Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

Unique Properties of Fluorine and Their Relevance 21
of C – F · · · C = O contacts with the F · · · C distance below the sum of the van der Waals radii
and the C – F · · · C = O torsion angle toward 90 ° . A similar but intramolecular orthogonal
polar interaction between a CF
3
groups and a spatially close amide C = O group, which
plays a role in the folding of an extended indole molecule, has recently been reported
[111] .
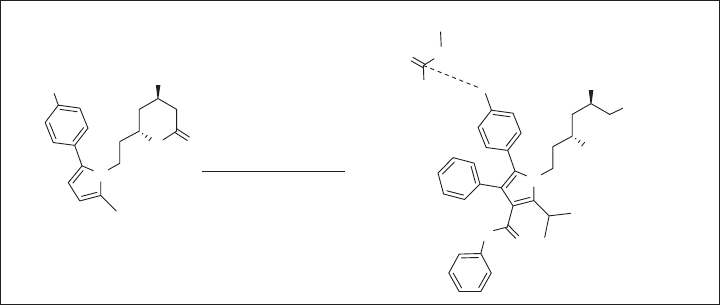
Figure 1.15 illustrates an example of the polar interaction of a C – F bond with the
guanidinium carbon of the Arg residue in an enzyme, revealed by the X - ray crystal struc-
ture of atorvastatin - HMG - CoA reductase (HMG - CoA = 3 - hydroxy - 3 - methylglutaryl -
coenzyme A; the rate - determining enzyme in the biosynthesis of cholesterol). As mentioned
above (see section 1.1 ), atorvastatin is the best - selling cholesterol - lowering drug, which
binds to HMG - CoA reductase tightly (IC
50
= 8 nM). In the early stage of the drug discov-
ery, a series of phenylpyrrole - hydroxylactones were evaluated for their inhibitory activity
against HMG - CoA reductase, as shown in Figure 1.15 a. Then, the SAR study on the
4 - substituted phenyl analogues identifi ed that the 4 - fl uorophenyl analogue (R = F) was
the most active; that is, fl uorine was better than hydrogen, hydroxyl or methoxy group
at this position [112] . This fi nding eventually led to the discovery of atorvastatin. The
X - ray crystal structure of atorvastatin – HMG - CoA reductase [113] shows a strong
C – F · · · C(NH
2
)( = NH) polar interaction between the 4 - fl uorophenyl moiety and the
Arg590 residue of the enzyme with a very short F · · · C distance (2.9 Å ), as illustrated in
Figure 1.15 b [101] .
Several other examples of similar polar interactions between a C – F bond in a ligand
and the guanidinium ion moiety of an Arg residue in protein have been found in PDB.
However, no linear C – F · · · H – N interactions are identifi ed, refl ecting the poor hydrogen -
bond accepting ability of a C – F bond. Instead of hydrogen bonding, a C – F bond is found
to orient either parallel or orthogonal to the plane of the guanidinium ion moiety, bearing
a delocalized positive charge. These examples confi rm the fl uorophilic character of the
guanidinyl side - chain of an Arg residue.
NH
NH
2
HN
2.9 Å
F
H
OH
OMe
Cl
IC
50
(µM)
R
2.8
13
6.3
28
3.2
•1/2Ca
2+
Arg590
(a) (b)
N
F
HN
O
OH
OH
COO
–
N
R
O
OH
O
Figure 1.15 (a) In vitro activity of HMG - CoA reductase inhibitors. (b) Orthogonal polar
interaction of the fl uorine substituent of atorvastatin with Arg590 in HMG - CoA reductase.
22 Fluorine in Medicinal Chemistry and Chemical Biology
1.1.8 Isostere
A variety of natural and synthetic peptides exhibit biological activities, which can be
developed as pharmaceutical drugs or biochemical agents. However, those peptides con-
sisting of amide linkages are, in most cases, easily deactivated through cleavage of key
amide bonds by hydrolytic enzymes. Accordingly, it is very useful if a key amide bond is
replaced with noncleavable bond, keeping the characteristics of the amide functionality.
