Nuclear Medicine Resources Manual
Подождите немного. Документ загружается.

CHAPTER 5. GUIDELINES FOR GENERAL IMAGING
400
(b) With caprylic acid (n-octanoic acid)
The following procedure is used:
—Dilute 2 mL of antiserum to 6 mL with 60mM acetate buffer, pH4.
—Adjust the pH to 4.8 using NaOH/HCl.
—Add 1 mL caprylic acid (= 136 mg) and stir continuously for 30 min at
room temperature.
—Centrifuge at 3000 rev./min for 45 min at 20ºC.
—Adjust the pH of the supernatant to 5.7 using NaOH.
—Dialyse against three changes of 15mM acetate buffer of pH5.7 to remove
the caprylic acid.
(c) Preparation with diethylaminoethyl (DEAE) cellulose chromatography
The following procedure is used:
—First prepare purified antibody using ammonium sulphate precipitation
as described in (a) above.
—Load the DEAE cellulose column with phosphate buffer of pH6.
—Add 2 mL of antibody solution in phosphate buffer of pH6.
—Run column with phosphate buffer of pH6 and collect 1 mL fractions.
—The fractions containing purified antibodies are located by UV
spectroscopy at 280nM.
Protocol 7: Direct iodination of protein using chloramine T
(a) Preparation of
125
I-T4 and
125
I-T3
The following procedure is used:
(1) Suspend 2 mg of T3 in a few millilitres of phosphate buffer of pH7.4 and
add (one molar) N.NaOH dropwise until the T3 is dissolved. Transfer to a
20 mL flask and make up to volume with phosphate buffer.
(2) For iodination, aliquot in 15 mL (1.5 mg) volumes and store at –20ºC, in
polypropylene vials.
(3) To a vial containing 15 mL of T3, add:
—20 mL 0.5M of phosphate buffer of pH7.4;
—10 mL (1 mCi) of sodium
125
I-iodine;
—10 mL of chloramine T solution in 50mM phosphate buffer of pH7.4.
(4) Mix for 20 s.
5.12. RADIOIMMUNOASSAY PROTOCOLS
401
(5) Add 10 mL (10 mg) of sodium metabisulphite in 50mM phosphate of
pH7.4 and 100 mL potassium iodide (10 mg/mL) containing 1% BSA.
(6) Vortex mix and count vial with contents.
(7) Transfer contents to column and count empty vial.
(8) The separation column is Sephadex G-25 Fine (approximately 2 g
Sephadex) in a 15 cm × 0.9 cm column. Equilibrate and elute with 50mM
NaHCO
3
of pH9.0 at a flow rate of 10–15 mL/hour.
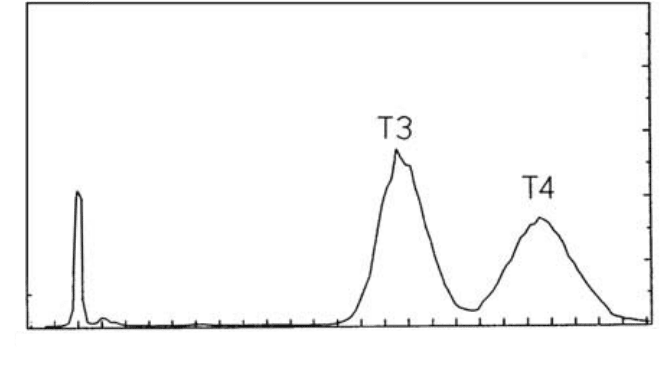
(9) Collect 10 min fractions. Count each fraction and plot the counts against
fraction number, to derive the chromatographic profile. Calculate the
proportion of radioactivity in each peak eluted (see the examples shown
in Fig. 5.3).
(10) Pool the desired fractions containing T3 and T4 and adjust the pH of each
of them to 7.5 by dropwise addition of NaHCl. Dilute each to a
radioactive concentration of 5–10 m Ci/mL, adding also phosphate buffer
(pH7.4), cysteine hydrochloride and mannitol to give a final concen
-
tration of 50mM phosphate buffer, 4% (wt/vol.) mannitol and 0.1%
cysteine.
