Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

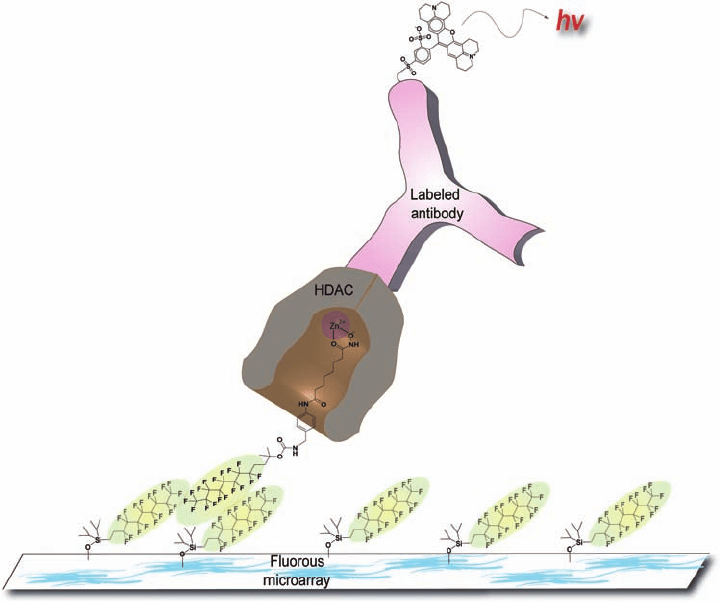
Plate 16.2 Fluorous small-molecule microarrays. Small-molecule histone deacetylase
(HDAC) binders are noncovalently immobilized onto a glass slide coated with fl uorocarbon
compounds. An antibody labeled with a fl uorescent dye recognizes HDAC proteins.
(Source: Vegas, A. J., Brander, J. E., Tang, W. et al., Fluorous-based small-molecule microarrays
for the discovery of histone deacetylase inhibitors, Angew. Chem. Int. Ed. (2007), 46, 7960–
7964. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.)
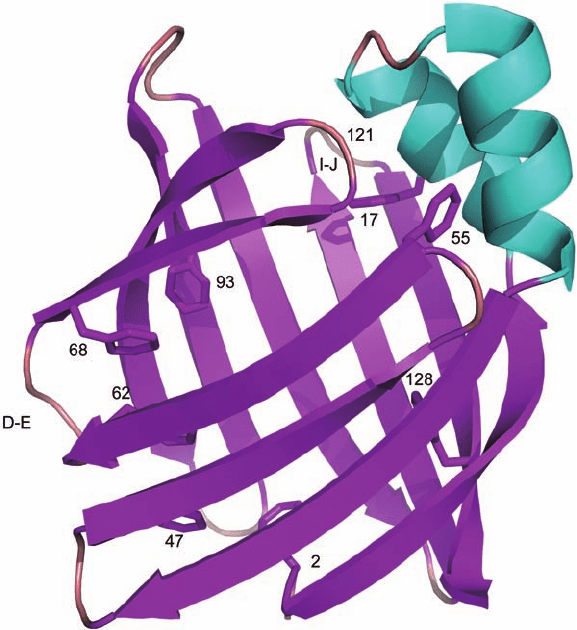
Plate 16.3 The structure of apo-IFABP (PDB code: 1IFB). Eight Phe residues (shown in stick
representation), the D
–E and I–J regions, and location of G121 are indicated by labels. The
structure was generated using MacPyMOL (DeLano Scientifi c LLC, Palo Alto, CA, U.S.A.).
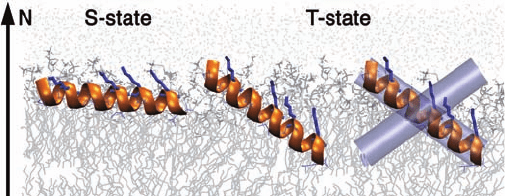
Plate 16.4 Illustration of the S-state and T-state of the antimicrobial peptide PGLa in a DMPC
membrane. At low concentrations, PGLa adopts an S-state. At high concentrations, PGLa
assumes a tilted T-state. PGLa forms a dimer in the T-state (shown in purple).
(Source: Glaser, R. W., Sachse, C., Durr, U. H. N. et al., Concentration-dependent realignment
of the antimicrobial peptide PGLa in lipid membranes observed by solid-state F-19-NMR,
Biophys. J. (2005) 88, 3392–3397. Reproduced with permission from the Biophysical
Society.)
