Nielsen H. RNA: Methods and Protocols
Подождите немного. Документ загружается.

44 Jacobsen, Eriksen, and Nielsen
ically active total RNA from tissues enriched in ribonucleases
by homogenization in the chaotropic salt guanidine thiocyanate
(GuSCN) and 2-mercaptoethanol followed by ethanol precip-
itation or by sedimentation through a cesium chloride cush-
ion. GuSCN effectively denature secondary–tertiary protein and
nucleic acid structures (4). In addition to pr omoting efficient
cell lysis, its use in extraction buffers at a high (4 M) concen-
tration also leads to concomitant inhibition of endogenous pro-
teases and nucleases, including RNases (2, 5). The method was
further modified by Chomczynski and Sacchi (3)toasingle-
step extraction of total RNA by the acid-guanidine thiocyanate-
phenol-chloroform protocol. At the low pH used in this proto-
col, the RNA is displaced to the water phase while the DNA is
selectively solubilized in the phenol phase thus eliminating the
ultra-centrifugation step of the guanidinium-CsCl method (2).
Yet another method has applied extraction with buffer-saturated
phenol followed by proteinase K treatment to prevent RNA
degradation (6).
Since most eukaryotic mRNAs contain tracts of poly(A) tails
at their 3
-termini, polyadenylated mRNA can be selected by
oligo(dT)-cellulose chromatography. Although peptide nucleic
acid (PNA) analogues have recently been used for poly(A)
+
RNA isolation (7), oligo(dT) continues to be the most exploited
affinity ligand in mRNA sample preparation (3). A single-
step poly(A)
+
RNA isolation method has been described using
streptavidin-coated superparamagnetic beads (8). While the direct
method significantly reduces the handling and purification time,
the need for high salt concentration in the stabilization of
the dT-A duplexes often results in co-purification of non-
polyadenylated RNAs. Moreover, the poly(A) selection is carried
out directly in crude cell lysates without the presence of RNase
inhibitors, thereby increasing the mRNA susceptibility to RNA
degradation.
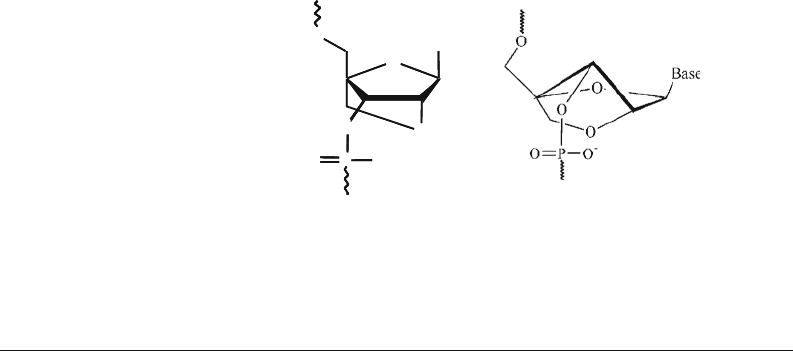
Locked nucleic acid (LNA) oligonucleotides (see Fig. 4.1)
comprise a class of bicyclic RNA analogues having an exception-
ally high affinity towards their complementary DNA and RNA
target molecules (9, 10). We have developed a method for highly
efficient isolation of intact poly(A)
+
RNA based on LNA-T’s
increased affinity to complementary poly(A) tracts (11). This
allows for direct isolation of poly(A)
+
RNA from 4 M GuSCN-
lysed cell extracts. In addition, the LNA substituted oligo(dT)
probe enables efficient isolation of poly(A)
+
RNA from extracted
total RNA samples in a low salt binding buffer. Here, we describe
the protocol for isolation of poly(A)
+
RNA from 4-M GuSCN
lysates by the combination of a biotinylated LNA oligo(T) cap-
ture probe and paramagnetic streptavidin beads.
Poly(A)
+
Isolation 45
O
Base
P
O
O
-
O
O
O
LNA
≡
Fig. 4.1. Two representations of the chemical structure of an LNA nucleotide. The
right
hand side
shows the LNA nucleotide in the 3
-endo (N-type) conformation.
