Moss Tom. DNA-protein interactions: principles and protocols
Подождите немного. Документ загружается.

390 Robert and Coulombe
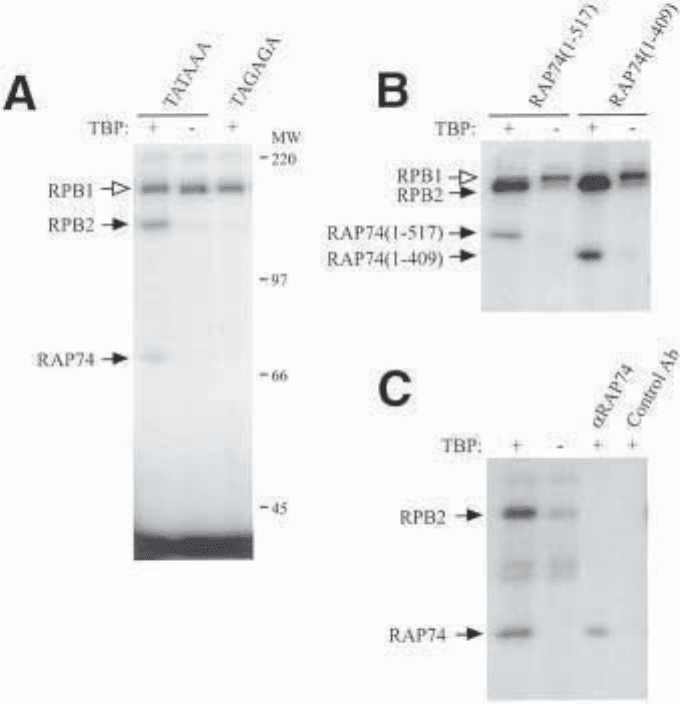
Fig. 3. Typical SDS-PAGE gels of photocrosslinked proteins. (A) Crosslinking
reactions assembled either in the absence of TBP or using a probe with a mutated
TATA box give identical results. Crosslinking reactions were performed with TFIIB,
RAP30, RAP74, TFIIE34, TFIIE56, and RNA Pol II in the presence (+) or in the
absence (–) of TBP using either a wild-type (TATAAA) or a mutated (TAGAGA)
TATA element. In the example shown here (the photonucleotide derivative is placed
at position –15 of the adenovirus major late promoter), the crosslinking of both the
second largest subunit of RNA Pol II (RPB2) and RAP74 are considered to be specific
(dark arrowhead). The crosslinking of RPB1 is considered as nonspecific (open arrow-
head) because it is not affected by the omission of TBP or the use of a probe with a
mutation in the TATA box. The positions of the molecular weight markers (MW) are
indicated. (B) The use of truncated polypeptides as a tool to identify the photo-
crosslinked polypeptides. Crosslinking reactions were performed using TFIIB, RAP30,
TFIIE34, TFIIE56, and RNA Pol II in the presence (+) or in the absence (–) of TBP,

Photocrosslinking of RNA Pol II Complexes 391
2. The specific primer must be designed in such a manner that T
4
DNA polymerase
only adds a few nucleotides. In the example shown in Fig. 1, the incorporation is
restricted to positions –4 to –1 by omitting dATP from the reaction (see refs. 1–4
for additional examples). The success of this step can be monitored by analysis
of the reaction products on a sequencing gel.
3. The addition of dNTPs in large excess is crucial because it is necessary to limit
primer extension to the incorporation of standard dNTPs, preventing the addition
of any more radiolabeled and photoreactive nucleotides.
4. The use of a standard dark room is not necessary at this stage. As a rule, we find
that conditions that provide just enough light to be able to work are acceptable.
5. A conventional red light can be used.
6. An example of gel photoprobe purification is shown in Fig. 2. The position of the
band corresponding to the photoprobe can be easily identified because the size of
the DNA fragment generated by digestion with restriction enzymes is known.
