Okafor N. Modern Industrial Microbiology and Biotechnology
Подождите немного. Документ загружается.

!' Modern Industrial Microbiology and Biotechnology
amino acid will occur. The mutants used for the production of aminoacids other than
glutamic acid are produced from L- glutamic acid producing bacteria. This is because
these bacteria assimilate carbon efficiently and also because they do not degrade the
amino acid which they excrete.
21.4.1 Production of Amino Acids by Auxotrophic Mutants
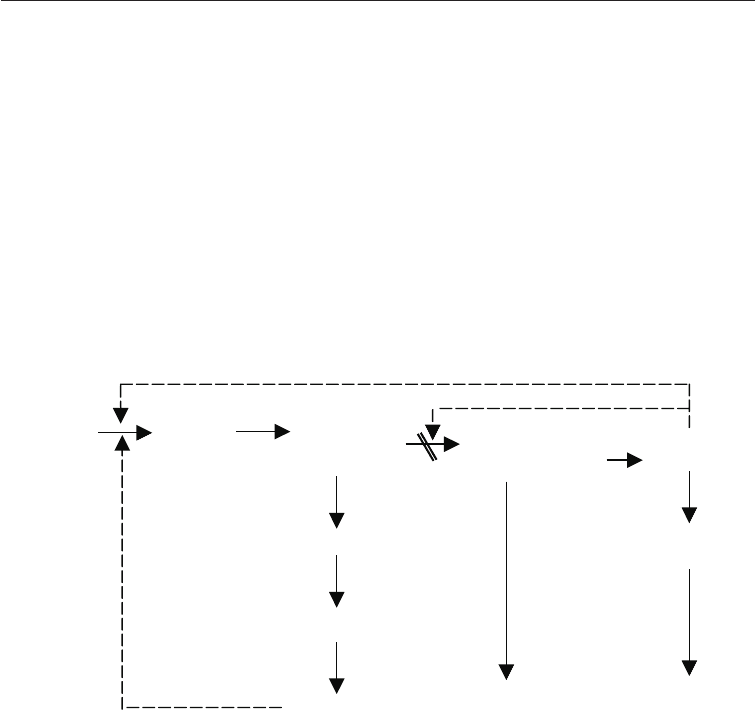
Table 21.4 shows the amino acids produced by the use of auxotrophic mutants. The first
to be produced was L- lysine using limiting concentrations of either L- homoserine or L-
methionine plus L- threonine with a mutant strain of Corynebacterium glutamicum. In the
wild type of this organism concerted feed back inhibition is by both lysine and threonine.
Inhibition does not occur when only one is present. In this particular mutant absence of
biosynthetic homoserine derived from aspartic acid causes lysine to accumulate. This is
illustrated in Fig. 21.6.
--------------------------------- Feed back inhibition
______________________ Biosynthetic pathway
aspartic aspartyl aspartyl
Acid phosphate semialdehyde L-homoserino L-threonine
dihydrodopicolinate a-ketobutyrate
diaminopimelate
L- lysine L-methionine L-isoleucine
L-
Fig. 21.6 Accumulation of Lysine in a Mutant Strain of
Corynebacterium glutamicum
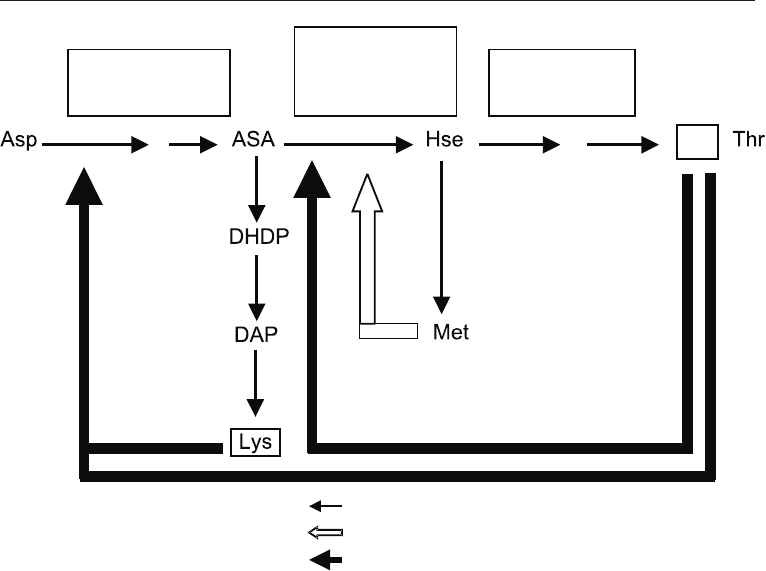
21.4.2 Production of Amino Acids by Regulatory Mutants
Regulatory mutants have a feed-back insensitive key enzyme and hence continues to over
produce the required amino acid. Examples are given in Table 21.4. In order to obtain
such mutants mutations are induced to produce organisms whose growth is not
inhibited by analogues of the amino-acid to be overproduced. A good example is the case
of lysine production by Brevibacterium flavum. In this organism the L- lysine pathway is
regulated at aspartate kinase which is the only enzyme sensitive to feed back inhibitation
by lysine. Mutants resistant to lysine analogues therefore over produce the amino acid
(Fig. 21.7).