2. Materials
1. 100 μM stock of biotinylated LNA oligo(T) capture probe
(Exiqon) (see Note 1).
2. Lysis buffer: 4 M guanidinium thiocyanate, 25 mM Na-
citrate, pH 7.0, 0.5% (w/v) sodium N-lauroyl sarcosinate
(see Note 2).
3. Binding buffer: 20 mM Tris-HCl, pH 7.5, 0.5 M NaCl,
1 mM EDTA, pH 7.5, 0.1% (w/v) sodium N-lauroyl sar-
cosinate.
4. Washing buffer: 20 mM Tris-HCl, pH 7.5, 0.1 M NaCl,
1 mM EDTA, pH 7.5, 0.1% (w/v) sodium N-lauroyl sar-
cosinate (see Note 3).
5. TE buffer: 10 mM Tris-HCl, 1 mM EDTA, pH 7.5.
6. Quartz sand, baked at 220
◦
C for 12 h.
7. Pestle.
8. Streptavidin-coated magnetic particles (e.g. Roche).
9. Magnetic separator (e.g. the PickPen system from BioNo-
bile).
10. Yeast tRNA, diluted to 1 μg/μL in TE-buffer.
11. Thermomixer (Eppendorf).
12. Siliconized, RNase-free microcentrifuge tubes (e.g. from
Ambion, ABI).
13. 3 M sodium acetate solution, pH 5.5.
14. Glycogen carrier, 5 mg/mL (Ambion, ABI).
15. 96% ethanol.
16. 70% ethanol.
17. RNase-free distilled/deionized water (dH
2
O).

46 Jacobsen, Eriksen, and Nielsen
3. Methods
3.1. Sample
Preparation
1. Thaw the cell or tissue sample (e.g. cells stored in RNAlater
(Ambion, ABI)) (see Note 4).
2. Centrifuge at 4,000×g for 2 min and carefully remove the
supernatant.
3. Add 200 μL of lysis buffer containing 10 mM dithiothreitol
(DTT) and vortex briefly (see Note 5).
4. Add a small spatula quartz sand covering 3–5 mm of the bot-
tom of a 1.5-mL microcentrifuge tube and disrupt the tis-
sue/cells for 2 min on ice using a pestle in order to homog-
enize the sample.
5. Dilute the cell extract corresponding to 10
6
cells/50 μLin
lysis buffer containing 10 mM DTT.
6. Heat the lysate at 65
◦
C for 30 min on a thermomixer at
moderate mixing avoiding the debris to precipitate.
7. Incubate for 10 min on ice and centrifuge the tube briefly
(e.g. at 16,100×g for 1 min) and transfer the supernatant
to a clean tube or directly proceed directly to the poly(A)
+
RNA capture (see pkt. 3.3).
3.2. Pre-blocking and
LNA-Binding of
Streptavidin-Coated
Magnetic Particles
1. Pipette 60 μL of streptavidin-coated magnetic particles in
suspension into a microcentrifuge tube for each sample
preparation.
2. Use a magnetic separator to collect the particles on the inside
of the tube wall and remove the supernatant without dis-
turbing the particles.
3. Release the particles by removing the tube from the mag-
netic separator and add 100 μLof1μg/μL yeast tRNA in
TE-buffer.
4. Keep the particles in suspension and incubate at room tem-
perature (RT) for 5 min in order to pr e-block the particles.
5. Wash the particles in 100 μL of TE-buffer using the mag-
netic separator to collect the particles and remove the super-
natants.
6. Add to each tube 100 μL of binding buffer and add 200
pmol biotinylated LNA oligo(T) (see Note 3).
7. Incubate for 5 min at 37
◦
C at moderate mixing to avoid
sedimentation.
8. Collect the particles using the magnetic separator, remove
supernatant and release the particle into 200 μL of binding
buffer.
Poly(A)
+
Isolation 47
9. Repeat the washing step. Avoid the particles to dry out,
completely.
3.3. Poly(A)
+
RNA
Isolation
1. Collect the streptavidin-coated particles using the magnetic
separator and remove the super natant.
To the tube containing particles transfer the cell-free extract
and release and resuspend the particles.