7. Fresh probes (less than a week old) give the best results.
8. Because some of the general transcription factors and RNA Pol II bind non-
specifically to DNA, it is important to discriminate between specific and nonspe-
cific crosslinking signals. For the adenovirus major late promoter, it is
well-documented that mutations of two bases in the TATA box (TATAAA to
TAGAGA) completely abolish pre-initiation complex formation. The comparison
of crosslinking signals obtained with probes containing either a wild-type or a
mutated TATA box permits differentiation between specific and nonspecific sig-
nals. However, this strategy is not simple, as it doubles the number of probes to be
synthesized. Because the basic function of the TATA box is to bind TBP, the
specificity can be assessed by comparing crosslinking reactions performed in the
presence or the absence of TBP. The absence of TBP was found to give the same
result as the use of a probe with a mutated TATA box (1–4). An example is shown
in Fig. 3A. The crosslinking signal at the top of the gel (RPB1) is considered as
nonspecific because its intensity is not affected by either a mutation in the TATA box
or the absence of TBP. The two additional crosslinking signals (RPB2 and RAP74)
are specific because their intensities are significantly affected by both mutation of
the TATA box and the omission of TBP in the crosslinking reaction.
9. Identification of the photocrosslinked polypeptides is a central issue in the
method. The main difficulty comes from the fact that several factors have a M
r
between 30 and 40 kDa. At least three different means can be used to identify the
using full-length (1–517) or truncated (1–409) RAP74. The different mobilities of
RAP74 fragments are diagnostic for RAP74 contact at this promoter position. (C) Immuno-
precipitation of photocrosslinked polypeptides. Crosslinking reactions were performed
using TFIIB, RAP30, RAP74, TFIIE34, TFIIE56, and RNA Pol II in the presence (+)
or in the absence (–) of TBP. The photocrosslinked polypeptides were either processed
normally (first two lanes) or immunoprecipitated using an antibody directed against
RAP74 or a control antibody.
392 Robert and Coulombe
crosslinking signals. First, SDS-PAGE analysis provides direct information on
the size of photocrosslinked polypeptides (see Fig. 3A). Second, the use of trun-
cated forms of a factor can be useful. An example is shown in Fig. 3B in which
all factors included in the crosslinking reactions are the same, except for RAP74,
which is either full-length (lanes 1 and 2) or truncated in its C-terminus (lanes 3
and 4). The different fragments of RAP74 migrate with different mobilities,
allowing the identification of the photocrosslinked polypeptide. Third, the
photocrosslinked polypeptides can be identified after immunoprecipitation with
a specific antibody. Following nuclease treatment, the crosslinking products
are immunoprecipitated and then submitted to SDS-PAGE analysis. An example
is shown in Fig. 3C, where the photocrosslinked polypeptides shown in lane 1
have been immunoprecipitated using an antibody raised against RAP74 (lane 3)
or a control antibody (lane 4). This shows that the photocrosslinked polypeptide
is RAP74.
10. In the crosslinking reactions, we routinely use 200 ng of each recombinant
human (rh) TFIIB, rhRAP30, rhRAP74, rhTFIIE34, and rhTFIIE56, 100 ng of
calf thymus RNA Pol II, 50 ng of natural human TFIIH, 50–200 ng of rhTFIIA,
and 200 ng of recombinant yeast TBP. The amounts of the different protein fac-
tors should be optimized for each different combination of proteins and for each
protein preparation.
11. The poly(dI.dC–dI.dC) stock should be diluted just prior to use. The exact dilu-
tion should be determined experimentally in order to favor specific versus non-
specific signals without adversely affecting the intensity of the specific signals.
12. Irradiation time with UV light should be optimized by performing a time-course
with the particular system to be used. We use a UV Stratalinker 2400 (Stratagene)
with 254-nm bulbs.
13. From this point on, normal light conditions can be used.
14. The amounts of antibody to be used should be optimized. Purified antibodies
provide the best results.
15. Detailed procedures for SDS-PAGE electrophoresis have been described (e.g.,
see ref. 6).
16. The use of BioMax (Kodak) screens and films is recommended.
Acknowledgments
We thank members of our laboratory for valuable discussions and Will
Home for critical reading of the manuscript. B.C. is the recipient of funding
from the Medical Research Council of Canada, the Cancer Research Society
and the Fonds de la Recherche en Santé du Québec. F.R. holds a studentship
from the FCAR.
