Moss Tom. DNA-protein interactions: principles and protocols
Подождите немного. Документ загружается.

564 Schultz, Bischler, and Lebeau
staining. The transferred specimen placed on the grid held by forceps is washed
with a drop of buffer that is quickly removed. The buffer is then replaced by a
drop of a 1–2% aqueous solution of uranyl acetate and the grid is dried by touch-
ing a piece of filter paper with the edge of the grid (see Notes 3 and 4).
3. The crystallization experiments have then to be evaluated in terms of protein
concentration on the lipid layer and degree of organization (see Notes 11 and 12).
When the specimen is large enough (> 50 kDa), individual molecules can be
identified visually during electron microscopy inspection. To ascertain that the
specimen is specifically bound to the lipid layer and not in a nonspecific way to
the carbon film, it is useful to locate breaks in the lipid layer in order to observe
a difference in binding efficiency with the underlying carbon film. A frequently
observed intermediate step in specimen ordering is the formation of symmetry-
related oligomers that arise when a particular set of protein–protein interactions
is energetically favored. The formation of oligomers probably favors further
organization because interactions between symmetry related surfaces will propa-
gate forming linear polymers or 2-D crystals. Once larger crystalline areas are
obtained, electron micrographs are recorded and the extent of order is evaluated by
optical diffraction.
3.4. Feedback Loops
1. If the protein is not concentrated on the lipid film, it is advisable to act on the
lipid region involved in protein recognition, on its environment, or on the buffer
composition. In the case of a specific lipid, the linker may be too short to allow
the ligand to be recognized by the protein. Alternatively, the surface potential
created by the lipid layer may have a repulsive effect on the protein and it may be
of importance to modify the environment by the addition of a dilution lipid.
Finally, the ionic strength of the buffer may be too high and screen electrostatic
interactions between the protein and the charged lipid (see Note 5).
2. When the protein tends to form close-packed arrays, which do not evolve toward
organized protein patches, it is advisable to reduce the kinetics of protein con-
centration either by increasing the viscosity of the medium by adding glycerol
(up to 40%) or by reducing the temperature or the protein concentration. The
specific or charged lipid can also be diluted with a neutral lipid to reduce the
local concentration of ligand or the charge density of the surface (see Note 10).
3. The experiment has to be evaluated further in terms of macromolecular organiza-
tion. Higher degrees of order are recognized visually during the electron micros-
copy inspection of the specimen by the appearance of patches of ordered arrays
(Fig. 4A). Once larger crystalline areas are obtained, electron micrographs are
recorded and the extent of order is evaluated by optical diffraction. A large num-
ber of parameters such as the pH, the ionic strength, the buffer composition, the
presence of divalent cations, the protein concentration, the presence of glycerol,
the incubation temperature, or the incubation time can be modified to improve
crystal order (see Note 6). At this stage, the homogeneity of the specimen sus-
pension may be crucial.
2-D Crystallization of Protein Complexes 565
4. In the case of streptavidin, the method for preparing the sample for electron
microscopy and, in particular, the transfer mode proved to be essential to recover a
large number of highly ordered crystals (15). More generally, the manipulation of
one-molecule-thick assemblies during transfer to the electron microscope is likely to
introduce at least some of the defects observed in 2-D crystals such as rotational and
translational distortions, fragmentation, and other forms of disorders (see Notes 7–9).
5. An improvement of the interpretable resolution once the specimen diffracts to
about 0.5 nm
–1
will probably need a change in the method of specimen preserva-
tion from negatively stained to frozen hydrated samples (17).
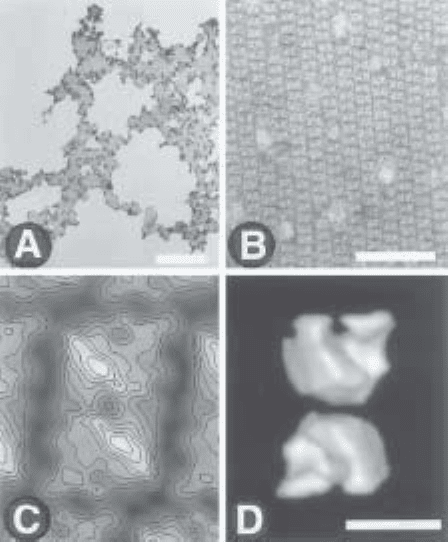
Fig. 4. Evaluation and exploitation of a 2-D crystallization experiment. Histidine-
tagged yeast RNA polymerase I was incubated with nickel chelating lipids. (A) Low-
magnification electron microscopy image showing the organization of the protein
complex into domains. The bar represents 5 µm. (B) A higher magnification reveals
ordered RNA polymerase arrays. The bar represents 50 nm. (C) A noise-free image is
obtained by averaging multiple molecular images. The stain excluding protein
densities are in white and represented as lines of equal densities. (D) A 3-D model of
the protein complex can be calculated by combining several views of the macro-
molecule obtained by tilting the crystals in the microscope. The bar represents 10 nm
in (C) and (D).