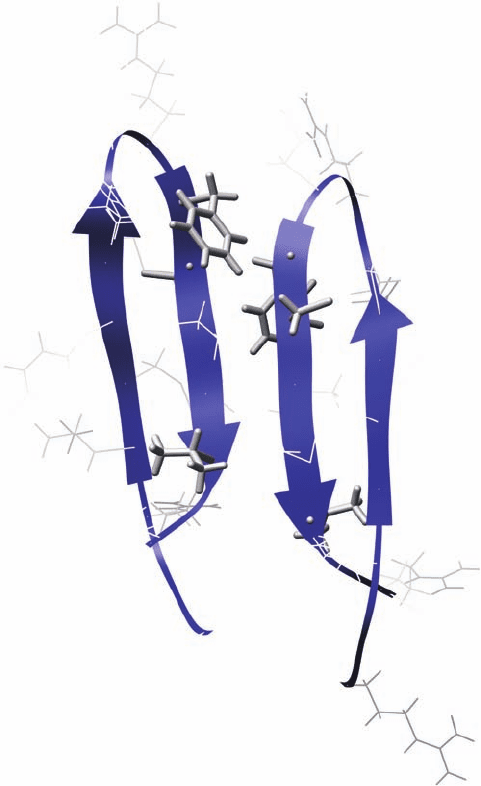
Plate 16.5 The dimer structure of PG-1 (PDB code: 1ZY6) in POPC bilayers as determined
by solid-state NMR. Isotopically labeled amino acids are shown in stick format; Phe12 was
labeled with
19
F, Cys12 with
15
N and
13
C, and Val16 with
13
C. Inter- and intramolecular dis-
tances were used to determine the relative position of the two monomers.
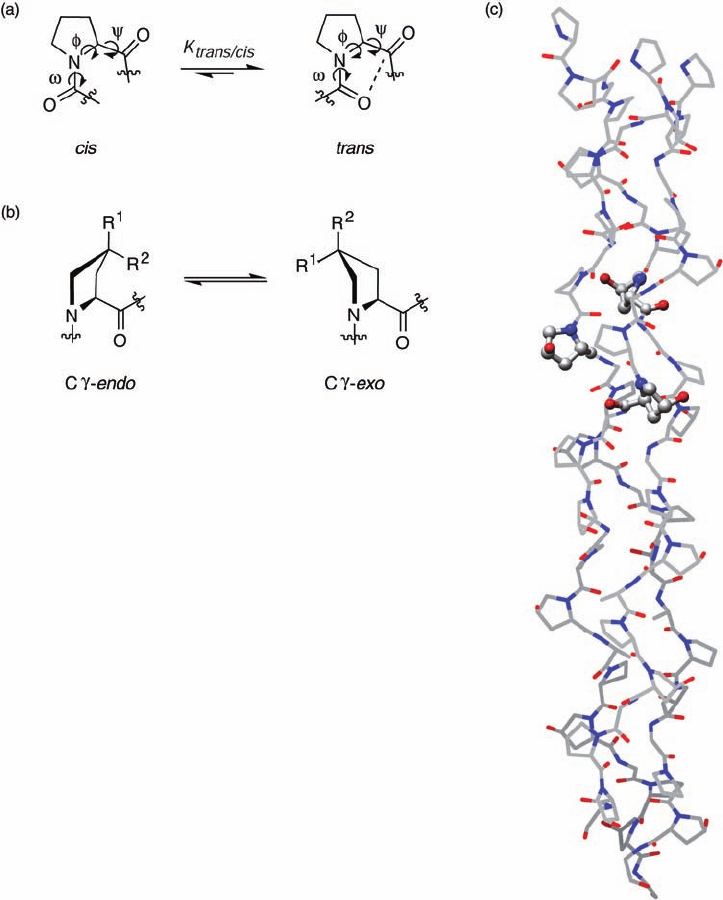
Plate 16.7 Fluorinated prolines in collagen. (a) The trans/cis isomerization of amide bonds
and main-chain angles of proline residues. The n → π* interaction, depicted by a dashed line,
helps stabilize backbone dihedral angles. (b) Electron-withdrawing groups at Cγ infl uence the
ring conformation of proline residues through the “gauche effect.” The Cγ-endo pucker is
favored when R
1
= H and R
2
= F, OH, or H. The Cγ-exo pucker is favored when R
1
= OH or
F, and R
2
= H. Preorganization of the pyrrolidine ring contributes to the thermal stability of
collagen and mimics. Flp: R
1
= H and R
2
= F; fl p: R
1
= F and R
2
= H; Hyp: R
1
= H and
R
2
= OH; hyp: R
1
= OH and R
2
= H. (c) Model structure of collagen (PDB code: 1CAG).