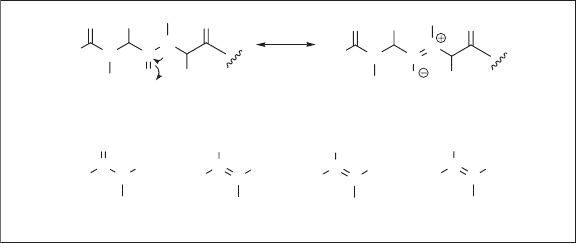
An amide linkage in a peptide such as 29a has an imidate - like zwitterioninc resonance
structure 29b . Since the contribution of this resonance structure is signifi cant, free rotation
around the C – N bond is partially restricted because of the substantial double - bond char-
acter of the C – N bond (see Figure 1.16 ). Accordingly, it might be possible to replace an
amide linkage with its isostere (i.e., a molecule having the same number of atoms as well
as valence electrons [114] ), regarded as a “ peptide isostere. ”
The fi rst and simplest attempt at the “ peptide isostere ” was made by replacing an amide
bond of enkephalin with a trans - olefi n unit [115] , but it did not give a desirable effect.
However, computational analysis of a model amide, N - methylacetamide ( 30b ) and its iso-
steres, by semiempirical molecular orbital calculation revealed that fl uoroolefi n 30c resem-
bled 30a much more closely than 30b [116] . To confi rm this fi nding based on a semiempirical
method, the ab initio analysis of 30a , ( E ) - 2 - butene ( 30b ), ( Z ) - 2 - fl uoro - 2 - butene ( 30c ) and
( Z ) - 1,1,1 - trifl uoro - 2 - methyl - 2 - butene ( 30d ) was carried out using the Gaussian 03 program
(B3LYP/6 – 311++G * * ) [117] by one of the authors (T. Y.), which gave electrostatic poten-
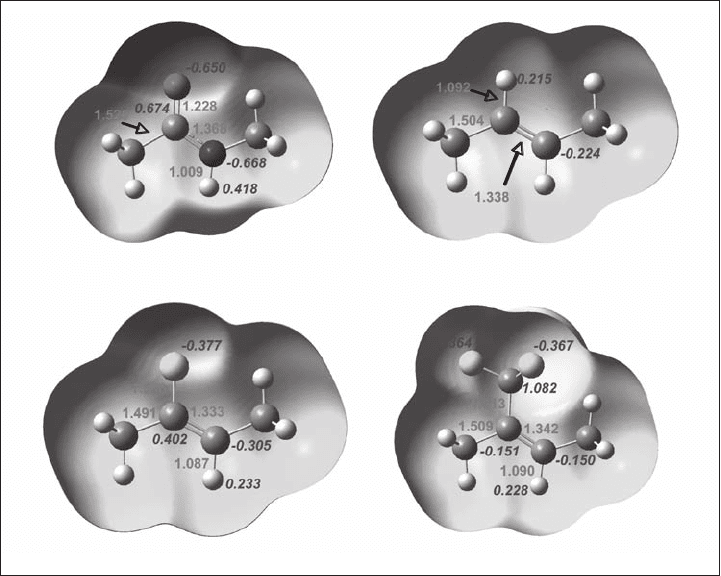
tials of these compounds. The results are illustrated in Figure 1.17 .
As readily anticipated, 30a has a very negative oxygen, a highly positive carbonyl
carbon, a highly negative nitrogen, and a very positive NH hydrogen. In sharp contrast,
30b has a nonpolarized negative C = C bond and only weakly positive CH hydrogen. Thus,
it is apparent that 30b does not mimic 30a electronically. In contrast, 30c has an appro-
priately polarized C = C double bond, a negative fl uorine atom in place of the oxygen atom
of amide 30a , and a positive CH in place of the NH moiety of amide 30a . Thus, 30b
indeed mimics 30a electronically. Finally, 30d has a nonpolarized C = C bond, a modestly
positive CH hydrogen, and three negative fl uorine atoms. Thus, 30d mimics 30a electroni-
cally to some extent, although sterically the CF
3
group is much bulkier than an oxygen
atom.
R
1
O
N
HO
C
R
3
N
O
HH
R
2
••
R
1
O
N
HO
C
R
3
N
OR
2
29a 29b
H
3
C
C
O
N
CH
3
H
30a
H
3
C
C
H
C
CH
3
H
30b
H
3
C
C
F
C
CH
3
H
30c
H
3
C
C
CF
3
C
CH
3
H
30d
Figure 1.16 Resonance structures of amide 29 , amide 30a , and its isosteres 30b – d .
