Moss Tom. DNA-protein interactions: principles and protocols
Подождите немного. Документ загружается.

Calorimetry of Protein–DNA Complexes 513
constant and T is the absolute temperature. For the simple equilibrium describ-
ing the interaction of protein P with DNA, D:
P + D ⇔ PD then K
A
=
[PD]
(1)
[P][D]
Techniques such as equilibrium dialysis, ultracentrifugation, the gel-shift,
or radio-ligand binding assays yield direct values for [P], [D], and [PD] and,
therefore, values for K
A
and ∆G at any fixed temperature. More indirect, mostly
spectroscopic, methods allow the construction of a binding isotherm in terms
of the degree of saturation, Y:
Y =
∆p
i
=
[PD]
=
K
A
[P]
(2)
∆p
max
[D] + [PD] 1 + K
A
[P]
where ∆p
i
is the signal change obtained at some particular addition of P to a
constant amount of D and ∆p
max
is the maximal signal change at full saturation
(Y = 1). If the experiment is repeated at different temperatures, K
A
(T) and ∆G(T)
are obtained. ∆H(T) and ∆S(T) can be derived from the integrated form of the
van Hoff equation. Further, the s derivative of ∆G(T) with respect to tempera-
ture yields ∆C
p
. This noncalorimetric analysis of binding data (sometimes
referred to as a van Hoff analysis) can be flawed. First, the analysis assumes a
model, which may or may not be correct, to describe the interaction of the
components. Second, for technical reasons, experiments can be performed only
over a limited temperature range and the experimental errors propagate into
large errors in ∆H, ∆S, and especially ∆C
p
when the extrapolation from the
reference temperature to any other temperature is large.
1.2. The ITC Experiment
Modern mixing microcalorimeters allow precise measurements of the
enthalpy of binding, ∆H
app
, between 5°C and 80°C. The basic principle is that
one binding partner (protein, P) is titrated at a constant temperature into a
known amount of the other binding partner (DNA, D) placed in the sample cell
of the calorimeter. Heat is produced or released when binding occurs. The instru-
ment measures the electrical power (in units of J/s) required to maintain a small
temperature difference between the sample cell and the reference cell, which is
filled with buffer. The contents of the sample cell are stirred to allow rapid mixing
and effective heat transfer over the surface of the cell. If K
A
is high and the degree
of saturation is still low, electrical power peaks of similar magnitude appear in
the thermogram at each addition. Integration with respect to time yields the
apparent heat change, ∆q
i,app
, between additions i and i–1 as ∆q
i,app
= q
i
– q
i
–1.
As the fractional saturation increases, ∆q
i,app
gradually decreases and even-
tually all binding sites become saturated. Small nonspecific heat effects, ∆q
i,ns

514 Read and Jelesarov
registered after complete saturation may be caused by the heat of protein
dilution, an imperfect match between the buffer composition of the protein
solution and the DNA solution, or by other nonspecific effects. ∆q
i,app
is pro-
portional to the volume of the calorimetric cell, V
cell
, to the change in concen-
tration of the bound protein ∆[P]
i,bound =
[P]
i,bound
= [P]
i–1,bound
and to the
apparent molar enthalpy of association ∆H
app
. Thus,
∆q
i,app
= ∆q
i
+ ∆q
i,ns
(3)
= ∆[P]
i,bound
V
cell
∆H
app
∆H
app
at temperature T may therefore be calculated because V
cell
is known and
∆q
i,ns
may be obtained from a blank titration of protein into buffer.
For the binding of protein, P, with n identical and noninteracting sites to
DNA, D,
∆q
i
= ∆q
i,app
+ ∆q
i,ns
(4)
= nR V
cell
∆H
app
∆q
i
is the effective heat change caused by the formation of complex, PD, at
the i
th
step of the titration and R is the root of the quadratic equation
Y
i
2
– Y
i
(
1 +
1
+
[P]
i,total
)
+ n[P]
i,total
[D]
total
= 0 (5)
nK
A
[D]
total
n[D]
total
where the fractional saturation is defined by Y
i
= ∆[P]
i,bound
/ [D]
total
. [P]
i,total
is
the total concentration of protein, P, added until injection, i. A nonlinear
regression procedure based on Eq. 4 yields n, K
A
, and ∆H
app
at temperature T,
from a single titration experiment. More complicated systems with multiple
and interacting sites require a statistical mechanical treatment of the data
(11,12). ∆C
p
,
app
the apparent heat capacity change of association can be calcu-
lated from a plot of ∆H
app
versus temperature, because in general, ∆C
p
= ∆H / ∆T.