(11) Aliquot in 0.5 mL volumes and freeze dry.
(12) Store at 4°C. The product is stable for at least four weeks.
The procedure described above incorporates 40–60% of the initial
125
I
into T4 and 25–40% into T3, with only about 5% of the
125
I remaining
unreacted. Specific activities are about 600–1000 m Ci/mg for T4 and
200
-
400 mCi/mg for T3.
Tube number
10 20 30 40 50 90 100 110 120 130 140807060
0
FIG. 5.3. Typical result from a Sephadex G-25 column.

CHAPTER 5. GUIDELINES FOR GENERAL IMAGING
402
(b) Elution patterns of iodinated T3 and T4
The results are given in Table 5.21.
Protocol 8: Radioiodination using solid phase lactoperoxidase
The following procedure is used:
(a) Add to 10 mg antigen in an iodination vial:
—10 mL 0.5M phosphate buffer of pH7.4;
—1 mCi sodium
125
I-iodide.
(b) Add 10 mL (10–20 ng) solid phase lactoperoxidase and 5 mL H
2
O
2
(0.5nM).
(c) React for 10 min.
(d) Add 5 mL more of H
2
O
2
(0.5nM).
(e) React for 20 min.
(f) Add 100 mL of 0.1% sodium acid phosphate.
(g) Purify the products as described in Annex VII.
TABLE 5.21. ELUTION PATTERNS OF IODINATED T3 AND T4
Tu b e n o .
(rows of
ten)
(Number of counts)/10 s
1 2 3 4 5 6 7 8 9 10s
1–10
11–20 124 111 133 212 283 549 2 819 47 929 4 248 678
21–30 413 607 444 1 249 728 707 378 246 188 200
31–40 197 215 223 149 175 178 158 169 156 188
41–50 174 241 360 320 335 288 209 191 199 175
51–60 179 168 148 174 166 181 181 212 188 182
61–70 155 191 178 137 143 145 152 177 172 186
71–80 205 257 309 524 824 1 312 2 314 3 881 5 730 7 541
81–90 11 352 15 306 19 015 21 577 22 831 27 370 25 986 24 897 24 763 21 496
91–100 19 154 15 487 13 337 10 527 8 359 6 172 4 827 3 304 2 687 2 442
101–110 2 193 2 182 2 405 3 533 4 254 5 068 6 289 7 969 9 257 10 065
111–120 11 860 13 550 14 171 15 590 15 707 16 780 16 368 15 852 14 720 13 920
121–130 12 503 10 503 9 677 8 327 7 216 6 088 5 145 4 233 3 291 2 574
131–140 2 004 1 480 1 181 1 074 806 745 545 489 419 341
5.12. RADIOIMMUNOASSAY PROTOCOLS
403
Protocol 9: Iodination of peptides by the iodogen method
(a) Preparation of Ultrogel column
The following procedure is used:
—Fill the column with water or buffer.
—Pour in the correct amount of swollen Ultrogel using the reservoir.
—Allow the column to settle whilst running with water or buffer.
—Equilibrate column with buffer using a high enough reservoir for it to
compact.
—After use store in buffer or water with bacteriostat. The solution is stable
for at least six months.
(b) Preparation of iodogen coated tube
The following procedure is used:
—Make up a 20 mL solution of iodogen in chloroform or dichloromethane
(e.g. by making up a 1 mg/mL solution and diluting in the ratio 1:50).
—Pipette 100 mL of the iodogen solution into the bottom of a glass tube (e.g.
a glass LP4 tube cut to a depth of approximately 4.5 cm).
—Allow to dry in a fume cupboard. This takes approximately two hours.
(c) Iodination
The following procedure is used:
—Mark the test tubes for collection of 400 mL fractions. Number the tubes.
—Set the Ultrogel column reservoir at a height that gives flow rates of
6
mL/hour.
—Add 20 mL of peptide solution in 0.05M phosphate buffer of pH7.2 to the
iodogen coated tube.