Unique Properties of Fluorine and Their Relevance 23
30b30a
30d30c
Figure 1.17 Electrostatic potential of the model compounds 30a to 30d (color alteration
from red to blue describes the shift of the electronically rich to defi cient circumstance). See
color plate 1.17.
A fl uoroolefi n isostere was successfully introduced to a physiologically important
neuropeptide, Substance P ( 31 ) [118] . As Figure 1.18 shows, the ( E ) - fl uoroethene isostere
replaced the peptide linkage between the Phe
8
- Gly
9
residues of 31 to give Substance P
analogue 32a and its epimer 32b [119] . The neurokinin - 1 receptor binding assay of 32a
disclosed that 32a was almost as potent as the original peptide 31 . On the other hand, its
epimer 32b was 10 times less potent than 32a , as anticipated.
Another fl uoroolefi n isostere, ( E ) - trifl uoromethylethene, was also introduced to
antibiotic Gramicidin S ( 33a ), replacing the peptide linkage between Leu and Phe (two
sites) to give an analogue 33b , as illustrated in Figure 1.19 [120] . Variable - temperature
NMR measurements of 33a and 33b indicated that the NH(Leu) and NH(Val) protons were
forming intramolecular hydrogen bonds. In addition, NOESY experiments of 33a and 33b
showed interstrand NOEs between NH(Leu) and NH(Val) as well as NH(Leu) and H
α
(Orn)
in both compounds. Moreover, X - ray crystallographic analysis of 33c confi rmed the pres-
ence of a pair of intramolecular hydrogen bonds between NH(Leu) and C = O(Val) (1.96 Å
and 2.00 Å ). However, the replacement of the Leu - Phe amide linkage with the bulky ( E ) -
trifl uoromethylethene isostere caused a 70 ° twist in the plane of the C = C double bond
24 Fluorine in Medicinal Chemistry and Chemical Biology
N
H
Leu-Met-NH
2
Arg-Pro-Lys-Pro-Gln-Gln-Phe
R
1
R
2
F
O
N
H
H
N
Leu-Met-NH
2
Arg-Pro-Lys-Pro-Gln-Gln-Phe
H
O
O
Ph
IC
50
2 nM
20 nM
1.3 nM
31 Substance P
32a R
1
= Bn, R
2
= H
32b R
1
= H, R
2
= Bn
Figure 1.18 Substance P ( 31 ) and its fl uoroolefi n dipeptide isosteres ( 32a, b ).
O
N
H
H
N
O
N
H
H
N
O
(CH
2
)
3
NH
2
O
Ph
O
H
N
N
H
O
H
N
N
H
O
NH
2
(CH
2
)
3
O
Ph
N
O
N
O
O
N
H
H
N
O
N
H
O
(CH
2
)
3
NHR
CF
3
Ph
O
H
N
N
H
O
H
N
O
RHN(CH
2
)
3
CF
3
Ph
N
O
N
O
H
H
33a
33b (R = H)
33c
(R = Cbz)
Figure 1.19 Gramicidin S ( 33a ) and its CF
3
- containing isostere ( 33b ).
compared with the amide moiety in the original β - turn structure of 33a . This is very likely
attributable to the bulkiness of the CF
3
groups, which are diffi cult to be accommodated
inside the β - turn hairpin structure. In spite of some conformational difference, 33b was
found to possess high antibiotic activity against Bacillus subtilis equivalent to that of 33a
[120] . The use of the ( E ) - trifl uoromethylethene peptide isostere as β - turn promoter
and peptide mimetics in general has also been studied [121] . A fairly large dipole moment
of this isostere ( µ = 2.3 D) appears to play a key role in the improved mimicry of the
electrostatic potential surface of the amide linkage (dipole moment of the amide unit:
µ = 3.6 D) [120] .
Although fl uoroethene and trifl uoroethene peptide isosteres were successfully
employed as nonhydrolyzable amide substitutes, more recently trifl uoroethylamines have
emerged as highly promising peptide isosteres. The trifl uoroethylamine isostere has been
studied for partially modifi ed retro - and retro - inverso Ψ [NHCH(CF
3
)]Gly peptides [122,
123] . Furthermore, it has been demonstrated that the CF
3
group of the trifl uoroethylamine

Unique Properties of Fluorine and Their Relevance 25
isostere can function as the carbonyl group of an amide to provide a metabolically stable,
nonbasic amine that has excellent hydrogen - bonding capability due to the strong inductive
effect of the CF
3
group [124, 125] . These unique characteristics of the trifl uoroethylamine
isostere have been exploited and quite successfully applied to the optimization of cathepsin
K inhibitors, which are promising antiresorptive agents for treatment of osteoporosis [124] .
