Moss Tom. DNA-protein interactions: principles and protocols
Подождите немного. Документ загружается.

Calorimetry of Protein–DNA Complexes 533
19. Freire, E., Mayorga, O. L., and Straume, M. (1990) Isothermal titration. Anal.
Chem. 62, 950A–959A.
20. Breslauer, K. J., Freire, E., and Straume, M. (1992) Calorimetry: a tool for DNA
and ligand–DNA studies. Methods Enzymol. 211, 533–567.
21. Privalov, G., Kavina, V., Freire, E., and Privalov, P. L. (1995) Precise scanning
calorimeter for studying thermal properties of biological macromolecules in dilute
solution. Anal. Biochem. 232, 79–85.
22. Read, C. M., Cary, P. D., Preston, N. S., Lnenicek-Allen, M., and Crane-Robinson,
C. (1994) The DNA sequence specificity of HMG boxes lies in the minor wing of
the structure. EMBO J. 13, 5639–5646.
23. Wallace, R. B. and Miyada, C. G. (1987) Oligonucleotide probes for the screen-
ing of recombinant DNA libraries. Methods Enzymol. 152, 432–442.

Surface Plasmon Resonance 535
535
From:
Methods in Molecular Biology, vol. 148: DNA–Protein Interactions: Principles and Protocols, 2nd ed.
Edited by: T. Moss © Humana Press Inc., Totowa, NJ
36
Surface Plasmon Resonance Applied
to DNA–Protein Complexes
Malcolm Buckle
1. Introduction
1.1. The Relationship Between Refractive Index and Mass
Surface plasmon resonance (SPR) measures refractive index changes (∆n)
at or near a surface and relates these to changes in mass at the surface (Fig 1).
This relationship is given by the Clausius Mossotti form (Eq. 2) of the Debye
equation (Eq. 1):
ε – 1 N
(
α + µ
)
(1)
ε + 2
=
3ε
0
kT
ε – 1 Nα
(2)
ε + 2
=
3ε
0
where ε is the real part of the dielectric constant or permittivity constant related
to the refractive index by ε = n
2
, N is the number density given by N
A
ρ/M
a
(N
A
is Avogadro’s number, ρ is the density and M
a
is the molecular mass). It is
assumed that ∆n/∆C is a constant.
1.2. SPR Using the BIACORE Instrument
In physical terms, the detection system of this SPR machine consists of a
monochromatic, plane polarized light source, and a photodetector that are con-
nected optically through a glass prism (Fig. 1). A thin gold film (50 nm thick),
deposited on one side of the prism, is in contact with the sample solution. This
gold film is, in turn, covered with a long-chain hydroxyalkanethiol, which
forms a monolayer (approx 100 nm thick) at the surface. This layer essentially
serves as an attachment point for carboxymethylated dextran chains that create
a hydrophilic surface to which ligands can be covalently coupled. Light
536 Buckle
incident to the back side of the metal film is totally internally reflected onto the
diode-array detector. A property of this situation is that a nonpropagative eva-
nescent wave penetrates into the solution side of the prism away from the light
source. Free electrons in the gold layer enter into resonance with the evanes-