References
1. Coulombe, B., Li, J., and Greenblatt, J. (1994) Topological localization of human
transcription factors IIA, IIB, TATA box-binding protein and RNA polymerase
II-associated protein 30 on a class II promoter. J. Biol. Chem. 269, 19,962–19,967.
Photocrosslinking of RNA Pol II Complexes 393
2. Robert, F., Forget, D., Li, J., Greenblatt, J., and Coulombe, B. (1996) Topological
localization of transcription factors IIE and IIF immediately upstream the tran-
scription start site of a class II promoter. J. Biol. Chem. 271, 8517–8520.
3. Forget, D., Robert, F., Grondin, G., Burton, Z. F., Greenblatt, J., and Coulombe,
B. (1997) RAP74 induces promoter contacts of RNA polymerase II upstream and
downstream of a DNA bend at the TATA box. Proc. Natl. Acad. Sci. USA 94,
7150–7155.
4. Robert, F., Douziech, M., Forget, D., Egly, J. M., Greenblatt, J., Burton, Z. F., et
al. (1998) Wrapping of promoter DNA around the RNA polymerase II initiation
complex induced by TFIIF. Mol. Cell 2, 341–351.
5. Hampsey, M. (1998) Molecular genetics of the RNA polymerase II general tran-
scription machinery. Microbiol. Mol. Biol. Rev. 62, 465–503.
6. Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989) Molecular Cloning: A
Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory, Cold Spring
Harbor, NY.

UV Laser-Induced Protein–DNA Crosslinking 395
395
From:
Methods in Molecular Biology, vol. 148: DNA–Protein Interactions: Principles and Protocols, 2nd ed.
Edited by: T. Moss © Humana Press Inc., Totowa, NJ
27
UV Laser-Induced Protein-DNA Crosslinking
Stefan I. Dimitrov and Tom Moss
1. Introduction
1.1. The Method
Photochemical crosslinking is a powerful method for studying all types of
protein-nucleic acids interactions. In particular UV-induced crosslinking has
been successfully applied to the study of protein–DNA interactions (e.g., ref. 1, see
Chapters 23–26 and 43). Ultraviolet (UV) light is a zero-length crosslinking
agent. It is therefore not subject to the steric problems that can be associated
with chemical crosslinking agents and provides strong evidence for close pro-
tein–DNA interactions. However, crosslinking with conventional UV-light
sources requires exposure times ranging from minutes to several hours (e.g.,
see refs. 1–3), permitting protein redistribution and the crosslinking of UV-
damaged molecules. Because UV-laser irradiation is intense enough to induce
crosslinking after very short exposure times, artifactual crosslinking can be
avoided. The typically nanosecond or picosecond exposures times also allow
UV-laser-induced crosslinking to be applied to study the intermediate states in
rapid protein–DNA binding reactions (4,5).
Ultraviolet-crosslinking of protein to DNA occurs in two distinct steps. In
the first step, the bases of DNA are excited by light absorption. This excite-
ment rapidly gives rise to radicals of the bases, resulting in chemical cross-
linking and macromolecular damage, mainly to the DNA. The time to excite
the bases is simply the time of UV irradiation. Completion of the crosslinking
reaction then occurs in less than a microsecond (6). Most micro-conforma-
tional transitions of macromolecules take more than 100 µs (see ref. 7). Nano-
seconds or picosecond UV-laser irradiation therefore avoids almost all
possibility of artifactual rearrangement during the crosslinking reaction.
What is more, such a rapid crosslinking reaction can be used to freeze protein–
396 Dimitrov and Moss
DNA interactions, providing “time-lapse views” of the assembly of protein–
DNA complexes. Laser-induced reactions, as opposed to those generated by
conventional UV sources, proceed via the higher (S
n
and T
n
) excited states of
nucleotide bases, which are induced by the rapid sequential absorption of two
photons. This leads to a higher quantum yield of cationic radicals and to higher
crosslinking efficiencies (6,8–10). Although the photochemistry is still not
understood, UV-laser irradiation probably also induces mechanisms of
crosslinking that simply cannot occur when using conventional light sources
(6,10). In a typical UV crosslinking experiment, 5–15% of UV-laser-irradiated
protein–DNA complexes are crosslinked (6,10,11), nearly two orders of mag-
nitude higher than with conventional UV sources (4,5,8,10,12). UV-laser-
induced crosslinking produces exclusively protein–DNA adducts (4,6,8,9) and
is applicable to a broad range of protein–DNA complexes. Even complexes too
weak to be seen by methods such as gel shift (electrophoretic mobility shift
assay [EMSA]) or footprinting can be crosslinked. In fact, only nonspecific
protein–DNA interactions with association constants of less than 10
3
/M are not
crosslinked (4,6).