As in other binding experiments, the dissociation constant, K
D
(equal to 1/K
A
)
can only be reliably measured over a narrow concentration range. If the con-
centration of binding sites is much larger than K
D
, the binding isotherm is of
rectangular shape with a sharp step at saturation. In the case of the binding-site
concentration being much smaller than K
D
, the binding isotherm is very shal-
low and is of limited use for calculating the dissociation constant. In practice,
values of the ratio of the binding-site concentration to K
D
that range between
10 and 100 represent an optimal experimental window to obtain precise values
of the dissociation constant. Unfortunately, this window is not always acces-
sible to ITC. For very small K
D
values, the optimal concentrations are so low
that the released heat is below the sensitivity of the instrument, the specific
enthalpy of biomolecular interactions never being very large. For this reason,
there is an effective lower limit to the ITC measurement of K
D
to values of
Calorimetry of Protein–DNA Complexes 515
approx 10
–9
M (∆G about –50 kJ/mol). Alternatively, if K
D
is high, then very
high concentrations are required and the heat of binding may be obscured by
aggregation effects.
The two types of ITC measurement are normally obtained under different
conditions. The procedure to obtain n, K
A
, and ∆H
app
is best carried out as a
full titration at concentrations near the stoichiometric point of the interaction
(i.e., at a DNA concentration 10–100 times the estimated value for K
D
and with
the injection of up to a 2 M to 4 M excess of protein. However, the direct
measurement of ∆H
app
is best carried out in conditions where the system is
fully associated and the degree of saturation is low (i.e., at high DNA concen-
trations (>1000K
D
) and with the injection of low amounts of protein). The total
number of moles of added protein is then much less than the number of moles
of binding sites available and virtually all protein molecules are bound to DNA,
such that ∆[P]
i,bound
approximately equals ∆[P]
i,total
. Each injection of protein,
∆[P]
i,total
thus produces an approximately equal change in heat, ∆q
i,app
. If the
overall fractional saturation is still low after completion of a series of injec-
tions, then the average may be taken and Eq. 3 becomes
m m
1
∑∆qi
,app
=
1
∑
(
∆qi
+
∆qi
,ns
)
(6)
m
i=1
m
i=1
m
=
1
∑
(
∆[P]
i,bound +
V
cell
∆H
app
)
m
i=1
where m is the number of injections. If low amounts of protein are injected,
aggregation effects and nonspecific binding are also minimized.
1.3. The DSC Experiment
In a DSC experiment, the heat capacity of a macromolecule is measured as a
function of temperature. Typically, two thermally insulated cells, a sample cell
containing the macromolecule of interest in buffer and a reference cell contain-
ing buffer, are electrically heated at a known rate. At a temperature-induced
transition, which is typically endothermic, the temperature of the sample cell
will lag behind that of the reference cell. An electrical feedback mechanism is
used to maintain the reference cell at the same temperature as the sample cell.
This amount of compensatory electrical power (in units of J/s or Watts) at
temperature T divided by the heating rate is the apparent difference in heat capac-
ity between the cell containing the sample and the reference cell, ∆C
p,app
(T) (in
units of J/K). Because the sample containing cell has a smaller volume fraction of
buffer as compared to that in the reference cell, the partial molar heat capacity of
the dissolved macromolecule C
p,f
(T) at temperature T (J/mol/K) is given by

516 Read and Jelesarov
CT
p,φ
(T) =
C
p,buffer
(T)V
φ
˚M∆C
p,app
(T)
(7)
V
buffer
m
where C
p,buffer
(T) and V
buffer
are the partial molar heat capacity and molar vol-
ume of buffer, respectively. V
f
˚ and M are the partial molar volume and the
molar mass of the macromolecule, respectively, and m is the mass of macro-
molecule in the sample cell.