—Add 10 mL of low specific activity Na
125
I.
—Leave to react for 20 min with occasional gentle shaking.
—Transfer the reaction mixture into another tube containing 200 mL
phosphate buffer of pH7.2 and leave for 5 min.
—Place diluted reaction mixture into a 60 cm × 0.9 cm column of Ultrogel
ACA 54 and run column slowly (6 mL/hour) using phosphate buffer
containing BSA. Collect about 50 fractions of about 400 mL each.
—Count the radioactivities of the fractions and plot the elution profile.
CHAPTER 5. GUIDELINES FOR GENERAL IMAGING
404
Protocol 10: Typical protocol for conjugate iodination of steroids
(a) Activation of steroid derivative
The following procedure is used:
—Take 2.4 mg steroid in 50 mL dioxane.
—Add 10 mL of a 1/5 solution of tri-n-butylamine in dioxane.
—Add 10 mL of a 1/10 solution of isobutylchloroformate in dioxane.
—React for 20 min at 10ºC.
—Add 3.5 mL dioxane to stop the reaction.
(b) Iodination of histamine
The following procedure is used:
—Take 220 ng of histamine in 10 mL phosphate buffer.
—Add 0.5 mCi of Na
125
I (5 mL).
—Add 50 mg of chloramine-T in 10 mL phosphate buffer.
—React for 30 s.
—Add 300 mg sodium metabisulphite to stop the reaction.
(c) Conjugation
The following procedure is used:
—Add 50 mL activated steroid to iodinated histamine.
—Add 10 mL of 0.1M NaOH.
—React on ice for one hour.
—Add 10 mL of 0.1M NaOH.
—React for 1 hour on ice.
—Acidify with 1 mL of 0.1M HCI.
—Extract excess unconjugated histamine and related products with 1 mL
toluene/ethyl acetate.
—Neutralize with 1 mL of 0.1M NaOH.
—Add 1 mL of phosphate buffer.
—Extract product with 1 mL toluene/ethyl acetate.
(d) Purify on TLC
The following procedure is used:

5.12. RADIOIMMUNOASSAY PROTOCOLS
405
—Develop with chloroform/methanol/acetic acid (90/10/1).
—Locate by autoradiography and scrape off product band.
—Dissolve in ethanol.
Protocol 11: Iodination of antibodies (rabbit IgG) by the N-bromosuccinimide
method
The antibodies must be pure for iodination. Purification can be done by
ammonium sulphate or caprylic acid precipitation, followed by isolation of IgG
using a DEAE or protein A sepharose chromatography.
The optimal specific activity for iodinated IgG is approximately 12 mCi/
mg. The final specific activity of the product can be altered by adjusting the
amount of protein added, the amount of Na
125
I added, the amount of N-bromo-
succinimide added and the reaction time:
(a) Equilibrate a small Sephadex G-25 column with 0.05M phosphate buffer.
(b) Make up a solution of N-bromosuccinimide (200 mg/mL) in 0.05M
phosphate buffer.
(c) To a small tube (e.g. an Eppendorf tube):
—Add 10 g IgG (e.g. 10 mL of a 1 mg/mL solution).
—Add 10 mL of 0.5M phosphate buffer.
—Add 10 mL of the low activity Na
125
I provided.
—Add 5 mL of N-bromosuccinimide solution, mix and react for 20 s.
—Add 200 mL of 0.05M phosphate buffer to dilute the reaction mixture
(some authors add excess tyrosine to, in effect, stop the reaction).
—Immediately apply the mixture to the chromatography column and run
with 0.05M phosphate buffer. Collect about 30 fractions of ten drops
each.
—Count each fraction for 1 s. Plot the elution profile.
—Add about 5–10 mg of BSA to the fractions saved (e.g. 20 mL of 30%
BSA).
Calculate the specific activity of the label:
Specific activity =
labelled counts ¥ µCi Na
125
I
= mCi/mg.