2. Incubate at 37
◦
C for 5 min on a thermomixer at gentle mix-
ing in order to bind the poly(A)
+
RNA to the particles (see
Note 6).
3. Collect the particles in the magnetic separator remove super-
natant and release the particle into 200 μL of washing
buffer.
4. Repeat the washing step twice.
5. Remove as much as possible of the supernatant without dis-
turbing the particle pellet and add 50 μLofdH
2
Otothe
tube.
6. Incubate at 65
◦
C for 10 min in or der to elute the poly(A)
+
RNA from the particles (gentle mixing) and leave on ice for
5min.
7. Collect the particles with the magnetic separator and care-
fully transfer the supernatant containing the poly(A)
+
RNA
to a clean tube.
8. Centrifuge the tube briefly (e.g. at 16,100×g for 1 min) and
transfer the supernatant to a clean tube without transfer of
remaining magnetic particles.
3.4. Ethanol
Precipitation
1. Precipitate the poly(A)
+
RNA b y addition to the sample of
0.1× volume of 3 M sodium acetate, glycogen carrier to
150 μg/mL and 2.5 vols of 96% ethanol. Leave at –20
◦
C
overnight (see Note 7).
2. Centrifuge the tube at 16,100×g for 30 min at 4
◦
C.
3. Remove the supernatant and wash the pellet with ice-cold
70% ethanol.
4. Dry the pellet at RT.
5. Dissolve the pellet in a small volume of dH
2
O; centrifuge
briefly to collect droplets. The poly(A)+ RNA can now be
quantitated and adjusted to the appropriate concentration
for subsequent use.
3.5. Analysis of
Purified Poly(A)
+
RNA
LNA oligo(T) capture of poly (A)
+
RNA has successfully been
applied to several cell types, including yeast, C. elegans and human
cells (11). Figure 4.2 illustrates the isolation of poly(A)
+
RNA
directly from 4 M GuSCN-lysed human cells and the subsequent
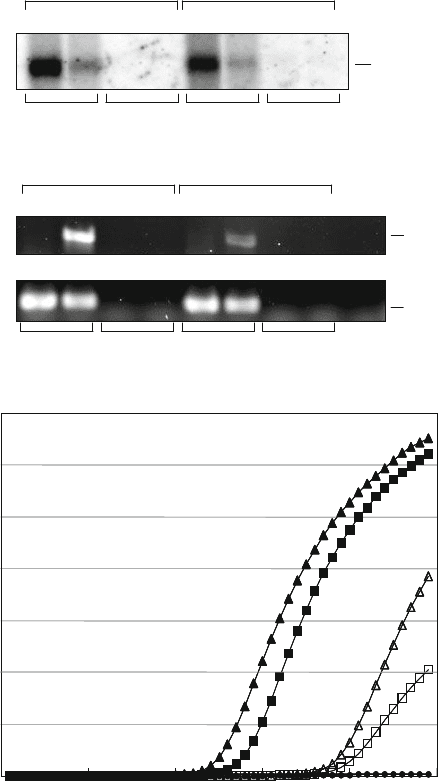
48 Jacobsen, Eriksen, and Nielsen
mdr1
C
Cycle no.
ΔRn
0
2
4
6
8
10
12
14
10 20 30 40 50
5’ - biotin
5’
– NH
2
LNA_2.T DNA - dT
20
GAPDH
1.3
kb
A
12121212
LNA_2.T DNA-dT
20
DNA-dT
20
LNA_2.T DNA - dT
20
B
neg.