The excess molar heat capacity function is the heat absorbed in the melting
transition above that of the intrinsic heat capacity of the macromolecule. Inte-
gration of the excess molar heat capacity function with respect to temperature
yields the enthalpy of the melting transition:
T
2
∆H = 兰具C
p
(T)典dT (8)
T
1
The intrinsic heat capacity of the initial (folded) and final (unfolded) states
of the macromolecule must be estimated within the melting transition. For
monomeric proteins, the heat capacity of the folded state is an approximate
linear function of temperature (13,14) and the unfolded state is a shallow para-
bolic function of temperature (13).
The relationship of the partial molar heat capacity to the excess molar heat
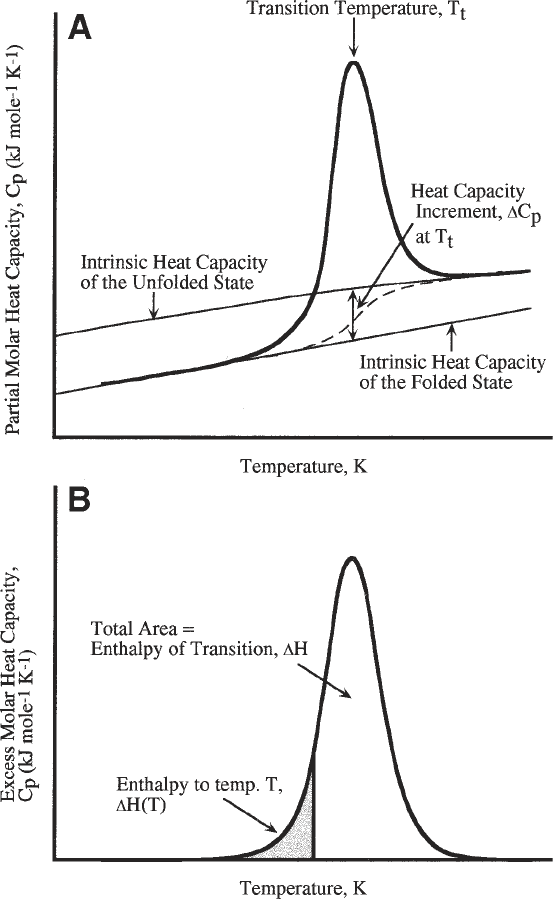
capacity functions are schematically shown in Fig. 1.
1.4. Preliminary Characterization of the Sox-5–DNA Interaction
It must be emphasized that the in vitro interaction of the protein with DNA
must be characterized prior to the calorimetry experiments. Preliminary
knowledge of the number of (independent) binding sites, the stoichiometry of
the interaction, and the magnitude of the association constant are all required
for the optimal design of the ITC experiments (see Subheading 1.2.). For DSC,
the experiments must be performed with a fully associated complex (at low
temperature) and therefore at a concentration well above the estimated K
D
.
Furthermore, the protein–DNA complex and its components need to be soluble
at this concentration, even at raised temperatures, and all temperature-induced
conformational transitions must be fully reversible. Knowledge of the mode of
interaction is also required to obtain van Hoff enthalpies from the DSC data.
Site-selection and DNAse I footprinting assays (Chapter 3) defined the
Sox-5 target sequence as 5’-AACAAT-3' within a DNAse I protected region of
approx 14 nucleotides on both DNA strands (15,16). This determined the
sequence and size of the DNA duplexes that were to be used. A circular permu-
tation assay showed that the binding of Sox-5 to these duplexes was able to
introduce a DNA bend of similar magnitude to that obtained with the complete
Calorimetry of Protein–DNA Complexes 517
Fig. 1. Schematic representation of (A) the partial molar heat capacity function as
would be observed for the melting of a single domain monomeric protein. Over the
transition region the intrinsic heat capacity function is an interpolation of the folded
and unfolded states, weighted in proportion to their relative contributions (dashed line).
(B) replots the C
p
/T function above that of the intrinsic heat capacity of the system.
This is known as the molar excess heat capacity function.
518 Read and Jelesarov
Sox-5 protein (6,16). The dissociation constant and the stoichiometry of the
Sox-5–DNA interaction were determined from circular dichroism (CD) and
gel-shift assays (Chapters 2 and 34):
1. The binding of Sox-5 to a 12-bp DNA duplex (10 µM) was followed by CD,
monitoring the ellipticity of the positive DNA peak at 280 nm upon successive
additions of the protein. The CD data were fitted to a 1:1 binding model with an
estimated K
D
of lower than 100 nM (6). This same estimate was obtained at tem-
peratures between 10°C and 37°C, suggesting no significant variation of binding
constant with temperature.