As Figure 1.20 shows, trifl uoroethylamine analogue 34a exhibits 1000 time better potency
than the corresponding ethylamine analogue 34b . Pentafl uoroethylamine analogue 34c
possesses a somewhat reduced activity, but still in a comparable level to 34a . Tosylmethyl-
amine analogue 34d shows virtually the same activity as that of 34c . The docking study
using the cathepsin K crystal structure has revealed that 34a forms a critical hydrogen
bond with the carbonyl oxygen of Gly66, as anticipated. The fact that the CF
3
group of
this isostere is attached to an sp
3
carbon provides fl exibility in the orientation of the NH
bond to form hydrogen bonding in the most favorable manner [124] . Optimization of the
aromatic group of 34a led to the discovery of the exceptionally potent inhibitor 35a ,
exhibiting an IC
50
of 5 pM or less. The corresponding amide 35 is also extremely potent
(IC
50
= 0.015 nM) but is metabolically labile. Thus, 35a has been identifi ed as the most
potent and promising drug candidate in this study [124] .
It has been shown that 2,3 - difl uorotoluene ( 36 ) serves as a nonpolar shape mimic of
thymine ( 37 ) and difl uorotoluene deoxyriboside ( 38 ) is, surprisingly, an excellent nonpolar
nucleoside isostere of thymidine ( 39 ) (see Fig. 1.21 ) [126, 127] . Thus, the nucleotide of
36 was incorporated into DNA by several high - fi delity DNA polymerases [126, 128] ,
35a (~0.005 nM)
N
HN
N
H
H
N
O
CN
N
H
H
N
R
Br
O
CN
34a (R = CF
3
: 0.9 nM)
34b (R = CH
3
: 988 nM)
34c (R = CF
3
CF
2
: 2.4 nM)
34d (R = p-CH
3
-C
6
H
4
SO
2
: 2.5 nM)
CF
3
35 (0.015 nM)
N
HN
N
H
H
N
O
CN
O
Figure 1.20 Cathepsin K inhibitors 34 and 35 , bearing trifl uoroethylamine isosteres. Numbers
in parentheses are IC
50
values.
O
HO
HO
F
Me
F
3736
N
H
40
N
N
N
NH
2
N
H
NH
O
Me
O
F
Me
F
38
O
HO
HO
39
N
NH
O
Me
O
Figure 1.21 Difl uorotoluene ( 36 ), difl uorotoluene deoxyriboside ( 38 ), thymine ( 37 ), thymi-
dine ( 39 ) and adenine ( 40 ).
26 Fluorine in Medicinal Chemistry and Chemical Biology
forming a positional pair with adenine ( 40 ) in place of 37 without perturbing the double
helix structure, which was confi rmed by X - ray crystallography (see Fig. 1.21 ) [127] . It
has also been shown that difl uorotoluene does not form appreciable hydrogen bonds with
adenine, and hence it is nonselective in base pairing and destabilizing DNA in the absence
of DNA polymerases [128] . The probability of thymidine triphosphate choosing a wrong
partner is less than 0.1% and that of the triphosphate of 38 is less than 0.3% [126] . Thus,
the results clearly indicate that 36 is virtually a perfect shape mimic of 37 for DNA
polymerases.
Since these surprising fi ndings, fi rst made in 1997, challenge the Watson – Click base -
pairing principle in DNA (although the difl uorotoluene isostere is only a minor part of
DNA), there has been a debate on the interpretation of the results, including the polarity
of 36 and possible F · · · HN as well as CH · · · N hydrogen bonding interactions [129] . However,
the X - ray crystallographic data [127] as well as extensive computational analysis [130]
unambiguously confi rmed that 36 is indeed a nonpolar thymine isostere. Thus, these
fi ndings have opened a new avenue of research on a variety of nonpolar nucleoside
isosteres [128] .