Three hydroxyprolines at Yaa positions from each peptide chain are shown in ball-and-stick
representation. Carbon = gray; Nitrogen = blue; Oxygen = red.
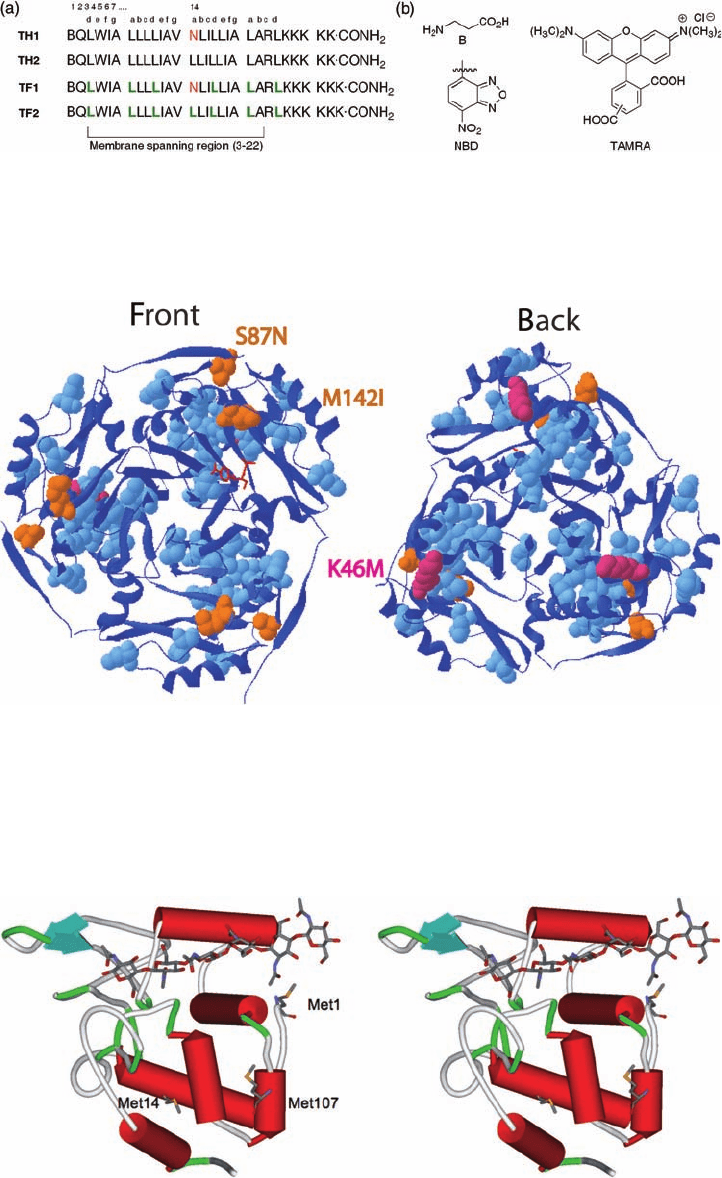
Plate 16.9 (a) Sequences of transmembrane peptides TH1, TF1, TH2, and TF2. Asn (N)
residues are assumed form interhelical hydrogen bonds in the core. L (green): hexafl uoroleu-
cine. (b) Structures of β-alanine, NBD (donor fl uorophore), and TAMRA (acceptor
fl uorophore).
Plate 16.12 Front and back views of the CAT trimer. The three stabilizing mutations in L2-A1
are S87N, M142I in orange, and K46M in pink. Trifl uorolecuines/leucines are colored blue
and chloramphenicol red.
(Source: Montclare, J. K., Tirrell, D. A., Evolving proteins of novel composition. Angew. Chem.
Int. Ed. (2006) 45, 4518–4521. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Repro-
duced with permission.)
Plate 17.3 Cartoon stereo diagram of the x-ray structure of wild-type bacteriophage lambda
lysozyme as solved for the (GlcNAc)
6
complex and showing the position of the three methio-
nine residues in the enzyme (PDB 1d9u) [32].