(labelled + free counts) ¥ mass IgG(mg)
CHAPTER 5. GUIDELINES FOR GENERAL IMAGING
406
Protocol 12: Preparation of
125
I-thyroxine with product separation by HPLC
(a) Apparatus and procedure
The procedures described in the following are used with the equipment
noted:
(1) The following equipment is required: A high pressure pump, for example
an Altex model 110A, a Rheodyne No. 7125 syringe loading injector, a
flow-through radioactivity detector and recorder, a fraction collector, a
column, an ultrasphere 5
m ODS, 4.6 mm × 4.5 cm (Beckman), 1.5 mL
conical microfuge tubes, snap-top volumetric glassware, pipettes and tips,
and disposable plastic ware.
(2) A manual counter (e.g. mini-assay type 6-20) is used for radioactive
counting; the bottom of the sample tube holder should be 17 cm above
the bottom of the counting well.
(3) Assemble the column and detector into the HPLC system, pump water
through the system for 15 min at 1 mL/min and open the sample loop so
this is also washed through.
(4) Note that this washing with water is most important.
(5) Transfer to pumping the eluent for a further 10 min through the column,
including the sample loop, then reduce the flow to 0.5 mL/min and leave
it running into the sink.
(6) Load the fraction collector with tubes, set to collect 30 drop fractions,
turn on and bring the arm to the start position, set the ratemeter to 3 ×
10
4
counts/s, linear with a time constant of 3.3 s, and the recorder to
15
cm/hour.
(b) Iodination
Perform the iodination (using chloramine-T) as described in Annex VII,
using T3, free acid, as the starting material.
(c) Purification
The following procedure is used:
(1) Insert the column outlet into the fraction collector drophead, start the
eluent flow at 0.5 mL/min and observe the fraction collector to see if it is
operating correctly.
(2) Switch on the chart recorder and check that it is operating.
5.12. RADIOIMMUNOASSAY PROTOCOLS
407
(3) Load approximately 0.2 mL of eluent buffer into a 1 mL disposable
syringe fitted with the injection needle, followed by the iodination
mixture and another 0.2 mL (approximately) of eluent.
(4) With the sample loop injector in the inject position, slowly load the
sample from the syringe into the sample loop.
(5) Count the vial again to check the residual activity, and record the counts.
(6) Mark the chart recorder and turn the injection valve to the load position,
watch for the first peak to appear on the chart recorder, then turn the
loop back to the inject position and leave the pump running.
(7) Using a Pasteur pipette, rinse the vial with water into the disposal sink
and again count the vial to record the solid waste activity.
(8) After 20–30 min when all the product peaks should have been eluted,
stop the pump. Transfer the column outlet back to the wastewater outlet
and the eluent back to water, continue washing with water for at least 30
min, and open the sample loop so that this is also washed.
(9) Remove the syringe and injection needle, and wash these with water; also
wash through the channels of the injection valve.
(10) Wash the column with methanol for 15 min before turning off.
(d) Dispensing and drying
The following procedure is used:
(1) Count each of the collected fractions for 1 s in the holder of the mini-
assay, and calculate the percentage of radioactivity in the iodine, T3 and
T4 peaks.
(2) For pooling, use the fractions corresponding to T4 but omit one fraction
from the beginning and one from the end of the peak. Calculate the total
activity.
(3) Pool the selected T4 fractions into diluent buffer and dilute to give a
radioactive concentration of 10 mCi/mL.
(4) Count 10 mL of the diluted T4 solution for 10 s in the well of the mini-
assay. There should be approximately (25 000 counts)/(10 s
· 10 mL)
(=
10 mCi/mL at 70% efficiency) but no less than 20 000 counts.
(e) Aliquot
Aliquot into 0.5 or 1.0 mL fractions (5–10 mCi) and freeze dry.
This is a typical protocol, obtained through the courtesy of Dr. R.
Edwards, Director, NETRIA, St. Bartholomew’s Hospital, London, UK, which
CHAPTER 5. GUIDELINES FOR GENERAL IMAGING
408
describes the preparation of
125
I-thyroxine, including details of the HPLC
procedure.