1.1.9 Difl uoromethylene as Isopolar Mimic of the Oxygen Component of
P – O – C Linkage
Difl uoromethylene has been recognized as an isopolar mimic of the oxygen component
of P – O – C or P – O – P linkage in phosphates, which can be used to generate nonhydrolyz-
able phosphate analogues of nucleotides, enzyme substrates, and enzyme inhibitors [131 –
137] . For example, a difl uoromethylene linkage was successfully introduced to a
protein - tyrosine phosphatase (PTP) inhibitor of insulin receptor dephosphorylation [132]
(see Figure 1.22 ). A comparison of a hexamer peptide inhibitor bearing a phosphonometh-
ylphenylalanine (Pmp) residue ( 41a ) with that having a phosphonodifl uoromethylphenyl-
alanine (F
2
Pmp) residue ( 41b ) revealed that 41b was 1000 times more potent than 41a ,
retaining high affi nity for the SH2 domain of PTP [132] . The marked difference in potency
observed for 41a and 41b can be ascribed to the fact that the difl uoromethylene group
increases the acidity of the phosphonic acid moiety and maintains appropriate polarity.
41a (X = CH
2
: 100 µM)
41b (X = CF
2
: 0.10 µM)
O
H
N
N
H
O
H
N
N
H
O
H
N
AcHN
CO
2
H
O O
CO
2
H
CO
2
H
X
O
P
O
OH
OH
X
P
O
R
OH
OH
pKa
6.4 (X = O)
7.6 (X = CH
2
)
6.5 (X = CHF)
5.4 (X = CF
2
)
Figure 1.22 Nonhydrolyzable phosphate mimics 41 and their estimated pKa values. Numbers
within the parentheses are IC
50
values.
Unique Properties of Fluorine and Their Relevance 27
A similar incorporation of a P – CF
2
unit as a P – O bond surrogate to sphingomyelin
was reported [134] for the development of nonhydrolyzable inhibitors of sphingomyelin-
ase (SMase), which cleaves sphingomyelin to release ceramide. As Figure 1.23 shows,
difl uoromethylene analogue 42b is twice as potent as methylene analogue 42a in the
inhibition of SMase from B. cereus [134] .
Norcarbovir triphosphate ( 43d ) is a potent inhibitor of HIV reverse transcriptase,
comparable to AZT and carvovir, but is amenable to enzymatic hydrolysis in vivo [135] .
Thus, methylenediphosphonate, fl uoromethylenediphosphonate, and difl uoromethylenedi-
phosphonate analogues of 43d were synthesized to block the enzymatic hydrolysis and
evaluated for their potency and stability in human fetal blood serum [135] . As Figure 1.23
shows, the methylenediphosphonate analogue ( 43a ) is inactive, and the fl uoromethylene-
diphosphonate analogue ( 43b ) shows only a moderate potency. Difl uoromethylenediphos-
phonate analogue 43c is the most active among these nonhydrolyzable analogues with IC
50
of 5.8 µ M, which is 10 times weaker than that of 43d . Nevertheless, 43c possesses > 40
times longer half - life (45 h) than the natural enantiomer of 43d (65 min) and > 500 times
more stable than AZT triphosphate (5 min) [135] .
The fl uoromethylene and difl uoromethylene linkages have also been incorporated
into an aspartyl phosphate, providing the fi rst synthetic inhibitors of aspartate semialde-
hyde dehydrogenase [136] as well as lysophosphatidic acid analogues, which increased
the half - lives of analogues in cell culture [137] .
The use of a difl uoromethylene unit as a surrogate of a carbonyl group is another
logical extension. In fact, difl uoromethylene analogues of the inhibitors of the rotamase
activity of FK506 binding protein 12 (FKBP12), which catalyzes cis – trans isomerization
of a peptidyl - prolyl bond, have been investigated [138] . As Table 1.8 shows, the K
i
values
of difl uoromethylene analogues, 44a and 44d , for the FKBP12 inhibition (rotamase activ-
ity) are comparable to or better than those of the carbonyl counterparts, 44b and 44e . It
should be noted that the corresponding simple methylene analogues, 44c and 44f do not
show any appreciable activity, which suggests that the gem - difl uoromethylene group par-
ticipates in some specifi c interactions with the protein. After optimization of the proline
and its ester moieties, the most potent inhibitor 45 ( K
i
19 nM) was developed. The X - ray
crystal structure of the 45 – FKBP12 complex strongly suggests that the two fl uorine atoms
participate in the moderate - to - weak hydrogen bonding interactions with the Phe36 phenyl
ring as well as Tyr26 hydroxyl group.