566 Schultz, Bischler, and Lebeau
6. To calculate a 3-D model, astigmatism-free and well-focused images of the
oriented or crystallized macromolecules must be recorded under minimal expo-
sure conditions and generally at low temperature. These images are analyzed to
calculate a noise-free image representing a projection of the macromolecular den-
sities (Fig. 4C). Because the particles are adsorbed on a planar surface, tilted
views are then recorded to recover the information normal to the lipid plane. The
images are then processed and the different views are merged into a 3-D model
(3) (Fig. 4D).
4. Notes
1. A good macroscopic indication that proteins bind to the lipid layers and that the
transfer is efficient is obtained by visual inspection of the carbon surface after
transfer. The original hydrophobic grid becomes hydrophilic, as assessed by the
change in its wetting properties.
2. Storage of the carbon-coated grids in hexane atmosphere may provide higher
reproducibility in the specimen transfer step by preventing adsorption of con-
taminating material.
3. Do not use phosphate buffers or buffers with high ionic strength, which precipi-
tate uranyl salts.
4. Other heavy metal solutions can be used for negative staining such as sodium
phosphotungstate or ammonium molybdate.
5. It is useful to check the specificity of the protein–lipid interactions. In the case of
charged lipids, the protein binding should be reduced by increasing the ionic
strength. In the case of functionalized lipids, the amount of transferred protein
should diminish by adding some competing ligand in solution. Note that in the
case of nickel-chelating lipids, it was observed that addition of small amounts of
imidazole prevented the nonspecific aggregation of the protein and allowed the
selection of the specific interaction with the polyhistidine tag (13).
6. Detergents should be avoided in the incubation buffer because they may solubi-
lize the lipid layer.
7. In some cases, it was observed that the grid side on which the carbon foil was
deposited affected crystal transfer (13). This effect may be related to the surface
roughness of the carbon foil and of the grid (18).
8. To strengthen the crystals, 1 µL of a 0.5% glutaraldehyde solution can be added
to the incubation drop before placing the electron microscopy grid in order to
crosslink the specimen.
9. Another method of specimen transfer is the loop method (19). A loop is formed
with a thin Pt/Pd wire (0.075 mm in diameter). The inside diameter must be
slightly larger than the outside diameter of the electron microscopy grid. The
loop is then lowered onto the drop, the entire loop makes contact with the drop
surface at the same time. This can be observed through drop deformation. So that
no excess subphase is picked up, the loop should not go through the monolayer
and into the subphase. The loop is then gently and carefully raised and lowered
onto a glow-discharged grid. The grid is held with forceps and is parallel to the
2-D Crystallization of Protein Complexes 567
film in the loop. The transfer is made by hydrophilic contacts between the carbon
foil and the crystal. The film is then broken by tilting the loop to increase the
angle between the film and the grid.
10. In order to better evaluate the organizational state of the molecule in the crystal-
lization experiment, it is useful to control its shape and size by direct adsorption
of the sample on a carbon film and negative staining. Such an experiment will
also give an insight into the aggregation state of the protein in solution.
11. The appearance of vesicular structures is often an indication for a too large excess
of lipids. The working lipid solution should then be diluted.
12. To remove excess lipids, a detergent solution at low concentration can be used
(19). Care must be taken during this step because the drop might migrate to both
sides of the grid and interfere with the staining process.
References
1. Henderson, R., Baldwin, J. M., Ceska, T. A., Zemlin, F., Beckmann, E., and
Downing, K. H. (1990) Model for the structure of bacteriorhodopsin based on
high-resolution electron cryo-microscopy. J Mol. Biol. 213, 899–929.
2. Kuhlbrandt, W., Wang, D. N., and Fujiyoshi, Y. (1994) Atomic model of plant
light-harvesting complex by electron crystallography. Nature 367, 614–621.