cent wave. In fact, such resonance implies that the amplitude vector character-
izing a transversal wave propagating along the gold surface (ks
→
p) is equal to
the component (kx
→
) of the evanescent wave. Because ε = n
2
, if ω is the fre-
quency of the wave and c the speed of light, then
ksp
c
12
12
=
−
+
ωε ε
εε
(3)
furthermore, given that for the evanescent wave,
kx
c
g
=
ω
θε
sin
(4)
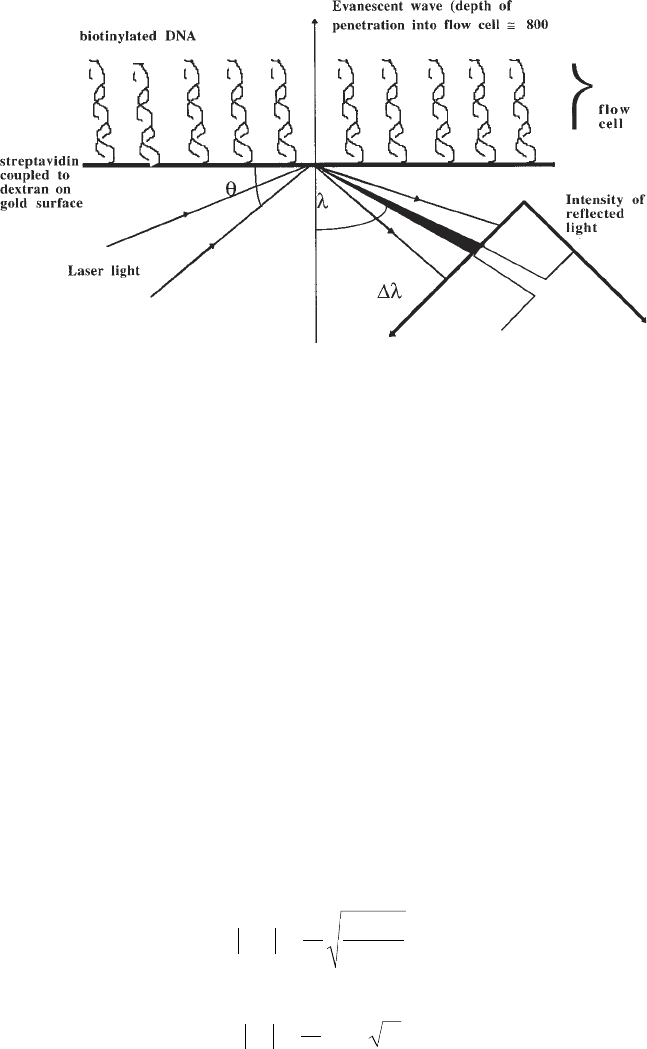
Fig. 1. Schema showing the principle of surface plasmon resonance. Light
from a laser source arriving through a prism at a gold surface at the angle of
total internal reflection (θ) induces a nonpropagative evanescent wave that pen-
etrates into the flow cell opposite the prism. The intensity of the reflected light
is continuously monitored. At a given angle (λ) dependent on the refractive
index of the solution in the flow cell, resonance between the evanescent wave
and free electrons in the gold layer results in a reduction in the intensity of
reflected light. The change in angle of reduced intensity (∆λ) reflects changes
in the refractive index (n) of the solution in the flow cell immediately adjacent
to the gold layer. A dextran surface coupled to the gold layer allows immobili-
zation of ligands (e.g., DNA) within the evanescent field.

Surface Plasmon Resonance 537
when resonance occurs,
|
ksp
|
=
|
kx
|
and the intensity of the reflected light
decreases at a sharply defined angle of incidence, the SPR angle, given by the
simple expression
sin·
()
0
12
g1 2
θ
εε
εε ε
=
+
(5)
Thus, θ
0
, the SPR angle at which a decrease in the intensity of reflected light
occurs, measures the refractive index of the solution in contact with the gold
surface and is dependent on several instrumental parameters (e.g., the wave-
length of the light source and the metal of the film). When these parameters are
kept constant, the SPR angle shifts are dependent only on changes in refractive
index of a thin layer adjacent to the metal surface. Any increase of material at
the surface will cause a successive increase of the SPR angle, which is detected
as a shift of the position of the light intensity minimum on the diode array. This
change can be monitored over time, thus allowing changes in local concentra-
tion to be accurately followed. The SPR angle shifts obtained from different
proteins in solution have been correlated to surface concentrations determined
from radio-labeling techniques and found to be linear over a wide range of
surface concentration. The instrument output, the resonance signal, is indicated
in resonance units (RU); 1000 RU correspond to a 0.1° shift in the SPR
angle, and for an average protein, this corresponds to a surface concentra-
tion change of about 1 ng/mm
2
(for nucleic acids, see Note 1). It is remark-
able that the present instrument (Biacore 2000) can measure a deviation of
10
–3
°, in other words, a variation of 10
–5
in the refractive index .