1.2. Practical Applications
Photocrosslinking induced by UV-laser irradiation has been applied to mea-
sure binding constants (6), to map the extent of protein–nucleic acid binding
sites (6), to determine protein–DNA (5,6) and protein–RNA (9) interactions,
and to identify protein–DNA contacts (13). It was found possible to study the
weak DNA-ATPase complex from the T4 DNA replication system by UV-
laser crosslinking despite this complex being invisible to DNase I footprinting
(6). UV-laser crosslinking has also provided data on the distribution of chro-
mosomal proteins in vivo (8). The presence of the histones and the high-mobil-
ity group 1 proteins on the Xenopus ribosomal DNA was determined and shown
to be regulated (8,14,15). Only the N-terminal domains of the histones were
found to be crosslinked to DNA, and hyperacetylation of these domains did not
affect their interaction with the DNA (14–16). An excellent guide to the prac-
tical application of in vitro laser protein–DNA crosslinking can be found in the
methodological review by von Hippel and co-authors (6). It is likely that new
developments in the use of femtosecond lasers will provide important improve-
ments to the use of UV-laser crosslinking (17 and see Chapter 63).
1.3. The Experimental Approach
Here, we describe a procedure used to induce histone–DNA crosslinking in
cell nuclei and to determine the DNA sequence distribution of the various his-
tone fractions. The procedures used are quite general and could well be applied
to study any type of protein–DNA complex. Indeed, we have essentially used a
UV Laser-Induced Protein–DNA Crosslinking 397
similar approach to determine the kinetics of the TBP–polymerase II promoter
interaction (11).
1. UV-laser irradiation of nuclei.
2. Isolation of the crosslinked protein–DNA complexes (see Note 1).
3. Detection of specific proteins crosslinked to bulk DNA using immunochemi-
cal techniques.
4. Immunoprecipitation of the crosslinked protein–DNA complex.
5. Identification and quantitation of the DNA sequences covalently attached to a
given protein using hybridization with specific DNA probes.
2. Materials
2.1. Laser Irradiation
When using a passively mode-locked picosecond neodymium–yttrium–
aluminum–garnet (Nd:YAG) laser (8), the parameters of the laser radiation
at 266 nm were as follows: pulse duration, 30 ps (in a Gaussian pulse shape
assumption), pulse energy 4 mJ; diameter of the beam, 0.5 cm; repetition
rate 0.5 Hz. The intensity of irradiation was controlled by focusing and
defocusing using fused silica lenses. The energy of radiation was measured
with pyroelectrical detectors calibrated with a Model Rj7200 energy meter
(Laser Precision Corp.). (The Nd:YAG lasers produce pulsed radiation at
1064 nm and the conversion to 266 nm [wavelength at which the samples
are irradiated] was performed by quadrupling the main frequency by means
of angle-matched KDP crystals.) A nanosecond Nd:YAG laser (Model
DCR-3J, Spectra-Physics Inc. [Mountain View, CA] or YAGMaster
YM1000/1200, Lumonics [Canada]) can also be used (6,11). Pulses in the
UV are about 5 ns in duration, and the energy per pulse at 266 nm was
typically 80 mJ (see Note 2).
2.2. Reagents and Solutions
2.2.1. Procedures Described in Subheading 3.1.
1. 8 M urea.
2. 1% sodium dodecyl sulfate (SDS).
2.2.2. Procedures Described in Subheading 3.2.
1. 10 mM Tris-HCl, pH 7.5, and 1 mM CaCl
2
.
2. 0.48 M Na phosphate buffer, pH 6.8.
3. 0.12 M Na phosphate buffer, pH 6.8.
4. 0.12 M Na phosphate buffer (pH 6.8) 2 M NaCl, and 5 M urea.