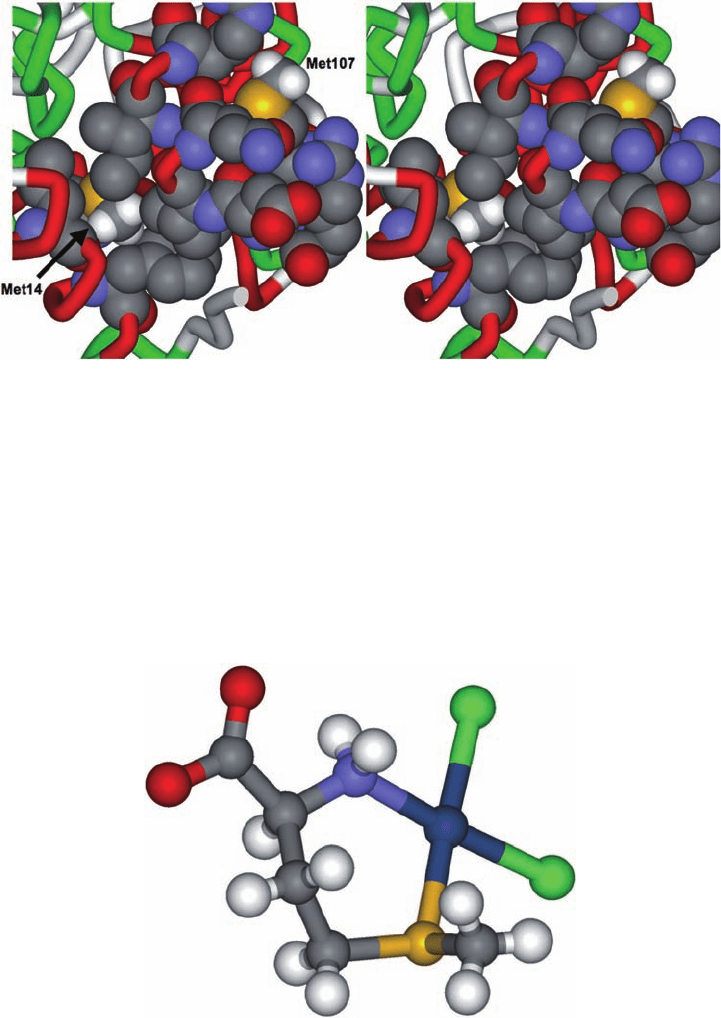
Plate 17.4 Close-up of the detailed interactions between Met14 and adjacent residues and
the location of Met107 (PDB 1d9u) [32].
Plate 17.7 Crystal structure of the methionine–PtCl
2
complex (coordinates from Wilson
et al. [37]).
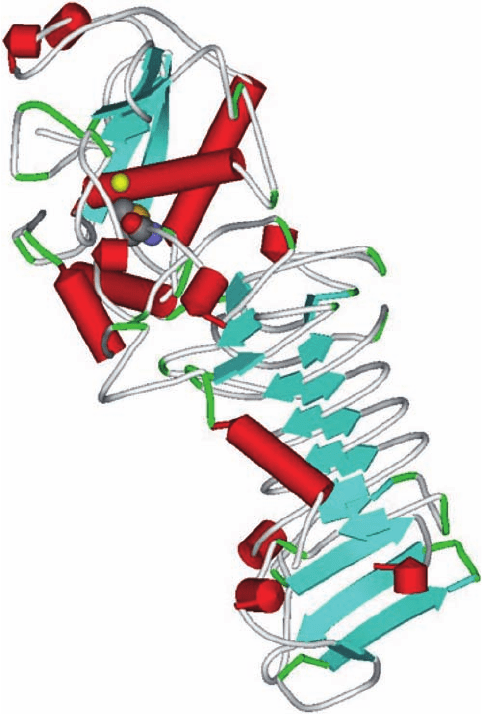
Plate 17.8 Ribbon diagram of the P. aeruginosa alkaline protease showing the active site
Zn
2+
and the invariant methionine residue (PDB 1kap. [38]).