2. A gel-shift assay using a radioactively labeled 12-bp DNA duplex (at 1 nM) was
titrated in molar excess with Sox-5 (6). This indicated that the primary binding
site is characterized by a K
D
of about 35 nM at 4°C. No secondary protein binding
was observed with <1 µM Sox-5.
These assays thus indicate a bimolecular interaction of Sox-5 with the DNA,
with a single primary binding site characterized by a K
D
in the low nanomolar
range. Furthermore any secondary binding of Sox-5 to DNA, if present, must
have a K
D
of >10 µM (i.e., at least two orders greater than the primary binding
site). Thus, for the DSC measurements, typically at a concentration of 100–
500 µM complex, the preformed Sox-5–DNA complex is fully associated and
the effect of secondary binding is negligible.
Given the estimated K
D
value, the ITC measurement of a full binding iso-
therm under optimal conditions requires a DNA concentration of between
approx 0.35 and 3.5 µM (see Subheading 1.2.). Preliminary titration experi-
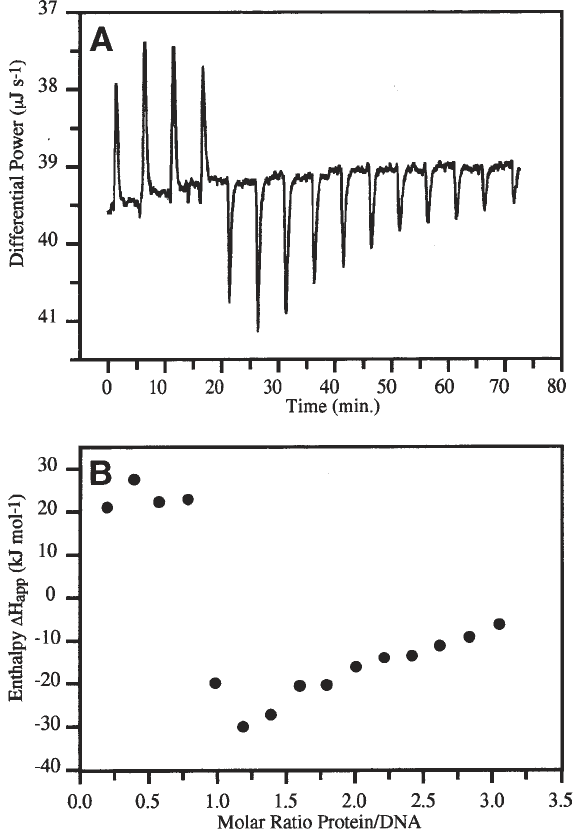
ments however showed only a small heat effect of association (a result of the
temperature dependency of ∆H
app
which passes through zero at 17°C) and the
presence of a secondary binding event, with a large exothermic effect, after
saturation of the primary binding site (Fig. 2). It was concluded that measure-
ment of the entire binding isotherm was not possible by ITC.
However, conditions could be chosen that were optimal for the direct deter-
mination of ∆H
app
by ITC (i.e., total association at partial saturation (see Sub-
heading 1.2.). At the chosen DNA concentration of 60 µM, total association of
injected Sox-5 in an ITC experiment is achieved (the ratio of the DNA concen-
tration to the estimated K
D
being approx 1700). The fractional molar saturation
was always kept lower than 0.5 to avoid any secondary binding effects. Because
high concentrations were to be used, the total heat effect was greater and more
easily detectable, which meant that ∆H
app
values could be accurately obtained
at temperatures close to 17°C.
Additional experiments included low-speed analytical ultracentrifugation at
different temperatures to demonstrate that the free Sox-5 is monomeric. For
the free DNA, UV melting was performed as an initial check on its melting
temperature and for two-state melting of the duplexes. UV melting of the
Calorimetry of Protein–DNA Complexes 519
Sox-5–DNA complex showed a single cooperative transition with a melting
temperature above that of the free DNA.