1.3. Immobilization of DNA to a Surface
Although a variety of techniques exist for the immobilization of DNA on
the dextran surface, the most efficient for the majority of protein–DNA inter-
actions is the use of immobilized streptavidin that can then interact with a suit-
ably end-labeled DNA molecule. The streptavidin is immobilized via a
carbodiimide–N-hydroxyl succinimide coupling reaction to the carboxyl
groups of the dextran (Fig. 2). DNA is easily obtained either by direct purchase
of oligomers end-labeled with biotin, or, for larger fragments, direct poly-
merase chain reaction (PCR) from biotinylated oligomers. Unless a particu-
larly unusual configuration is required, biotin is generally present at one end of
the DNA molecule and on one strand if the DNA is double stranded. The end
biotinylated DNA is then flowed across the surface and allowed to bind to the
desired final concentration (Fig. 3).
1.4. Protein Binding to Immobilized DNA
The protein is flowed across the immobilized DNA in a buffer and at a tem-
perature suitable for the interaction being studied (Fig. 4). A range of concen-
538 Buckle
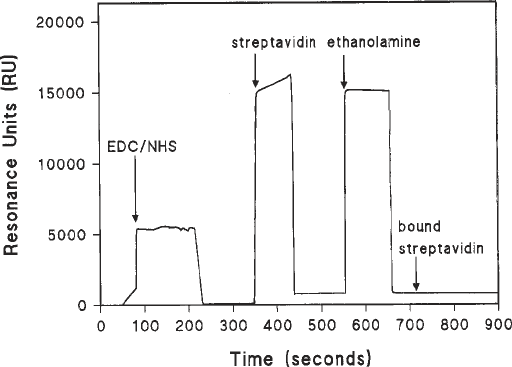
Fig. 2. Coupling of streptavidin to a flow cell activated by carbodiimide and
hydroxyl succinimide. The figure shows a sensorgram of a carboxymethylated dextran
surface (CM5) activated by carbodiimide and N-hydroxylsuccinimide prior to coupling
with streptavidin. Sharp changes in the resonance units (RU) reflect bulk refractive
index changes as a result of differences in the buffer. Ethanolamine is used to block all
unreacted activated carboxyl groups. The difference between the final RU value and
the initial value presents an accurate measure of the amount of streptavidin covalently
coupled to the surface.
trations should be investigated. A flow rate in excess of 10 µL/min is advised
and a sufficient contact time during the association phase to saturate the immo-
bilized DNA (as seen by a steady-state plateau for the RU values) at protein
concentrations in excess of the anticipated K
d
for the interaction. The dissocia-
tion phase should also be allowed to continue for at least sufficient time to
allow over a third of the complex to dissociate.