5. CsCl.
6. Microccocal nuclease.
7. Hydroxyapatite (Bio-Rad).
398 Dimitrov and Moss
2.2.3. Procedures Described in Subheading 3.3.
1. Phosphate-buffered saline (PBS).
2. PBS–0.05% Triton X-100 (PBS-T).
3. 1% bovine serum albumin (BSA) in PBS-T.
4. PBS, 0.4% Triton X-100.
5. 0.3% 4-chloronaphtol, 0.03% H
2
O
2
in 50 mM Tris-HCl, pH 7.5, and 150 mM NaCl.
6. Nitrocellulose filter (Schleicher & Schuell).
2.2.4. Procedures Described in Subheading 3.4.
1. IgGsorb (The Enzyme Center, Malden, MA).
2. Antibody buffer: 50 mM HEPES, pH 7.5, 2 M NaCl, 0.1% SDS, 1% Triton X-100,
1% Na-deoxycholate, 5 mM EDTA, and 0.1% BSA.
3. Rinse buffer: 50 mM HEPES, pH 7.5, 0.15 M NaCl, 5 mM EDTA.
4. 3.5 M KSCN and 20 mM Tris-HCl, pH 7.5.
5. RNase A (1 mg/mL).
6. Pronase (1 mg/mL).
7. Ethanol.
8. 10 mM Tris-HCl, pH 7.5, and 0.25 mM EDTA.
2.2.5. Procedures Described in Subheading 3.5.
1. Zeta Probe blotting membranes (Bio-Rad).
2. Pre-hyb buffer: 6X SSC, 10X Denhardt’s solution, 0.1 mg/mL denatured
Escherichia coli DNA, 1% SDS, 0.2% Na-pyrophosphate, and 50% formamide.
3. 0.5X SSC and 0.5% SDS.
4. 0.1% SSC.
3. Methods
3.1. Irradiation Techniques
One to two milliliters of the nuclei/cell suspension are placed in a standard rect-
angular fused silica cuvet, thermostated at 4°C and the sample constantly stirred
(see Note 3). The optical density of the solution should be kept in the range of
2 <A
260
<5 (i.e., optically thick samples (see Note 4). In the case of a picosecond
laser, the conditions of irradiation should be such that 10–20 photons are absorbed
per nucleotide (about 500 mJ of incident light per 1 OD
260
of optically thick sample)
at a constant laser intensity 0.7 GW/cm
2
. In the case of the nanosecond UV laser
use 250 mJ of incident light per 1 A
260
of optically thick sample. Dependent on the
power of the laser used, it may be necessary to irradiate with multiple pulses.
3.2. Separation of Covalently Crosslinked
Histone–DNA Complexes
1. Digest the irradiated nuclei with microccocal nuclease (5 U/A
260
unit, 15 min,
37°C) in 10 mM Tris-HCl, pH 7.5, 1 mM CaCl
2
This is simply to reduce the DNA
size (see Notes 1 and 5).
398 Dimitrov and Moss
2.2.3. Procedures Described in Subheading 3.3.
1. Phosphate-buffered saline (PBS).
2. PBS–0.05% Triton X-100 (PBS-T).
3. 1% bovine serum albumin (BSA) in PBS-T.
4. PBS, 0.4% Triton X-100.
5. 0.3% 4-chloronaphtol, 0.03% H
2
O
2
in 50 mM Tris-HCl, pH 7.5, and 150 mM NaCl.
6. Nitrocellulose filter (Schleicher & Schuell).
2.2.4. Procedures Described in Subheading 3.4.
1. IgGsorb (The Enzyme Center, Malden, MA).
2. Antibody buffer: 50 mM HEPES, pH 7.5, 2 M NaCl, 0.1% SDS, 1% Triton X-100,
1% Na-deoxycholate, 5 mM EDTA, and 0.1% BSA.
3. Rinse buffer: 50 mM HEPES, pH 7.5, 0.15 M NaCl, 5 mM EDTA.
4. 3.5 M KSCN and 20 mM Tris-HCl, pH 7.5.
5. RNase A (1 mg/mL).
6. Pronase (1 mg/mL).
7. Ethanol.
8. 10 mM Tris-HCl, pH 7.5, and 0.25 mM EDTA.