control
mdr1
256
bp
LNA_2.T
5’
- biotin
5’ - NH
2
12121212
738
β-ACT
Fig. 4.2. Analysis of poly(A)
+
RNA isolated directly from 4 M GuSCN-lysed human K562 and K562/VCR erythroleukemia
cells by LNA oligo(T) capture. a Northern blot analysis of the poly(A)
+
RNA samples selected from 4 M GuSCN-lysed
human K562 (1) and K562/VCR (2) cells, respectively, using the 5
-biotinylated or 5
-NH
2
-modified LNA_2.T affinity
probe and the corresponding DNA oligo-dT
20
control probes. The filter was hybridized with a
32
P-labelled DNA fragment
for the mouse GAPDH mRNA. b Ca. 100 ng of poly(A)
+
RNA purified from the human K562 (1) and K562/VCR (2) cell
lines was used as template for RT-PCR assays for human
mdr1
and β
-actin
. The amplicon sizes were 256 and 738 bp
for the β
-actin
and
mdr1
mRNAs, respectively. The RT-PCR products were electrophoresed in a 1% native agarose
gel and visualized by staining with Gelstar. A negative PCR control without template w as performed for each assay. c
Representative amplification plots of quantitative real-time RT-PCR assays for the human
mdr1
transcript using mRNA
samples isolated from human erythroleukemia cells as template. The poly(A)
+
RNAs were selected either using the
biotinylated LNA_2.T affinity probe from K562 cells (
solid triangle
) and K562/VCR cells (
solid square
); or by the 5
-NH
2
-
modifed LNA_2.T affinity probe from K562 cells (
open triangle
) and K562/VCR cells (
open square
). The plots relate the
PCR cycle number to the change of detected, baseline-corrected fluorescence (R
n
). The small,
solid circle
depicts the
fluorescence generated from the no template control reaction.

Poly(A)
+
Isolation 49
characterization by norther n blotting analysis, RT-PCR and
quantitative real-time PCR. The cells were a human ery-
throleukemia cell line derived from a chronic myeloid leukaemia
patient in blast crisis (K562) and similar cells selected for resis-
tance to the chemotherapeutic drug vincristine (K562/VCR).
The yield was approximately 300 ng of poly(A)
+
RNAfrom1
× 10
6
K562 cells with two different kinds of LNA p robes (5
-
biotinylated or 5
-NH
2
-modified LNA_2T), whereas no mRNA
could be captured with the DNA-dT
20
control probes. Northern
blot analysis of the poly(A)
+
RNA samples r evealed a single 1.3-
kb mRNA species for the human GAPDH gene in the K562 and
K562/VCR sample preparations selected with both LNA_2.T
affinity probes (see Fig. 4.2a). RT-PCR assays for the human β-
actin mRNA revealed single cDNA fragments of the expected size
in all four LNA_2.T-selected mRNA templates, whereas no PCR
products were detected after 30 cycles of amplification from the
DNA-dT
20
-selected control samples (see Fig. 4.2b). In contrast,
RT-PCR for the human multidrug resistance gene mdr1 gener-
ated the 738-bp PCR amplicon in the K562/VCR cell line, but
not in the drug-sensitive K562 cell line, implying that the mdr1
gene is overexpressed in K562/VCR cells, presumably reflect-
ing their significantly increased resistance to the chemotherapeu-
tic drug vincristine. This result was corroborated by quantita-
tive real-time RT-PCR that revealed an average increase of four
orders of magnitude in mdr1 expression relative to control β-
actin mRNA in the vincristine-resistant K562/VCR cell line com-
pared to the sensitive K562 cells (see Fig. 4.2c). It is our estimate
that given the average yield of 300 ng per 1 × 10
6
cells and a
fivefold dilution of the cDNA reaction made for qRT-PCR, a sin-
gle LNA oligo(T) sample preparation would allow quantification
of 33 different mRNAs in triplicate using real-time PCR assays.
The fact that we were successful in substituting the biotinylated
LNA_2.T affinity probe with the NH
2
-modified LNA_2.T probe
strongly suggests that the LNA oligo(T) method is amenable to
automation for streamlined, high-throughput expression profil-
ing by real-time PCR by covalently coupling the probe to solid,
pre-activated surfaces, such as microtitre plate wells or magnetic
particles.
4. Notes
1. The LNA described in the present work is a 20-mer oligo-
dT with a 5
-biotin and every second residue substituted
with an LNA-residue. This LNA is referred to as LNA_2.T:
5
-Biotin-T
L
T T
L
T T
L
T T
L
T T
L
T T
L
T T
L
T T
L
T T
L
T
50 Jacobsen, Eriksen, and Nielsen
T
L
T-3
. LNA oligo(T) capture probes can be synthesized at
other lengths and with varying degrees of LNA substitution.