1.5. Binding Curve Analysis
1.5.1. Stoichiometry and Equilibrium Analysis
For an immobilized DNA fragment (D), the interaction with a mobile pro-
tein (P) can be written as
k
a
D + P
↔
DP (Scheme 1)
k
d
A classical Langmuir adsorption isotherm requires that the fraction of avail-
able sites on the DNA occupied by the protein (θ
D
) be given by
Surface Plasmon Resonance 539
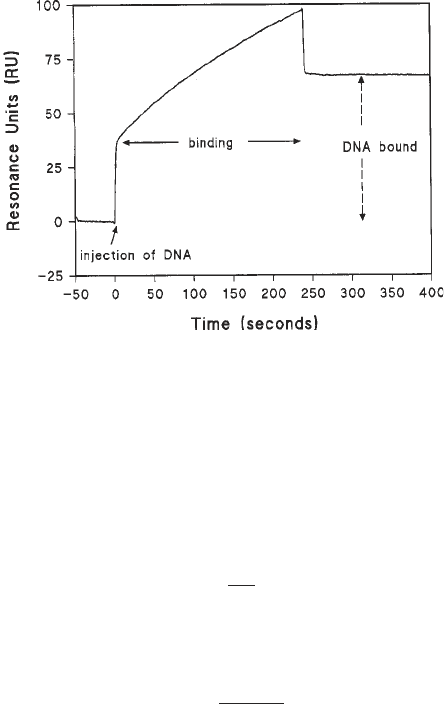
Fig. 3. Immobilization of a biotinylated DNA fragment to a streptavidin surface. In
this example, a 200-bp fragment of DNA (10 µg/mL) containing a single biotin label
at one 5' end was flowed at 20 µL/min in HBS buffer over the streptavidin surface. The
initial bulk refractive index change was followed by a gradual increase reflecting DNA
binding to the streptavidin. At the end of the DNA injection phase, the bulk refractive
index change was recovered and the difference in absolute RU values compared to the
initial value reflects the number of molecules of DNA now bound to the surface.
θ
D
=
DP
(6)
D
t
Furthermore, in such a simple case, the equilibrium association constant K
a
is
given by the expression
θ
D
=
KaP
(7)
1 + KaP
Thus, in an SPR experiment, the steady-state level of bound protein at a
given concentration of total protein should be calculated from the asymptote of
the sensorgram and the RU values converted into moles of bound protein.
Assuming that in the continuous-flow system typical of Biacore SPR machines,
[P]
T
= [P], a plot of θ
D
against [P]
T
should allow a direct fit by Eq. 7 to give an
estimation of K
a
, from which we obtain K
d
= 1/K
a
.
1.5.2. Kinetic Analysis
The protein that is injected across the surface should after an infinite time
arrive at an association equilibrium giving a signal R
eq
, and the resonance sig-
nal R at time t during this process following injection at t = 0 when R = R
0
,
should, in simple instances, obey the expression
540 Buckle
R
t
= R
0
– (R
eq
– R
0
) (1 – e
–k
obs
t
) (8)
Similarly, for the dissociation of the bound protein,
R
t
= R
0
+ (R
eq
– R
0
) (e
–k
off
t
) (9)
assuming that the bound molecule completely dissociates from the immobi-
lized ligand. Consequently, the observed reaction rate k
obs
for the interaction is
given by
k
obs
+ k
on
[P] + k
off
(10)
There is thus a linear relationship between the value for k
obs
and the total
concentration of protein [P]. The value for k
obs
can be obtained from a direct fit
of the association phase using Eq. 8, or by linear regression of a semi-log plot.
It thus follows that linear regression analysis of the dependence of k
obs
on [P]
allows the calculation of k
on
and k
off
using Eq. 7. If we assume that the reaction
is in fact activation controlled (were it otherwise, then the association rate
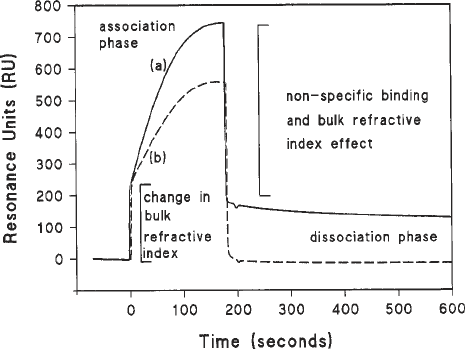
Fig. 4. Protein binding to immobilized DNA. In this example, purified RNA poly-
merase (120 nM) from Eschericia coli was injected at 20 µL/min across an immobi-
lized 203-bp DNA fragment containing a promoter sequence (continuous line [a]).