3. Amos, L. A., Henderson, R., and Unwin, P. N. (1982) Three-dimensional struc-
ture determination by electron microscopy of two-dimensional crystals. Prog.
Biophys. Mol. Biol. 39 , 183–231.
4. Uzgiris, E. E. and Kornberg, R. D. (1983) Two-dimensional crystallization tech-
nique for imaging macromolecules, with application to antigen-antibody-comple-
ment complexes. Nature 301, 125–129.
5. Darst, S. A., Ribi, H. O., Pierce, D. W., and Kornberg, R. D. (1988) Two-dimen-
sional crystals of Escherichia coli RNA polymerase holoenzyme on positively
charged lipid layers. J. Mol. Biol. 203, 269–273.
6. Schultz, P., Celia, H., Riva, M., Darst, S. A., Colin, P., Kornberg, R. D., et al.
(1990) Structural study of the yeast RNA polymerase A. Electron microscopy
of lipid-bound molecules and two-dimensional crystals. J. Mol. Biol. 216,
353–362.
7. Ribi, H. O., Reichard, P., and Kornberg, R. D. (1987) Two-dimensional crystals
of enzyme-effector complexes: ribonucleotide reductase at 18-A resolution. Bio-
chemistry 26, 7974–7979.
8. Lebeau, L., Regnier, E., Schultz, P., Wang, J. C., Mioskowski, C., and Oudet, P.
(1990) Two-dimensional crystallization of DNA gyrase B subunit on specifically
designed lipid monolayers. FEBS Lett. 267, 38–42.
9. Avila-Sakar, A. J., and Chiu, W. (1996) Visualization of beta-sheets and side-
chain clusters in two-dimensional periodic arrays of streptavidin on phospholipid
monolayers by electron crystallography. Biophys. J. 70, 57–68.
10. Lebeau, L., Schultz, P., Celia, H., Mesini, P., Nuss, S., Klinger, C., et al. (1996)
Specifically designed lipid assemblies as tools for two-dimensional crystalliza-
tion of soluble biological macromolecules, in Handbook of Nonmedical Applica-
568 Schultz, Bischler, and Lebeau
tions of Liposomes, vol. II (Barenholz Y. and Lasic D. D., eds.), CRC, Boca Raton,
FL, pp. 155–188.
11. Darst, S. A., Ahlers, M., Meller, P. H., Kubalek, E. W., Blankenburg, R., Ribi, H.
O., et al. (1991) Two-dimensional crystals of streptavidin on biotinylated lipid
layers and their interactions with biotinylated macromolecules. Biophys. J. 59,
387–396.
12. Kubalek, E. W., Le Grice, S. F., and Brown, P. O. (1994) Two-dimensional crys-
tallization of histidine-tagged, HIV-1 reverse transcriptase promoted by a novel
nickel-chelating lipid. J. Struct. Biol. 113, 117–123.
13. Bischler, N., Balavoine, F., Milkereit, P., Tschochner, H., Mioskowski, C., and
Schultz, P. (1998) Specific interaction and two-dimensional crystallization of his-
tidine tagged yeast RNA polymerase I on nickel-chelating lipids. Biophys. J. 74,
1522–1532.
14. Mosser, G. and Brisson, A. (1991) Structural analysis of two-dimensional arrays
of cholera toxin B- subunit. J. Electron Microsc. Tech. 18, 387–394.
15. Kubalek, E. W., Kornberg, R. D., and Darst, S. A. (1991) Improved transfer of
two-dimensional crystals from the air/water interface to specimen support
grids for high-resolution analysis by electron microscopy. Ultramicroscopy 35,
295–304.
16. Fukami, A. and Adachi, K. (1965) A new method of preparation of a self-perfo-
rated micro plastic grid and its application. J. Electron Microscopy, 14, 112–118.
17. Dubochet, J., Adrian, M., Chang, J. J., Homo, J. C., Lepault, J., McDowall, A. W.,
et al. (1988) Cryo-electron microscopy of vitrified specimens. Quart. Rev.
Biophys. 21, 129–228.
18. Schmutz, M. and Brisson, A. (1996) Analysis of carbon film planarity by reflected
light microscopy. Ultramicroscopy 63, 263–272.
19. Asturias, F. J. and Kornberg, R. D. (1995) A novel method for transfer of two-
dimensional crystals from the air/water interface to specimen grids. EM sample
preparation/lipid-layer crystallization. J. Struct. Biol. 114, 60–66.