Fig. 2. ITC titration experiment of Sox-5 into a 12-bp DNA duplex. (A) Fifteen
injections of 540 µM Sox-5 into 10 µM of 12-bp DNA at 9°C. The injection rate was
5 µL in 8 s, with a time interval of 5 min between injections. (B) a plot of ∆H
app
against
ratio of Sox-5 to DNA showing the primary endothermic and secondary exothermic
reactions that occur on either side of the stoichiometric point.
520 Read and Jelesarov
2. Materials
2.1. Calorimeters
Isothermal titration calorimetry was performed on an OMEGA or MCS-ITC
titration calorimeter (MicroCal Inc., Northampton, MA). Technical details on
the construction of mixing microcalorimeters, their performance and sensitiv-
ity, and on the theory of data analysis have been described elsewhere (17–20).
Differential scanning calorimetric experiments were usually carried out on
a Nano-DSC calorimeter (Calorimetric Science Corp., Utah). The instrument’s
performance and data acquisition are detailed in ref. 21. In brief, the instru-
ment operates over the temperature range –20°C to 130°C at any set heating/
cooling rate. The sample and reference calorimetric cells are of approx 900 µL
volume. Control of the calorimeter and scan acquisition is achieved via the
supplied DSC_Acquisition program running on a PC computer connected to
the calorimeter. In experiments in which the amount of sample was limiting, a
new version of the Nano-DSC calorimeter having cells of approx 300 µL vol-
ume was used.
2.2. Reagents and Solutions
2.2.1. Calorimetry
1. For calorimetric experiments, it is important to ensure that the protein and DNA
samples are homogeneous, because even a few percent of contaminating species
might cause significant heat effects. Homogeneity of the samples is most reliably
verified by mass spectrometry.
2. Concentrations must be accurately determined because this affects the DSC and
ITC data (see Subheading 3.4.). The preparation of equimolar mixtures (of
complementary oligonucleotides or Sox-5-DNA complex) can then be made
precisely, without further purification. Dilutions are made by weight on a preci-
sion balance.
3. The DSC and ITC experiments with the Sox-5–DNA complex used a working
buffer of 100 mM KCl, 10 mM potassium phosphate, and 1 mM EDTA (pH 6.0)
(see Note 1). All buffer solutions were prepared from the highest-quality reagents
and ultrapure water. For ITC experiments, the solutions were filtered through
a 0.45-µm membrane and thoroughly degassed prior to use.
2.2.2. Preparation of Sox-5 HMG Box–DNA Complexes
and Components
1. DNA oligonucleotides were synthesized using phosphoramidite chemistry and
purified on a Mono Q HR16/10 column fitted to a Pharmacia FPLC system, elut-
ing with a linear 0.1 M to 1.0 M NaCl gradient in 10 mM Tris-HCl, 1 mM EDTA,
and 20% (v/v) acetonitrile (pH 7.0). Fractions containing oligonucleotide were
precipitated with 3 vol of ethanol at –20°C overnight, then centrifuged down and
Calorimetry of Protein–DNA Complexes 521
redissolved in water. Oligonucleotides were extensively dialyzed using
Spectrapor tubing (molecular-weight cutoff 500 Daltons) against three changes
of 1 L working buffer at 4°C.
2. DNA duplexes were prepared by mixing equimolar amounts of the complemen-
tary oligonucleotides in working buffer and annealed by heating to 95°C in a
water-bath followed by slow cooling to 4°C over a period of approx 4 h. DNA
duplexes were then extensively dialyzed using 3000-Dalton molecular-weight
cutoff tubing against three changes of 1 L working buffer at 4°C.
3. The HMG box of mouse Sox-5 (amino acids 182 to 260, [15]) was expressed as
a fusion protein in pGEX-2T, using E. coli BL21 (DE3) plysS cells. After affinity
purification with glutathione-agarose and thrombin cleavage while still
attached to the column (22), reverse-phase high-performance liquid chromatog-
raphy (HPLC) was used to purify the protein. Protein was redissolved in water
and refolded by extensive dialysis against three changes of 1 L working buffer
at 4°C.
4. The Sox-5–DNA complex was prepared by the mixing together of equal volumes
of the components (at equimolar concentration) in working buffer at 4°C. Sox-5
was added in 10% aliquots, at 5-min intervals, to the DNA. The complex was
then extensively dialyzed using 3000-Dalton molecular-weight cutoff tubing
against three changes of 1 L working buffer at 4°C. The accuracy of the concen-
trations of protein and DNA used to form the 1:1 complex may be checked by
trial additions of Sox-5 to DNA at various protein:DNA ratios followed by elec-
trophoresis on a nondenaturing polyacrylamide gel.