Substitution of a DNA oligo(dT)
20
oligonucleotide with
LNA-T results in significantly increased thermal duplex sta-
bilities in all LNA oligo(T) designs measured, corresponding
to an increase in melting temperature ranging from +2.8 to
+6.0
◦
C per LNA thymidine monomer. A fully substituted
LNA-T
20
has a T
M
of above 95
◦
C, indicating an excep-
tionally high thermal stability that would not allow efficient
elution of the captured poly(A)
+
RNA from the affinity lig-
and. By comparison LNA_2.T shows a T
M
of 70.8
◦
Cand
an increase of 30
◦
C compared to an all-DNA control probe.
Thus, the LNA_2.T affinity probe represents an adequate
compromise between increased duplex thermal stability and
melting of the dA-T duplexes in elution buffer.
2. The optimal GuSCN concentration for the reference DNA
oligo-dT
20
probe was found to be 0.5 M GuSCN in
accordance with previous results reported with oligo(dT)
chromatography (12). In contrast RNA recovery with the
LNA_2.T probe was not affected by increasing the GuSCN
concentration in the binding buffer showing comparable
yields of ca. 80% in the entire range from 0.5 to 4 M
GuSCN.
3. A high recovery of 70–100% has been observed for both
the reference DNA-dT
20
and LNA_2.T affinity probes in
the high salt concentration range of 0.2–0.5 M NaCl in
the binding buffer. However, in the low salt range of
50–100 mM, a significantly decreased recovery has been
observed with the reference DNA-dT
20
probe, while the
recovery is between 80 and 90% with the LNA oligo(T)
affinity ligand, indicating that a low salt, high hybridization
stringency window can be employed in combination with
the LNA oligo(T) affinity probe without compromising the
mRNA yield.
4. The protocol can also be employed using 50 μgofpurified
whole cell RNA as the starting material.
5. Dithiotreitol (DTT) should be added to the lysis buffer
immediately before use. Stock solution of 1 M DTT in
dH
2
O can be stored at –20
◦
C in aliquots.
6. We were successful in exploiting the biotin–streptavidin cou-
pling chemistry in our mRNA isolation procedure by lim-
iting the hybridization time to 5 min in order to prevent
streptavidin from denaturation even in the presence of 4 M
GuSCN. This is in accordance with previous studies report-
ing that streptavidin is highly resistant to denaturation by
guanidine hydrochloride (13–15). Alternatively, we have
Poly(A)
+
Isolation 51
demonstrated the utility of the LNA oligo(T) sample prepa-
ration method employing a 5
NH
2
-modified LNA_2.T
affinity probe coupled covalently to pre-activated magnetic
particles, thus overcoming the potential problem of denatu-
ration by GuSCN.
7. The ethanol precipitation step can be carried out in many
different ways depending on the experimental situation. As
an example, the precipitation time can be shortened to
15 min by placing the samples in a dry ice/ethanol bath
followed by a high-speed centrifugation at 4
◦
C.
References
1. Aviv, H., Leder, P. (1972) Purification of
biologically active globin messenger RNA
by chromatography on oligothymidylic acid-
cellulose. Proc Natl Acad Sci USA 69,
1408–1412.
2. Chirgwin, J. M., Przybyla, A. E., MacDon-
ald, R. J., Rutter, W. J. (1979) Isolation
of biologically active ribonucleic acid from
sources enriched in ribonuclease. Biochem-
istry 18, 5294–5299.
3. Chomczynski, P., Sacchi, N. (1987)
Single-step method of RNA isolation
by acid guanidinium thiocyanate-phenol-
chloroform extraction. Anal Biochem 162,
156–159.
4. von Hippel, P. H., Wong, K. Y. (1964) Neu-
tral salts: the generality of their effects on the
stability of macromolecular conformations.
Science 145, 577–580.
5. MacDonald, R. J., Swift, G. H., Przybyla,
A. E., Chirgwin, J. M. (1987) Isolation of
RNA using guanidinium salts. Methods Enzy-
mol 152, 219–227.
