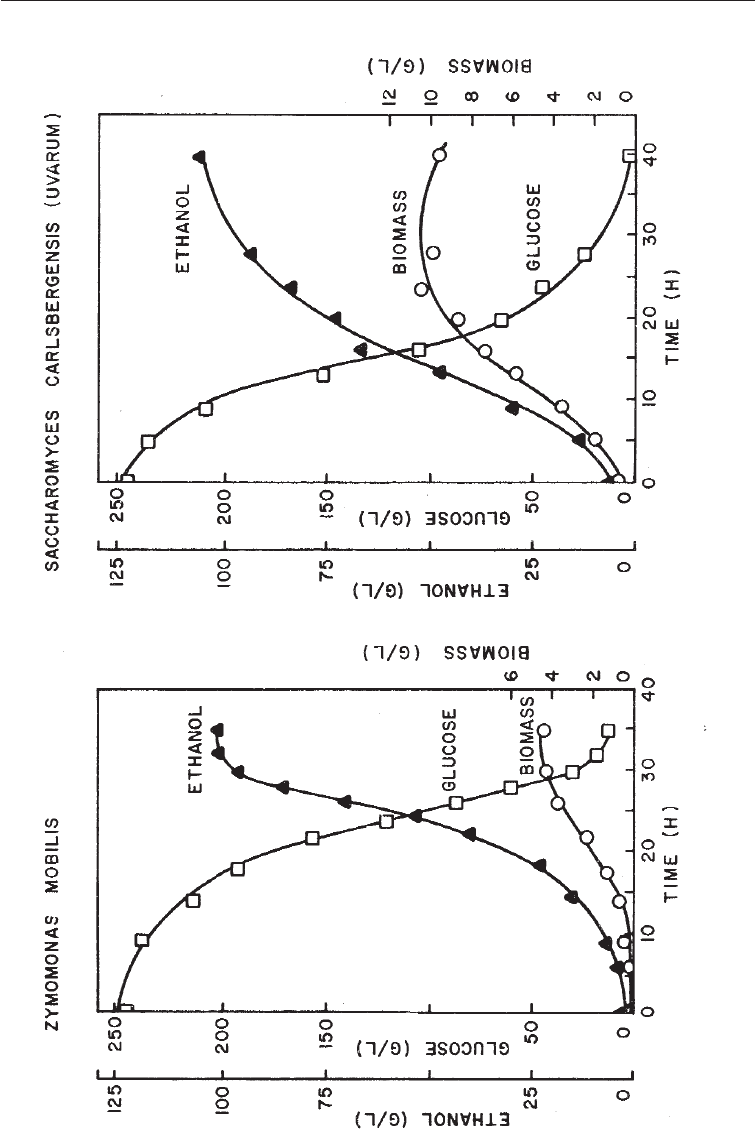
(b) Higher ethanol yields and lower biomass than with yeasts. This deduction is
based on Fig. 20.5 where, although the same quantity of alcohol is produced by
the two organisms in 30-40 hours, the biomass of Zymomonas required for this
level of production is much less than with yeast. The lower biomass appears to
be due to the lower energy available for growth. Zymomonas utilized glucose by
the Enthner-Duodoroff pathway (Fig. 5.4) which yields one mole of ATP/mole
glucose, whereas yeasts utilize glucose anaerobically via the glycolytic
pathway (Fig. 5.1) to give two ATP/mole glucose. Its use does not appear to
have gained general acceptance.
(c) Ethanol tolerance is at least as high or even higher [up to 16% (v/v)] in some
strains of the bacterium than with yeast.
(d) Zymomonas also tolerates high glocuse concentration and many cultures grow
in sugar solutions of up to 40% (w/v) glucose which should lead to high
ethanol production.
(e) Zymomonas grows anaerobically and, unlike yeasts, does not require the
controlled addition of oxygen for viability at the high cell concentrations used
in cell recycle.
(f) The many techniques for genetic engineering already worked out in bacteria
can be easily applied to Zymomonas for greater productivity.
!%& Modern Industrial Microbiology and Biotechnology
Fig. 20.5 Alcohol Production by Yeast and
Zymomonas mobilis

Production of Organic Acids and Industrial Alcohol !%'
SUGGESTED READINGS
Charrington, C.A, Hinton, M., Mead, G.C., Chopra, I. 1991. Organic Acids: Chemistry,
Antibacterial Activity and Practical Applications. Advances in Microbial Physiology. 32, 87 –
108.
Ho, N.W.Y. 1980. Ann. Repts. Ferm. Proc. 4, 235-266.
Kosaric, D.C.M., Ng, I.R., Steart, G.S. 1980. Adv. Appl. Microbiol. 26, 137-227.
Lockwood, L.B. 1979. In: Microbial Technology. H.J. Peppler, D. Perlman, (eds.) 2
nd
Edit.
Academic Press, New York, USA, pp. 256-288.
Narayanan, N., Pradip, K., Roychoudhury, P.K., Srivastava, A. 2004. L (+) lactic acid fermentation
and its product polymerization. Electronic Journal of Biotechnology 7, Electronic Journal of
Biotechnology [online]. 15 August 2004, vol. 7, no. 3 [cited 23 March 2006]. Available from:
http://www.ejbiotechnology.info/content/vol2/issue3/full/3/index.html. ISSN 0717-3458.
Ward, W.P., Singh, A. 2003. Bioethanol Technology: Developments and Perspectives. Advances
In Applied Microbiology, 51, 53-80.