Production of Amino Acids by Fermentation !'
21.5 IMPROVEMENTS IN THE PRODUCTION OF AMINO
ACIDS USING METABOLICALLY ENGINEERED
ORGANISMS
The improvements of the microorganisms discussed above used classical mutation
techniques and screening procedures which relied on deleting competing pathways and
eliminating feed back regulations on the biosynthetic pathways. The mutagenic
procedure cannot however totally eliminate deregulation. The use of recombinant DNA
technology has enabled genetic modifications which have further improved existing
production strains through metabolic engineering (Chapter 7). As indicated in Chapter 7,
metabolic engineering involves the introduction of genes which will enhance the
production of a metabolic pathway. The pathways through which amino acids are made
by the organism are shown in Fig. 21.5. The genes limiting the production of the amino
acid are enhanced by gene amplication thus leading to a more rational improvement of
the organism.
Many examples exist of improvements in amino acid production through cloning of
genes (Table 21.5). Among the pathways which have been targeted for improvement
through gene cloning are:
Enzyme reaction
Repression
Feedback inhibition
ASA = aspartate semino-aldehyde; DADP = dihydrodipicolinate;
Hse = Homoserne; DAP = diaminopimelate
Aspartate
Kinase
-
Homosertine
dehydro
genase
Kinase
Homoserine
kinase
Fig. 21.7 Lysine Biosynthesis in
Brevibacteium flavum
and
Corynebacterium glutamicum

!' Modern Industrial Microbiology and Biotechnology
(i) the terminal pathways of the amino acid synthesis
(ii) the central metabolic pathway for producing the amino acid
(iii) the transport process for secreting amino acid
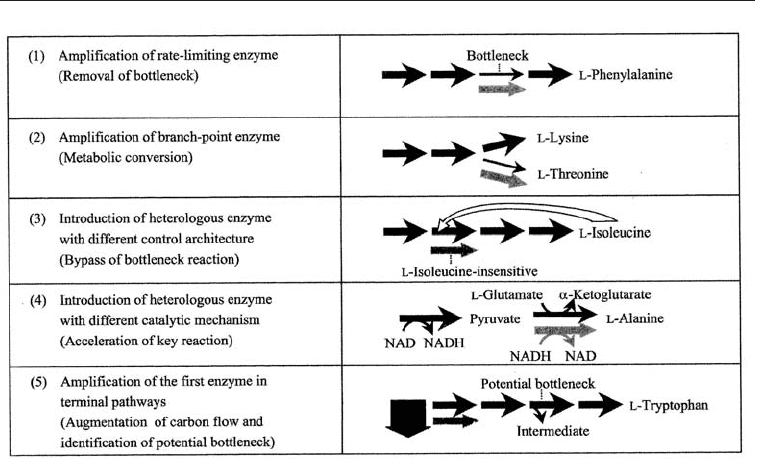
21.5.1 Strategies to Modify the Terminal Pathways
The strategies for modifying the terminal pathways are indicated in Fig. 21.8.
1. Amplification of rate limiting enzyme: The gene coding for the rate limting enzyme in
the biosynthetic pathway is amplified. Large increases have been observed when
this technique was applied to L-phenyl alanine production in Corynebacterium
glutamicum.
2. Amplification of branch-point enzyme: The gene coding for the branch-point enzyme
is amplified to redirect the common intermediate to another amino acid. It has been
used successfully in converting L-lysine to L-tryptophane and L-tyrosine to L-
phenylalanine.