AFM of DNA and Nucleoproteins 569
569
From:
Methods in Molecular Biology, vol. 148: DNA–Protein Interactions: Principles and Protocols, 2nd ed.
Edited by: T. Moss © Humana Press Inc., Totowa, NJ
39
Atomic Force Microscopy of DNA
and Protein-DNA Complexes
Using Functionalized Mica Substrates
Yuri L. Lyubchenko, Alexander A. Gall,
and Luda S. Shlyakhtenko
1. Introduction
Atomic force microscopy (AFM; also called scanning force microscopy
[SFM]) is a rather novel technique that offers unique advantages in the poten-
tial for the very high resolution of DNA and small ligands in the absence of
stains, shadows, and labels (1,2). Furthermore, the scanning can be performed
in air or liquid. The latter is particularly important for resolving fully hydrated
structures. The AFM is theoretically capable of resolving structural details at
the level of atomic dimensions, provided that the specimen is not dynamic.
A serious practical limitation to the application of AFM to structural and
conformational studies of DNA and its complexes with proteins and other bio-
logical macromolecules has been sample preparation. The macromolecules
must be tethered to the substrate surface in order to avoid resolution-limiting
motion caused by the sweeping tip during scanning. Progress in sample prepa-
ration for AFM studies of DNA has been achieved in a number of groups (3–7)
and some of these approaches have been applied to studies of a number of
protein–DNA complexes (3,4,8).
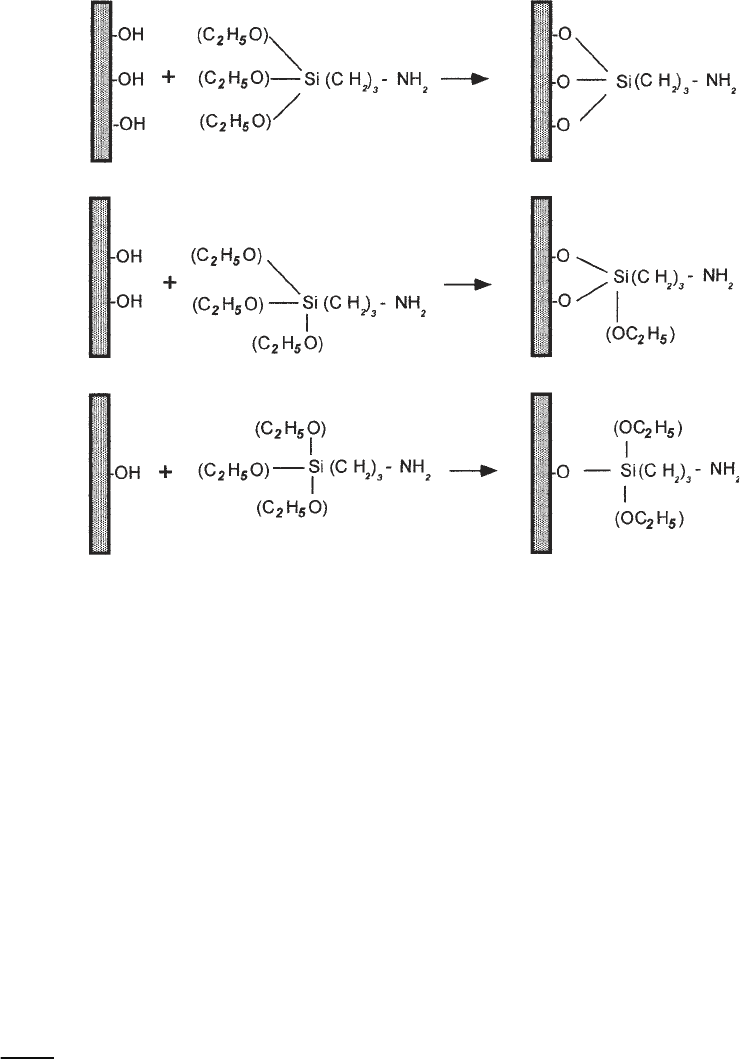
A versatile approach based on functionalization of surfaces with silanes was
suggested in refs. 9–11. A weak cationic surface is obtained if amino-
propyltrietoxy silane (APTES) is used to functionalize the mica surface with
amine groups (AP-mica). This technique in addition to imaging nucleic acids
under different conditions (10,12–14) was applied to imaging of a number of
nucleoprotein complexes (9,11,15–17). Here, we describe a sample prepara-
tion procedure for AFM using AP-mica substrates.