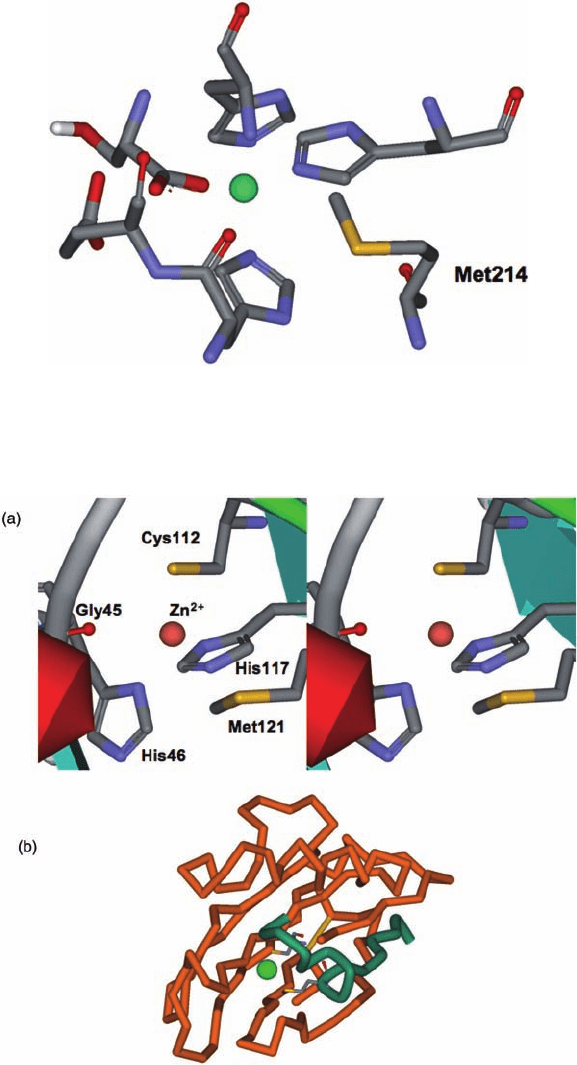
Plate 17.9 Close-up of the active-site region of P. aeruginosa alkaline protease showing Zn
2+
and protein ligands and the position of the thiomethyl group in close proximity to these side-
chains (PDB 1kap. [38]).
Plate 17.10 (a) Stereo view of the active-site structure of P. aeruginosa azurin (PDB 4azu)
[46]. (b) Tube diagram of the P. aeruginosa azurin showing the section of protein that was
contributed by intein ligation.
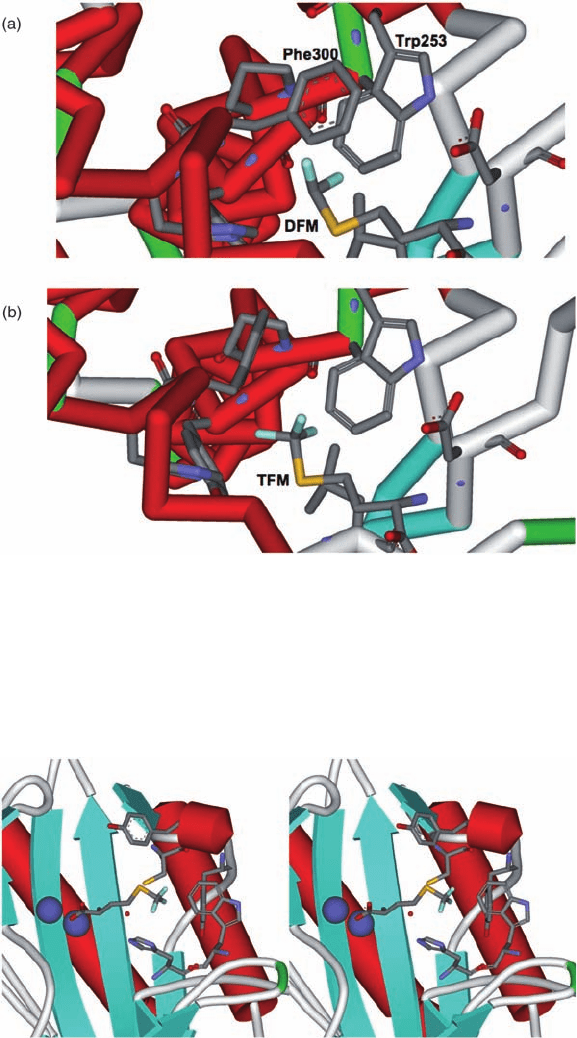
Plate 17.11 X-ray structures of (a) DFM (PDB 1pfv) [48] and (b) TFM (PDB 1pfw) [48]
complexes with Met-tRNA synthetase from E. coli.
Plate 17.12 Stereo view of the active-site interactions of TFM with E. coli methionine ami-
nopeptidase (PDB 1c22) [49].