The dotted line (b) shows the same protein flowing across a streptavidin surface with-
out immobilized DNA. Note the large bulk refractive index effects resulting princi-
pally from the presence of glycerol in the protein solution and nonspecific binding.
This is a complex phenomenon composed not only of electrostatic interactions with
the dextran but also necessary transient interactions with nonpromoter DNA. The dis-
sociation phase is characterized by a steady decrease in signal lending itself to the type
of analysis described in the text. The association phase is more complicated. In this
instance an example of how the association phase may be dealt with is given in ref. 7.
Surface Plasmon Resonance 541
would be of the order of 10
9
/M/s, which is well beyond the range of current
SPR devices), then
k
off
= k
on
K
d
(11)
Thus, the equilibrium dissociation constant (K
d
) can be obtained from the ratio
of the off and on rates.
There are many pitfalls to using SPR, which are covered in several fairly
recent reviews (2,3). In the case of the Biacore instrumentation, the new data
evaluation software deals with certain situations. What it cannot do is to deter-
mine the best strategy for setting up an experiment. In summary, certain
important points must be taken into account even in the simple analysis given
above. Several of these are covered in Notes 2–6.
2. Materials
1. An SPR device. In this chapter, a Biacore instrument is referred to either as the
classic Biacore or the Biacore 2000. It is recommended (but not essential) that
the machine be modified such that the two racks into which samples are placed in
the machine are separately thermostated. Rack 1 should be thermostated to 4°C;
rack 2 should be thermostated to the temperature at which the interaction is to be
measured. The protocols illustrated here require rack D in the first position and
rack A in the second position.
2. Streptavidin from Pierce resuspended in 0.22 µm filtered distilled water to a final
concentration of 5 mg/mL. This preparation may be stored at 4°C for up to 3 mo.
3. HBS buffer: 10 mM HEPES, pH 7.4, 150 mM NaCl, 3.4 mM EDTA, and 0.005%
Biacore surfactant.
4. N-Ethyl-N'-(diethylaminopropyl) carbodiimide (EDC) and N-hydroxyl
succinimide (NHS) purchased from Biacore as lyophilized powders are
resuspended in 0.22 µm filtered distilled water to a final concentration of
100 mM each.
5. 1 M ethanolamine hydrochloride (pH 8.5), purchased from Biacore, stored
at 4°C.
6. HBS buffer: 10 mM HEPES, pH 7.4, 150 mM NaCl, 3.4 mM EDTA, and 0.005%
surfactant P20.
7. Sensor chip surface CM5 research grade installed in the Biacore apparatus and
preprimed with HBS buffer.
8. Reaction vials for the Biacore (small, plastic = 7 mm; medium, glass = 16 mm;
large, glass = 2 mL) purchased from Biacore.
9. End biotinylated DNA suspended in HBS buffer to 10 µg/mL. This DNA can
either be purchased directly or constructed by polymerase chain reaction
(PCR) using templates and an oligomer primer carrying a biotin group (pur-
chased from Genset for example) as one of the primers. It is advisable to gel
purify or high-performance liquid chromatography (HPLC) purify the DNA prior
to immobilization.
542 Buckle
3. Methods
3.1. Immobilization of the Ligand on the Surface
3.1.1. Coupling of Streptavidin
1. Prime the apparatus with HBS buffer.
2. The thawed EDC solution, in an Eppendorf tube with the top removed, is placed
in rack 1 position a1 (r1a1).
3. The thawed NHS solution in an Eppendorf tube with the top removed, is placed in r1a2.
4. Streptavidin (5 mg/mL, 50 µL), in an Eppendorf tube with the top removed, is placed
in r1a3; 2 mL of filtered (0.2 µm) distilled water is placed in a large glass vial in r2f7.
5. 1 M, sodium acetate buffer (1 mL, pH 4.5) is placed in a large glass vial in r2f3.
6. 1 M ethanolamine (200 µL) is placed in a large tube in r2f4.