Table 21.5 Improvements in amino acid production through the cloning of different genes
Amino acid Microorganisms Gene donor Cloned gene Yield
produced or enzyme mg/mL
L-alanine E. coli B. stearothermophilus Ala dehydrogenase
D-alanine E. coli Ochrobactrum anthropi D-aminopeptidase 200
L-histidine C. glutamicum C. glutamicum His G, D, C, B 15
S. marcescens S. marcescens His G, D, B 43
L-isoleucine C. glutamicum C. glutamicum Hom dehydrogenase 11
B. flavum E. coli ilv A 21
L-lysine C. glutamicum C. glutamicum Lys A, dap A, B, D, Y
C. glutamicum E. coli Asp A
L-phenylalanine C. glutamicum C. glutamicum aro F, chorismate 28
mutase, PRDH
C. glutamicum E. coli aro G, Phe A
B. lactofermentum B. lactofermentum aro F, E, L, PRDH 21
L-proline S. marcescens S. marcescens Pro A, B 75
L-threonine E. coli E. coli Thr A, B, C 55
C. glutamicum C. glutamicum hom dehydrogenase, 51
hom kinase, Thr C
B. lactofermentum B. lactofermentum ppc, hom 33
dehydrogenase,
hom kinase
B. flavum E. coli Thr B, C 27
S. marcescens E. coli ppc 60
L-tryptophan E. coli E. coli Trp A, E, R, tna A 40
C. glutamicum C. glutamicum Trp E, aro F, 45
chorismate mutase,
PRDH
L-tyrosine C. glutamicum E. coli Aro F 9
Production of Amino Acids by Fermentation !'!
Fig. 21.8 Strategies to Modify Terminal Pathways for the Improved Production of Amino
Acids, (from Ikeda, 2003)
3. Introduction of a different enzyme able to produce the same end amino acid: The gene for
a different enzyme for the same end amino acid is introduced. The enzyme creating
the bottle neck is thus bypassed. This has been used for increased L-isoleucine
production in Corynebacterium glutamicum.
4. Introduction of a more functional enzyme than the native one: Introduction of an enzyme
which is more active than the native one thereby enhancing the production of the
amino acid. This has enhanced the production of L-alanine production by
Corynebacterium glutamicum when L-alanine dehydrogenase from Arthrobacter
oxydans was engineered into it .
5. Amplification of the first enzyme in the terminal pathway: The first enzyme in a
pathway diverging from central metabolism is amplified to increase the flow in
that pathway; any bottleneck is removed by the increased down the pathway. This
strategy has been applied to obtain increased yield of L-tryptophan by
Corynebacterium glutamicum.
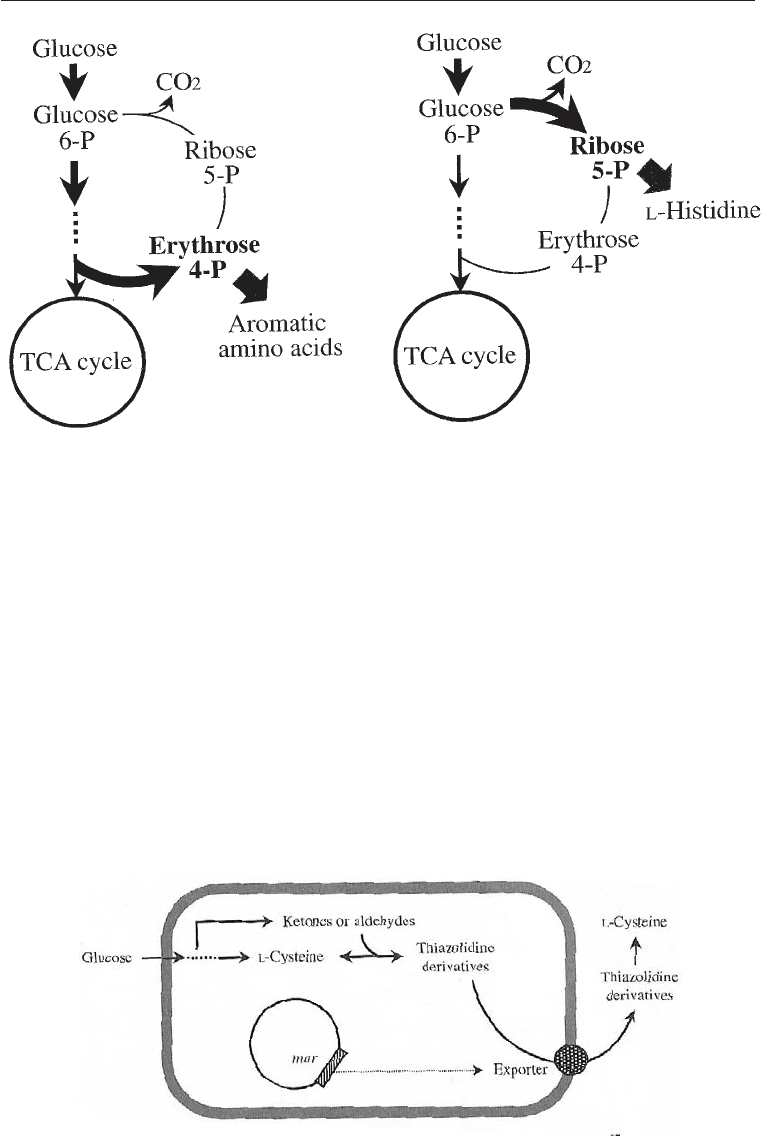
21.5.2 Strategies for Increasing Precursor Availability
A major aim of metabolic engineering for increased amino acid production is to channel
as much carbon as possible from sugar into the production of a desired amino acid. After
bottlenecks in the terminal pathway are removed, the main factor limiting increased
production is the shifting of intermediates to the central metabolic pathway. The
complete genetic sequence of Corynebacterium glumicum is available. One strategy is to
amplify the genes for the enzymes leading to the formation of aromatic amino acids
erythrose 4-P and to L histidine through 5-P (Fig. 21.9).