Protocol 13: Antibody coated tubes and wells
The following procedure is used:
—Dispense 300 mL per tube or 200 mL per well of a 1, 10 and 100 mg/mL IgG
solution in phosphate buffer of pH7.4. For blanks dispense 300 mL per
tube or 200 mL per well of buffer.
—Enclose in a container in a humid atmosphere and leave at 4ºC overnight.
—Aspirate IgG solution from the tubes and wells.
—Dispense 500 mL per tube or 250 mL per well wash buffer, and aspirate
again.
—Dispense 500 mL per tube or 250 mL per well 1% BSA solution to block
remaining binding sites. Two hours at room temperature is sufficient time
for blocking, but for convenience leave the tubes and wells containing 1%
BSA overnight at 4ºC.
—Aspirate or decant the 1% BSA solution.
—Add 300 mL per tube or 200 mL per well iodinated rabbit IgG in assay
buffer and incubate at room temperature for two hours.
—Aspirate and wash with 1 mL assay buffer.
—Count.
Protocol 14: Antibody coated cellulose
Activation procedure
Five grams of Sigmacell are weighed into a 50 mL conical flask fitted with
a ground glass stopper. Following which 0.61 g CDI and 25 mL acetone are
added and the mixture left to react for one hour at room temperature with
shaking. The activated imidazole-carbamate, cellulose, is recovered by
filtration over a glass microfibre filter, washed with three 100 mL aliquots of
acetone and allowed to air dry. The cellulose may be used immediately or
stored dry at –20ºC.
The procedure is as follows:
—Weigh 200 mg of activated cellulose into a polystyrene tube.
—Add 1 mL of 10 mg/mL IgG solution in barbitone buffer of pH8 and
vortex briefly to form a slurry.
—Leave the tube rotating overnight at room temperature.
5.12. RADIOIMMUNOASSAY PROTOCOLS
409
—Centrifuge at 2500 rev./min for 5 min at room temperature.
—Retain the supernatant for use again and to test for the protein concen-
tration.
—Resuspend the cellulose in 10 mL 0.5M bicarbonate buffer of pH8 and
rotate for 20 min.
—Centrifuge at 2500 rev./min for 5 min at room temperature.
—Resuspend the cellulose in 10 mL 0.5M bicarbonate buffer of pH8 and
rotate for 20 min.
—Centrifuge at 2500 rev./min for 5 min at room temperature.
—Resuspend the cellulose in 10 mL 0.1M acetate buffer of pH4 and rotate
for 60 min.
—Centrifuge at 2500 rev./min for 5 min at room temperature.
—Resuspend the cellulose in 10 mL 0.1M acetate buffer of pH4, sonicate
for 30 s and rotate overnight at room temperature.
—Centrifuge at 2500 rev./min for 5 min at room temperature (adding assay
buffer to maintain constant volume throughout the procedure).
—Resuspend the cellulose in a 5 mL assay buffer.
—Pipette 5, 10, 20, 50, 100 and 200 mL cellulose in duplicate into assay tubes.
—Add 100 mL labelled rabbit IgG solution and leave at room temperature
for 2 hours with occasional shaking to keep the cellulose in suspension.
—Add 1 mL of wash solution, centrifuge at 2500 rev./min for 5 min at room
temperature, decant the supernatant and count the cellulose pellets.
—Plot a dilution curve for the solid phase antibodies.
Protocol 15: Antibody coated magnetic particles
The activation procedure is as follows:
—Roll the bottle containing the magnetic particles for 30 min at room
temperature at 30 rev./min.
—Pipette or pour out the required amount (20 mL = 1 g = sufficient for IgG
isolated from 1 mL serum).
—Sediment the particles on a magnetic block and aspirate the supernatant.
Wash the particles three times with 20 mL water, by mixing gently with
water, sedimenting and aspirating the supernatant. Wash the particles five
times with acetone.
—Adjust the volume to 10 mL with acetone and add 0.12 g CDI.
—Mix gently by rolling for one hour at room temperature.
—Sediment the particles and wash four times with 40 mL of acetone, four
times with 40 mL of water and four times with 40 mL bicarbonate buffer
of pH8.