6. Frazier, M. L., Mars, W., Florine, D. L.,
Montagna, R. A., Saunders, G. F. (1983)
Efficient extraction of RNA from mammalian
tissue. Mol Cell Biochem 56, 113–122.
7. Phelan, D., Hondorp, K., Choob, M., Efi-
mov, V., Fernandez, J. (2001) Messenger
RNA isolation using novel PNA analogues.
Nucleosides Nucleotides Nucleic Acids 20,
1107–1111.
8. Hornes, E., Korsnes, L. (1990) Magnetic
DNA hybridization properties of oligonu-
cleotide probes attached to superparamag-
netic beads and their use in the isolation of
poly(A) mRNA from eukaryotic cells. Genet
Anal Tech Appl 7, 145–150.
9. Koshkin, A. A., Singh, S. K., Nielsen, P.,
Rajwanshi, V. K., Kumar, R., Meldgaard,
M., Olsen, C. E., Wengel, J. (1998)
LNA (Locked Nucleic Acid): synthesis
of the adenine, cytosine, guanine, 5-
methylcytosine, thymine and uracil bicy-
clonucleoside monomers, oligomerisation,
and unprecedented nucleic acid recognition.
Tetrahedron Lett 54, 3607–3630.
10. Obika, S., Nanbu, D., Hari, Y., Morio, K., In,
Y., Ishii, J. K., Imanishi, T. (1997) Synthesis
of 2
-O,4
-C methyleneuridine and cytidine.
Novel bicyclic nucleosides having a fixed C
3
-
endo sugar puckering. Tetrahedron Lett 38,
8735–8738.
11. Jacobsen, N., Nielsen, P. S., Jeffares, D. C.,
Eriksen, J., Ohlsson, H., Arctander, P., Kaup-
pinen, S. (2004) Direct isolation of poly(A)+
RNA from 4 M guanidine thiocyanate-
lysed cell extracts using locked nucleic acid-
oligo(T) capture. Nucleic Acids Res 32, e64.
12. Morrissey, D. V., Lombardo, M., Eldredge, J.
K., Kearney, K. R., Groody, E. P., Collins, M.
L. (1989) Nucleic acid hybridization assays
employing dA-tailed capture probes. I. Mul-
tiple capture methods. Anal Biochem 181,
345–359.
13. Green, N. M., Toms, E. J. (1972) The disso-
ciation of avidin-biotin complexes by guani-
dinium chloride. Biochem J 130, 707–711.
14. Green, N. M. (1975) Avidin. Adv Protein
Chem 29, 85–133.
15. Green, N. M. ( 1990) Avidin and strepta-
vidin. Methods Enzymol 184, 51–67.

Chapter 5
Genome Browsers
Elfar Torarinsson
Abstract
Genome browsers are important tools for studying genomes given the vast amounts of data available.
This chapter focuses on providing the reader with the skills necessary to perform relatively simple, yet
powerful, analysis relating to the structure of the transcription unit. Studying available data should be one
of the very first steps taken in designing experiments. This can save considerable time in your research or
as expressed by Alan Bleasby “Two months in the lab can easily save an afternoon on the computer.”
Key words: Genome browser, UCSC, Ensembl, track, view, comparative genomics, data,
bioinformatics, expression, regulation.
1. Introduction
Whole genome data are now available from a number of closely
and distantly related vertebrates. Many projects can greatly bene-
fit from relatively simple sequence comparisons of genomic data.
Here I describe some of the analysis relating to the structure of
the transcription unit that would help in design of experiments.
Genome browsers are great tools to access the vast amount
of genomic data available. There are three major genome
browsers available, UCSC, Ensembl, and NCBI. Each of these
browsers provides their own annotation of the common assem-
bled sequence. Focus will be on the genome browsers at UCSC
and Ensembl. I will describe how you easily can start using the
browsers (see Note 1). Once you are comfortable navigating in
the browsers it is quite simple to continue on your own to learn
more and e xploit the power of the browsers. Later we will deal
H. Nielsen (ed.), RNA, Methods in Molecular Biology 703,
DOI 10.1007/978-1-59745-248-9_5, © Springer Science+Business Media, LLC 2011
53