42a (X = CH
2
: 120 µM)
42b (X = CF
2
: 57 µM)
C
5
H
11
C
7
H
15
NH
X
P
O
NMe
3
O
OH
OO
N
NH
N
N
O
NH
2
P
Y
O
P
P
O O O
HO
HO
HO HO
O
43a (Y = CH
2
: >100 µM)
43b (Y = CHF: 34.8 µM)
43c (Y = CF
2
: 5.8 µM)
43d (Y = O: 0.5 µM)
Figure 1.23 SMase inhibitors ( 42 ) and FKBP 12 rotamase inhibitors ( 43 ). Numbers within
the parentheses are IC
50
values.
28 Fluorine in Medicinal Chemistry and Chemical Biology
1.1.10 High Electrophilicity of Fluoroalkyl Carbonyl Moieties
Successive substitution of hydrogen atoms in acetone with fl uorine atoms clearly lowers
the frontier orbital energy levels by 0.2 – 0.6 eV on the basis of ab initio computation (see
Table 1.9 ). It is worthy of note that the positive charge at the carbonyl carbon and the
negative charge at the oxygen atoms in these ketones, decrease as the number of fl uorine
atoms increases. The validity of the computational analysis is confi rmed experimentally
by the
13
C NMR chemical shifts of these carbons, which decrease from 206.58 ppm for
acetone to 172.83 ppm for hexafl uoroacetone, in good agreement with the carbonyl carbon
charges, as shown in Table 1.9 .
The substantial decrease in the HOMO (highest occupied molecular orbital) energy
level of trifl uoroacetophenone ( 46b ), compared with acetophenone ( 46a ) was observed
[139] by
13
C NMR when a 1:1 mixture of 46a and 46b was treated with BF
3
· O E t
2
. Thus,
a 16.7 ppm downfi eld shift took place only for the carbonyl carbon atom of 46a , showing
the coordination of the carbonyl group to the Lewis acid, while no change in the
13
C
chemical shift was observed for 46b (see Scheme 1.3 ). This marked difference in reactivity
is attributed to the substantially decreased basicity of the carbonyl oxygen in 46b caused
by the strong inductive effect of the trifl uoromethyl moiety. This selectivity was demon-
strated in the highly chemoselective reduction of 46a and 46b under two different reaction
conditions, as illustrated in Scheme 1.3 . Thus, the reduction of a 1:1 mixture of 46a and
46b with tributyltin hydride gave 1 - phenyl - 2,2,2 - trifl uoroethanol ( 48b ) as the sole product
in 40% yield, while the same reaction in the presence of one equivalent of BF
3
· OEt
2
at
− 78 ° C afforded 1 - phenylethanol ( 48a ) exclusively in 82% yield.
Table 1.8 FKBP 12 rotamase inhibition [138]
H
3
CO OCH
3
OCH
3
X
N
O
O
O
Y
H
3
CO OCH
3
OCH
3
N
O
F
F
O
O
N
44 45
Compound X Y
K
i
( µ M)
44a F
2
N 0.872
44b O N 4.00
44c H
2
N
–
a
44d F
2
CH 1.30
44e O CH 2.20
44f H
2
CH
–
a
45 0.019
a
No appreciable inhibition at 10 µ M.
Unique Properties of Fluorine and Their Relevance 29
Table 1.9 Calculated HOMO and LUMO levels of fl uorinated acetones
a
Compound Energy Level (eV) Charges
13
C NMR chemical
shift of C = O
HOMO LUMO C = O C = O
CH
3
C(O)CH
3
− 7.054 − 0.784
0.573
− 0.553
206.58
b
CH
2
FC(O)CH
3
− 7.507 − 1.247
0.544
− 0.547
205.62
c
CHF
2
C(O)CH
3
− 7.911 − 1.873
0.518
− 0.513
197.38
c
CF
3
C(O)CH
3
− 8.278 − 2.098
0.505
− 0.486
189.33
c
CF
3
C(O)CF
3
− 9.384 − 3.476
0.421
− 0.419
172.83
b
a
Computation was carried out by one of the authors (T.Y.) using the Gaussian 03W at the B3LYP/6 – 311++G * * level.
b
Ref. [132] .
c
Ref. [140] .