!'" Modern Industrial Microbiology and Biotechnology
21.5.3 Metabolic Engineering to Improve Transport of
Amino Acids Outside the Cell
The aim of strain improvement is to prevent feedback inhibition when the amino acid
accumulates intracellularly. One manner in which feedback inhibition can be avoided is
through increased efflux of the amino acid. A gene which codes for increased efflux has
been introduced into E.coli resulting in a vastly increased production of L-cysteine (Fig.
21.10).
21.6 FERMENTOR PRODUCTION OF AMINO ACID
21.6.1 Fermentor Procedure
Starting from shake flasks the inoculum culture is grown in shake flasks and transferred
to the first seed tank (1,000–2,000 liters) in size. After suitable growth the inoculum is
Fig. 21.10 Increased Efflux of Amino Acid in
E. coli
through Metabolic Engineering
Fig. 21.9 Strategies for Increasing Precursor Availability for the Production of Aromatic
Amino Acid and L-Histidine in
Corynebacterium glutamicum

Production of Amino Acids by Fermentation !'#
transferred to the second seed tank (10,000–20,000 liters), which serves as inoculum for
the production tank (50,000–500,000 liters).
The fermentation is usually batch or fed-batch (Chapter 9). In batch cultivation all the
nutrients are added at once at the beginning of the fermentation, except for ammonia
which is added intermittently to help adjust the pH, and fermentation continues until
sugar is exhausted. In a fed-batch process, the fermentor is only partially filled with
medium and additional nutrients added either intermittently or continuously until an
optimum yield is obtained. The fed-batch appears preferable for the following reasons:
(a) Most amino acid production requires high sugar concentrations of up to 10%. If all
were added immediately, acid would be quickly produced which will inhibit the
growth of the microorganisms and hence reduce yield.
(b) Where auxotrophic mutants are used, excess supply of nutrients leads to reduced
production due to overgrowth of cells or feed back regulation by the nutrient.
(c) During the lag phase of growth, the oxgen demand of the organism may exceed
that of the organism leading to reduced growth.
21.6.2 Raw Materials
The main raw materials used are cane or beet molasses and starch hydrolysates from
corn or cassava as glucose. In the US, the preferred carbon source is corn syrup from corn,
whereas in Europe and South America it is beet molasses.
As nitrogen source, inorganic sources such as ammonia or ammonium sulfate is
generally used.
Phosphates, vitamins and other necessary supplements are usually provided with
corn steep liquour.
21.6.3 Production Strains
Apart from the glutamatic acid bacteria already discussed, E. coli and Bacillus subtilis are
also good amino acid producing organisms. The glutamic acid bacteria previously
classified as four different species are now regarded one species. The optimum
temperature of C glutamicum is 30°C, whereas that of E. coli is higher. Hence, E. coli may be
prefered for production in tropical countries.