570 Lyubchenko et al.
The method of functionalization of mica is based on covalent attachment of
3-aminopropyltriethoxy silane to the surface of the mica, as shown schemati-
cally in Fig. 1. The amino groups of APTES are bound covalently to the freshly
cleaved mica surface, giving it properties similar to an anion-exchange resin
used in affinity chromatography. This group after being exposed to a water
solution becomes positively charged in a rather broad range of pH (aliphatic
amino groups have a pK of around 10.5). Therefore, DNA, which is a nega-
tively charged polymer, should adhere to this surface strongly. The binding of
DNA to AP-mica was monitored directly by the use of radiolabeled DNA. AFM
imaging of AP-mica showed that very low concentration of APTES (less than
1 µM) should be used to obtain smooth surface (9,10). Vapor deposition of
APTES allowed one to obtain the surface with mean roughness of several ang-
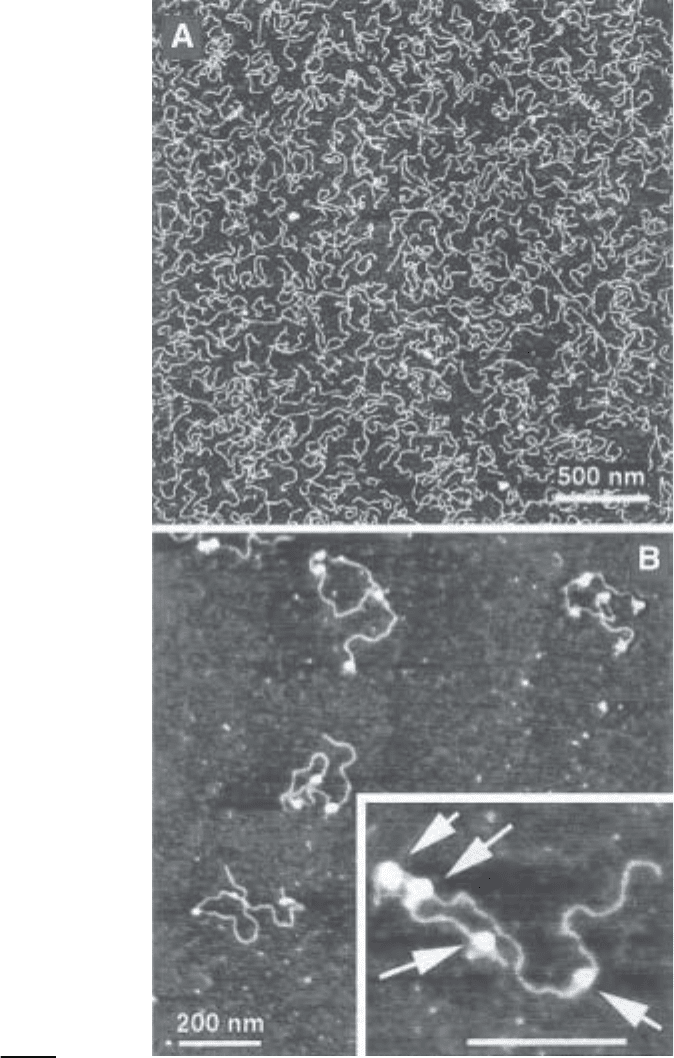
stroms (9–12), so the DNA and DNA–protein complexes can be visualized
easily (see Fig. 2A,B, respectively).
The features of this procedure of sample preparation are as follows (9,11):
• DNA binding to AP-mica is insensitive to the type of buffer and presence of
Mg
2+
or other divalent and miltivalent cations; hence sample preparation can be
done in a variety of conditions.
• Deposition can be done in a wide variety of pH and over a wide range of
temperatures.
• Once prepared, samples are stable and do not absorb any contaminants for months
with minimal precautions for storing.
• As low as 10 ng of DNA is sufficient for the preparation of one sample.
These characteristics of AP-mica were crucial for routine imaging nucleic
acids (DNA, dsRNA, kinetoplast DNA) and nucleoprotein complexes of dif-
ferent type (9,16,17).
2. Materials.
1. Chemicals: commercially available 3-aminopropyltriethoxy silane (e.g., Fluka,
Chemika-BioChemika (Switzerland), Aldrich (USA), United Chemical Technol-
ogy (USA), and N,N-diisopropylethylamine (Aldrich, Sigma). It is recommended
to redistill APTES and store under argon.