7. Two small clean plastic vials are placed in r2a1 and r2a2.
8. An empty large glass vial is placed in r2f5.
9. The following method is programmed into the Biacore or Biacore 2000, checked
for errors, and run.
DEFINE APROG mixing
FLOW 20
TRANSFER r1a1 r2a1 50 !rack1a1 = EDC
TRANSFER r1a2 r2a1 50 !rack1a2 = NHS
MIX r2a1 50 !rack2a1 = EDC/NHS mix
TRANSFER r2f7 r2a2 200 !rack2f7 = distilled water
TRANSFER r2f7 r2a2 290 !rack2f7 = distilled water
TRANSFER r2f3 r2a2 5 !rack2f3 = 1 M acetate pH 4.5
TRANSFER r1a3 r2a2 5 !rack1a3 = streptavidin (5 µg/mL)
MIX r2a2 50
END
DEFINE APROG bind
CAPTION activation
FLOW 20
* INJECT r2a1 50
-0:20 RPOINT EDC/NHS -b
* INJECT r2a2 30
-0:20 RPOINT streptavidin
* INJECT r2f4 35 !Ethanolamine (1 M)
-0:20 RPOINT ethanolamine
15:00 RPOINT bound
END
MAIN
FLOWCELL 1
APROG mixing
FLOWCELL 1
APROG bind
END
(See Note 3.)
Surface Plasmon Resonance 543
3.1.2. Immobilization of the DNA
1. Place the streptavidin-activated sensor chip surface CM5 research grade in the
Biacore apparatus and preprime with HBS buffer.
2. Select a surface pretreated with streptavidin.
3. Flow HBS buffer at 20 µL/min across the surface.
4. Inject the DNA solution across the surface, set the baseline to the point of injec-
tion, and monitor the change in RU during the injection phase. Ideally, between
20 and 100 RU of DNA should be immobilized (Fig. 3).
5. Wash the surface with a 50-µL injection of 1 M NaCl in filtered (0.2 µm) distilled
water.
6. Allow the surface to equilibrate in HBS buffer to a stable baseline, the difference
in RU between the beginning of the injection phase and the end of the wash
period reflects the amount of DNA bound. For stoichiometry, and availability of
sites, see Note 6.
7. Note that values in excess of 100 RU for DNA molecules of appro 100–1000 bp
are to be avoided for a number of reasons (see Notes 2–6).
3.2. Protein Binding to the Immobilized DNA
1. The protein should be prepared in the required buffer over a range of concentra-
tions, at least two orders of magnitude on either side of the suspected K
d
. The
detergent P20 should be present at concentrations of around 0.005% unless it has
been shown to have a deleterious effect on the interaction with the DNA.
2. Samples should be injected over the both the immobilized DNA surface and a
surface that has been treated with streptavidin and ethanolamine but no DNA as a
blank (Fig. 4).
3. The baseline should be stable with a slope inferior to 10 RU/min. If this is not the
case, then check the temperature of both the apparatus and the continuous-flow
buffer. If the problem persists, replace the sensor surface with an old used chip
and carry out a desorb and sanitize. Re-equilibrate the immobilized DNA chip in
new filtered (0.22-µm filters) buffer by running the prime command. If the prob-
lem persists, a potential reason may be degradation of the integrated fluid car-
tridge necessitating its replacement (see Note 8).
4. A typical sensorgram is shown in Fig. 4. Note that at low protein concentrations
it is very difficult to obtain steady-state saturation levels. Note also that there is a
nonspecific interaction with the control surface (curve b) and also a contribution
from the bulk refractive index effect and that both of these must be taken into
consideration when deducing kinetic or equilibrium values.
4. Notes
1. The relationship between mass and refractive index changes as measured by
changes in the angle at which resonance occurs in this system, although theoreti-
cally available through the additive properties of molar refractivity, has been
empirically established such that 10
–1
° is equivalent to 1000 resonance units (RU)