Production strains for amino acids are generally classified as wild-type, capable of
producing amino acids under defined conditions, but generally low-yielding in quantity,
auxotrophic or regulatory mutants, in which feedback regulations are bypassed by
partially starving them of their requirements or by removal of metabolic controls through
mutation, and by gentically modifying the organism by amplifying genes coding for rate-
limiting enzymes. The strains used belong to the last two categories and have been
developed using classical mutation methods or through genetic engineering (Chapter 7).
The selection of the strain is not only for high yields but also for those least producing
undesirable side products. For instance, when branched chain amino acids are
produced, it is essential that other branched chain amino acids do not occur as this
increases the cost of separation and extraction.

!'$ Modern Industrial Microbiology and Biotechnology
21.6.4 Down Stream Processing
After fermentation, the cells may be filtered using a rotary vacuum filter (Chapter 10).
Sometimes filtration can be improved by using filteraids. These filteraids, usually
kiesselghur, which are based on diatomaceous earth, improve the porosity of a resulting
filter cake leading to a faster flow rate. Before filtration a thin layer is used as a precoat of
the filter (normally standard filters).
The extraction method of the amino acid from the filtrate, depends on the level of purity
desired in the product. However two methods are generally used: the chromatographic
(ion exchange) method or the concentration-crystallization method.
Crystallization is often used as a method to recover the amino acid. Due to the amphoteric
character (contains both acidic and basic groups) of amino acids, their solubility is
greatly influenced by the pH of the solution and usually show minima at the isoelectric
point (zero net charge). Since temperature also influences the solubility of amino acids
and their salts, lowering the temperature can be used in advance as a means of obtaining
the required product. Precipitation of amino acids with salts, like ammonium and
calcium salts, and with metals like zinc are also commonly used. This is followed by acid
(or alkali) treatment to obtain the free or acid form of the amino acid.
Ion exchange resins have been widely used for the extraction and purification of amino
acids from the fermentation broth. The adsorption of amino acids by ion exchange resins
is strongly affected by the pH of the solution and by the presence of contaminant ions.
There are two types of ion exchange resins; cation exchange resins and anion exchange
resins. Cation exchange resins bind positively charged amino acids (this is in the
situation where the pH of the solution is lower then the isoelectric point (IEP) of the amino
acid), whereas anion exchange resins bind negatively charged amino acids (pH of the
solution is higher than IEP). Elution of the bound amino acid(s) is done by introducing a
solution containing the counterion of the resin. Anion exchange resins are generally
lower in their exchange capacity and durability than cation exchange resins and are
seldom used for industrial separation. In general, ion exchange as a tool for separation is
only used when other steps fail, because of its tedious operation, small capacity and high
costs.
SUGGESTED READINGS
Araki, K. 2003. Amino Acids Kirk-Othmer Encyclopedia of Chemical Technology. 2, 554-618.
Currell, B.R.C., Mieras, V.D., Biotol Partners. 1997. Biotechnological Innovations in Chemical
Synthesis Elsevier.
Ikeda, M. 2003. Amino Acid Production Processes. Advances in Biochemical Engineering/
Biotechnology, 79, 1–35.
Kelle, R., Hermann, T., Bathe, B. 2005. L-Lysine. In: Handbook of Corynebacterium glutamicm.
L Eggelin, and M Bott, (eds). Taylor and Francis, Boca Raton FI, USA, pp. 465-488.
Kimura, E. 2003. Metabolic Enginering of Glutamate Production. Advances in Biochemical
Engineering/Biotechnology, 79, 37–57.

Production of Amino Acids by Fermentation !'%
Mueller, U., Huebner, S. 2003. Economic Aspects of Amino Acid Production. Advances in
Biochemical Engineering/Biotechnology, 79, 137–170.
Pfefferle, W., Mockel, B., Bathe, B., Marx, A. 2003. Advances in Biochemical Engineering/
Biotechnology, 79, 59–112.
Sano, K. 1994. Host – Vector Systems for Amino Acid-Producing Coryneform Bacteria.
Improvement of Useful Enzymes by Protein Engineering. In: Recombinant Microbes for
Industrial and Agricultural Applications. Y Murooka, T. Imanaka, (eds). Marcel and Dekker,
New York, USA. pp. 485-507.