2. Mica substrate: any type of commercially available mica sheets (green or ruby
mica). Asheville-Schoonmaker Mica Co. (Newport News, VA) supplies both
thick and large (more than 5 × 7 cm) sheets suitable for making the substrates of
different sizes.
3. Water: Double glass distilled or deionized water filtered through a 0.5-µm filter.
4. 2-L glass desiccators and vacuum line (50 mmHg is sufficient).
5. Plastic syringes (5–10 mL) with a plastic tip for rinsing the samples.
6. Plastic syringes (1 mL) for imaging in liquid.
7. Gas tank with clean argon gas.
8. Vacuum cabinet for storing the samples.
AFM of DNA and Nucleoproteins 571
3. Methods
3.1. AP-Mica Preparation
1. Place two plastic caps (cut them from regular 1.5 mL plastic tubes) on the bottom
of a 2-L desiccator, evacuate then purge with argon.
2. Cleave mica sheets (approx 5 × 5 cm) to make them as thin as 0.1–0.05 mm and
mount at the top of the desiccator.
3. Put 30 µL of APTES into one plastic cap in the desiccator and 10 µL of N,N-
diisopropylethylamine (Aldrich) into the other cap and allow the functionalization
reaction to proceed for 1–2 h. Remove the cap with APTES and purge the desic-
cator with argon for 2 min.
4. Leave the sheets for 1–2 d in the desiccator to cure. The AP-mica is then ready
for the sample deposition. (See Note 1.)
The procedure allows one to obtain a weak cationic surface with rather uni-
form charge distribution. This is illustrated in Fig. 2A by the uniform distribu-
tion of DNA fragments that can be obtained.
Fig. 1. The reaction of aminopropyltriethoxy silane (APTES) with mica. Three pos-
sible types of reaction are illustrated.
572 Lyubchenko et al.

AFM of DNA and Nucleoproteins 573
3.2. Sample Preparation for AFM Imaging in Air
3.2.1. The Droplet Procedure
1. Prepare the solution of the sample (DNA, RNA, protein–DNA complex) in
appropriate buffer. DNA concentration should be between 0.1 and 0.01 µg/mL,
depending on the size of the molecules (see Notes 2 and 3).
2. Place 5–10 µL of the solution in the middle of AP-mica substrate (usually 1 × 1-cm
squares) for 2–3 min.
3. Rinse the surface thoroughly with water (2–3 mL per sample) to remove all buffer
components. A 10-mL plastic syringe is very useful for rinsing, but attach an
appropriate plastic tip instead of a metal needle.
4. Dry the sample by blowing with clean argon gas. The sample is ready for imag-
ing. Store the samples in vacuum cabinets or desiccators filled with argon.
3.2.2. The Immersion Procedure
This procedure is recommended if the deposition should be performed at
strictly controlled temperature conditions (0°C or elevated temperatures).
1. Prepare the solution (DNA, RNA, nucleoprotein complexes) and preincubate for
10–20 min to allow the temperature to equilibrate. Recommended concentration
of DNA is 0.2 –0.01 µg/mL, depending on the size of the molecules (see Notes 2
and 3).
2. Immerse a piece of AP-mica into the vials and leave it for 10–20 min to allow the
samples to adsorb to the surface.
3. Remove the specimen, rinse with water thoroughly, and dry under the argon flow.
The sample is ready for imaging.
4. The samples can be stored in vacuum cabinet or under argon.
3.3. AFM Imaging in Air
1. Mount the sample and start approaching the probe.
2. Both the contact and intermittent (tapping) modes can be used, but the latter is
preferable and allows one to obtain images of DNA and DNA–protein complexes
routinely. Our experience is mostly limited to a NanoScope III microscope
(MultiMode system, Digital Instruments, CA), but samples prepared on AP-mica
were imaged on other commercially available instruments (e.g., the microscopes
manufactured by Topometrix, Park Scientific Instruments, Molecular Imaging).
With the MultiMode system, any type of probe designed for noncontact imaging
can be used. NanoProbe TESP tips (Digital Instruments, Inc.) and conical sharp
Fig. 2. (previous page) AFM images of a 800-bp fragment (A) and reconstituted
chromatin (B). The concentration of DNA was 0.5 µg/mL in (A). Reconstituted chro-
matin was deposited onto the substrate after glutaraldehyde fixation. (The sample was
from D. Lohr [Arizona State University] and the images were taken in air with TM
AFM [NanoScope III]).