2.2.3. DNA Concentrations
1. 100 mM Tris-HCl (pH 8.0).
2. Snake venom phosphodiesterase I (PDE1, from Crotalus durissus terrificus, Sigma).
3. Methods
3.1. Isothermal Titration Calorimetry
Before a series of experiments, the calorimeter was calibrated either by
applying electrically generated heat pulses or by standardized chemical reac-
tions (e.g., the protonation of tris[hydroxymethyl] aminomethane or the bind-
ing of Ba
2+
to 18-crown-6 ether). It is recommended to equilibrate the jacket
with a circulating water-bath for about 10 h. at a temperature lower than the
temperature of the experiment by 3–5°C. Although such equilibration is not
strictly necessary when operating at above room temperature, it substantially
improves the baseline stability. The stirring speed during both the equilibra-
tion and the experiment was 350 rpm (see Note 2).
1. Sample and reference cells are first rinsed with dialysis buffer. The reference cell
is then filled with buffer and the sample cell filled with the DNA solution. The
system is heated to the working temperature and equilibrated until the differen-

522 Read and Jelesarov
tial power signal levels off. The injection syringe containing Sox-5 is inserted
into the sample cell, stirring is initiated, and the baseline is established over a
period of 30–60 min. Typically, a baseline drift (differential power signal drift)
of less than 20–30 nJ/min and an rms noise of less than 15–20 nJ/s (or nW) indi-
cates complete thermal equilibration of the system under stirring (see Note 3).
2. The experiment is started with a small injection of 1–2 µL. The reason for this is
that during the long equilibration period, diffusion through the injection ports
occurs, thus causing a change in protein concentration near the syringe needle
tip. The actual injection schedule is then executed. To measure the enthalpy of
Sox-5 binding to DNA, six to eight injections each of 8–12 µL and of 12–15 s
duration were performed, with a 5-min interval between injections (see Note 4).
Typical thermograms are depicted in Fig. 3A, traces a and c.
3. After completion of the experiment, the cells are thoroughly cleaned. Cleaning of
the calorimetric cells and filling syringes follows a standard laboratory protocol
(see Note 5).
4. The cells may then be filled with buffer and step 2 repeated. Injections of protein
into buffer will yield the heat associated with protein dilution and other non-
specific effects. Typical control thermograms are depicted in Fig. 3A, traces b
and d. Because the heats obtained in steps 2 and 4 are directly compared in the
data analysis, it is crucial that (1) the blank titration is performed at exactly the
same temperature as the main experiment, (2) the same protein solution and
dialysis buffer are used, and (3) an identical injection scheme is executed. If
any of the listed requirements is not fulfilled, the results of the experiment could
be misleading.
5. The titrations are performed at different temperatures to collect data on the tem-
perature dependence of the binding enthalpy.
3.1.1. ITC Data Analysis
If a complete binding isotherm has been recorded over an optimal concen-
tration range, the data can be subjected to a nonlinear least-squares analysis to
obtain a full set of parameters (∆H
app
, K
A
, and n) according to Eq. 4. In the
case of Sox-5 binding to DNA, the experiments were designed to measure only
the enthalpy and heat capacity changes. Thus integration of the differential
power peaks (see Note 6) collected as in step 2 of Subheading 3.1. yields
∆q
i,app
and the nonspecific heats ∆q
i,ns
are obtained by integration of the peaks
collected as in step 4 of Subheading 3.1. Under conditions of total association
∆[P]
i,bound
equals ∆[P]
i,total
and is easily calculated from the known concentra-
tion of protein in the injection syringe, the volume of each injection and the
Fig. 3. (opposite page) Representative records of a Sox-5 titration into DNA duplex
(A) and enthalpies of binding of Sox-5 to a 12-bp DNA duplex as measured by ITC
(B). (A) Trace a: six injections of 550 µM Sox-5 into 58 µM of 16-bp DNA at 9°C (i.e.,
up to a fractional saturation of 0.45); trace b: control titration of Sox-5 into buffer at