Ph
O
CH
3
Ph
O
CF
3
Ph
OH
CH
3
Ph
OH
CF
3
46a 46b
48a 48b
+
+
conditions
n-Bu
3
SnH (1.0 equiv)/
C
3
H
7
CN, rt, 5 h
n-Bu
3
SnH, BF
3
·OEt
2
(1.0 equiv)/
CH
2
Cl
2
, –78 °C, 1 h
40%
82%
0%
0%
Ph
O
CH
3
Ph
O
CF
3
47a 46b
+
BF
3
BF
3
·OEt
2
Scheme 1.3 Reactions of 46a and 46b with BF
3
· OEt
2
and n - Bu
3
SnH.
1.1.11 Use of Difl uoromethyl and Trifl uoromethyl Ketones as Transition State
Analogues in Enzyme Inhibition
Tetrahedral transition state analogues of ester and amide substrates are known to function
as effi cient enzyme inhibitors of hydrolytic enzymes such as serine and aspartyl proteases
as well as metalloproteinases (see Fig. 1.24 ) [141 – 144] . Although ketals of alkyl or aryl
ketones are usually not stable, those of difl uroalkyl or trifl uoromethyl ketones have con-
siderable stability, as exemplifi ed by their facile formations of the corresponding stable
hydrates [145, 146] . Therefore, substrate analogues, containing difl uoroalkyl or trifl uoro-
methyl ketone moiety in appropriate positions, have been studied as effective transition
state inhibitors of hydrolytic enzymes [141, 147 – 150] .
30 Fluorine in Medicinal Chemistry and Chemical Biology
For example, the rather simple difl uoroalkyl ketone 49c and trifl uoromethyl ketone
49b were designed as inhibitors of acetylcholinesterase (AchE), a serine esterase [151] .
As Figure 1.25 shows, these fl uoroketones exhibit excellent AchE inhibitory activities with
nanomolar level K
i
values, and 49c possesses 200,000 times higher potency ( K
i
= 1.6 nM)
than the corresponding alkyl ketone 49a [151] . This remarkable result can be readily
rationalized by taking into account the strong electrophilicity of the carbonyl group of
difl uoroalkyl and trifl uoromethyl ketones, described in the preceding section.
In a similar manner, a variety of transition state analogues bearing a trifl uoroacetyl
moiety as the key functional group have been designed and synthesized as the inhibitors
of human neutrophil elastase [152, 153] , human cytomegalovirus protease [154, 155] ,
human leukocyte elastase [156, 157] , and other enzymes. Some of these inhibitors exhibit
extremely high potency. For example, 51 has a K
i
of 3.7 pM against AchE [158] and 50
has an IC
50
of 0.88 nM against insect juvenile hormone esterase [159] (see Figure 1.26 ).
Difl uoroalkyl ketones have also been successfully employed as the key structure of
renin inhibitors. Renin is an aspartyl protease and transforms angiotensinogen (452 amino
acid residues for human) to angiotensin I, which is further cleaved by angiotensin -
converting enzyme (ACE) to angiotensin II, which exerts a vasoconstricting effect [160] .
Accordingly, renin inhibitors have been extensively studied for their use in controlling
hypertension. Although human angiotensinogen has 452 amino acid residues, the fi rst
dodecapeptide sequence is the most important for its activity (see Figure 1.27 ) [160] .
Enzyme-Ser-OH
Enzyme-Ser
O
H
N
R
FF
O
X
H
N
(AA)
n
R
O
NH
(AA')
m
Enzyme-Ser-OH
H
N
(AA)
n
R
NH
(AA')
m
OH
O
H
N
(AA)
n
R
NH
2
(AA')
m
OH
+
Enzyme-Ser
Enzyme-Ser-OH
H
N
R
OOH
X
F F
Tetrahedral transition state for hydrolysis
Stabel tetrahedral intermediate
Figure 1.24 Mechanism of peptide hydrolysis by a serine protease and enzyme inhibition
by forming stable tetrahedral intermediate.
CX
3
O
YY
49a (X = H, Y = H) 310,000
1.6
49b (X = F, Y = H)
49c (X = H, Y = F)
16
K
i
(nM)
N
O
O
Acetylcholine
Figure 1.25 Transition state inhibitors of AchE based on difl uoroalkyl and trifl uoromethyl
ketone substrate analogues.