!'& Modern Industrial Microbiology and Biotechnology
22.1 RATIONALE FOR USE OF ENZYMES FROM
MICROORGANISMS
Enzymes are organic compounds which catalyze all the chemical reactions of living
things – plants, animals and microorganisms. They contain mainly protein; some of them
however contain non-protein components, prosthetic groups. When excreted or
extracted from the producing organism they are capable of acting independently of their
source. It is this property of independent action which drew early attention to their
industrial use.
All enzymes have infrastructural backbones of protein. In some enzymes only proteins
exist, while in others, covalently attached carbohydrate groups may be present; often
these carbohydrate groups may play no part in the catalytic activity of the enzyme,
though they may contribute to the stability and solubility of the enzyme. Metal ions
known as co-factors and low molecular weight organic compounds, known as co-
enzymes may also be present. Co-factors and co-enzymes are important for the stability
and activity of the enzyme. They have a tendency to be detached and it is important to
provide conditions which ensure their retention.
Most industrial enzymes are obtainable from microorganisms. The advantages of
using microorganisms are numerous, in contrast with their production from plants (e.g.
malt diastase) and animals (e.g. pepsin) and are as follows:
(a) Plants and animals grow slowly in comparison with microorganisms;
(b) Enzymes form only small portions of the total plant or animal and large tracts of
land as well as huge numbers of animals would be necessary for substantial
productions. These limitations make plant and animal enzymes expensive.
Microbial enzymes on the other hand are not subject to the above constraints and
may be produced at will in any desired amount.
(c) By far the greatest attraction for the production of microbial enzymes, however, is
the great diversity of enzymes which reflects the diversity of microbial types in
Biocatalysts: Immobilized
Enzymes and Immobilized
Cells
22
+0)26-4

Biocatalysts: Immobilized Enzymes and Immobilized Cells !''
nature. Thus largely, though not entirely, because of the widely varying
environmental conditions in nature, microbial enzymes have been isolated which
operate under extreme environmental conditions. For example microorganisms
produce amylases functioning at temperatures as high as 110°C and proteases
operating at pH values as high as 11 or as low as 3.
(d) Finally, following from greater understanding of the genetic basis for the control of
physiological function in micro-organisms it is now possible to manipulate
microorganisms to produce virtually any desired metabolic product, including
enzymes.
22.2 CLASSIFICATION OF ENZYMES
Based on catalyzed reactions, the enzyme committee (EC) of the International Union of
Biochemistry and Molecular Biology (IUBMB) recommended the classification of
enzymes into six groups. The nomenclature of enzymes is based on the number assigned
to these six major groups, and the sub-groups found within the major groups. Enzymes
are also known by long-standing common names which are also widely used.
The IUBMB committee also defines subclasses and sub-subclasses. Each enzyme is
assigned an EC (Enzyme Commission) number. For example, the EC number of catalase
is EC 1.11.1.6. The first digit indicates that the enzyme belongs to oxidoreductase (class
1). Subsequent digits represent subclasses and sub-subclasses. Thus the enzyme rennet
used in cheese manufacture and also known as chymosin, has the number of EC 5.3.1.5.
The six major EC groups are as follows.
1. Oxidoreductases catalyze a variety of oxidation-reduction reactions. Common names
include dehydrogenase, oxidase, reductase and catalase.
2. Transferases catalyze transfers of groups (acetyl, methyl, phosphate, etc.). Common
names include acetyltransferase, methylase, protein kinase, and polymerase. The first
three subclasses play major roles in the regulation of cellular processes.
3. Hydrolases catalyze hydrolysis reactions where a molecule is split into two or more
smaller molecules by the addition of water. Some examples are:
Proteases: Proteases split protein molecules. They are further classified by their optimum
pH as acid, alkaline or neutral. They may also be classified on the basis of their active
centers into the following:
(i) Serine proteases: These have a residue in their active center and are specifically
inhibited by diisopropyl phosphofluoridate and other organophosphorus
derivates.
(ii) Thiol proteases: The activity of these depends on the presence of an intact-SH group
in their active center. They are specifically inhibited by thiol reagents such as
heavy metal ions and their derivatives, as well as alkylating and oxidizing agents.
(iii) Metal proteases: These depend on the presence of more of less tightly bound divalent
cations for their activity.
(iv) Acid proteases: Acid proteases contain one or more side chain carboxyl groups in
their active center.