2.2.5. Procedures Described in Subheading 3.5.
1. Zeta Probe blotting membranes (Bio-Rad).
2. Pre-hyb buffer: 6X SSC, 10X Denhardt’s solution, 0.1 mg/mL denatured
Escherichia coli DNA, 1% SDS, 0.2% Na-pyrophosphate, and 50% formamide.
3. 0.5X SSC and 0.5% SDS.
4. 0.1% SSC.
3. Methods
3.1. Irradiation Techniques
One to two milliliters of the nuclei/cell suspension are placed in a standard rect-
angular fused silica cuvet, thermostated at 4°C and the sample constantly stirred
(see Note 3). The optical density of the solution should be kept in the range of
2 <A
260
<5 (i.e., optically thick samples (see Note 4). In the case of a picosecond
laser, the conditions of irradiation should be such that 10–20 photons are absorbed
per nucleotide (about 500 mJ of incident light per 1 OD
260
of optically thick sample)
at a constant laser intensity 0.7 GW/cm
2
. In the case of the nanosecond UV laser
use 250 mJ of incident light per 1 A
260
of optically thick sample. Dependent on the
power of the laser used, it may be necessary to irradiate with multiple pulses.
3.2. Separation of Covalently Crosslinked
Histone–DNA Complexes
1. Digest the irradiated nuclei with microccocal nuclease (5 U/A
260
unit, 15 min,
37°C) in 10 mM Tris-HCl, pH 7.5, 1 mM CaCl
2
This is simply to reduce the DNA
size (see Notes 1 and 5).
UV Laser-Induced Protein–DNA Crosslinking 399
2. Add 0.12 M phosphate buffer (pH 6.8) 2 M NaCl, 5 M urea to stop the reaction
a. Load the material on a hydroxyapatite column (1 g hydroxyapatite/mg DNA)
equilibrated with 0.12 M phosphate buffer (pH 6.8) 2 M NaCl , 5 M urea.
b. Wash the column with 5 vol of the same buffer, then with 0.12 M phos-
phate buffer.
c. Elute the free DNA and the crosslinked complex with 0.48 M phosphate
buffer, pH 6.8.
4. Apply the eluted material on a preformed CsCl gradient (four layers, 2.2 mL
each, density (ρ) = 1.76, 1.57, 1.54, and 1.32 g/mL) and run in a SW 41 Beckman
rotor at 15°C for 35–40 h at 35,000 rpm.
5. Collect 250-µL fractions and monitor optical density at 260 nm.
6. The gradient profile should show a clear shoulder to the light side of the major
(free-DNA) peak. This shoulder is a highly enriched fraction of protein–DNA
crosslinked complexes.
7. Collect the material from the peak (or, better, from the shoulder only) and dialyze
extensively against 10 mM Tris-HCl, pH 7.5, and 0.25 mM EDTA.
3.3. Dot Immunoassay
for Abundant Crosslinked Protein–DNA Complexes
1. Dot the crosslinked material (about 0.5 µg DNA) on nitrocellulose filters.
2. Wash filters twice for 5 min in PBS-T with gentle shaking to remove
unbound antigen.
3. Repeat step 2 with PBS only.
4. Block the filters in BSA (1% in PBS-T) for 1 h at 37°C.
5. Incubate overnight at 4°C with a suitable dilution of specific antibody in PBS-T,
and 1% BSA.
6. Wash three times with PBS-T then three times with PBS only with gentle
shaking.
7. Incubate with peroxidase-conjugated goat anti-rabbit IgG (Sigma, 1:1000 dilu-
tion) in PBS containing 1% BSA for 4 h at 37°C.
8. Repeat step 6.
9. Develop filters in 0.3% 4-chloro-1-naphtol, 0.03% H
2
O
2
in 50 mM Tris-HCl,
pH 7.5, and 150 mM NaCl.
3.4. Immunoprecipitation of Crosslinked Protein–DNA Complex
1. Suspend 0.05 mL IgGsorb in 0.5 mL of 1% BSA in PBS and shake for 30 min at
room temperature to block the sites of nonspecific absorption.
2. Centrifuge for 30 s in a microcentrifuge (see Note 8) and suspend IgGsorb
directly in 0.5 mL of a mixture of the specific antibody and the crosslinked DNA–
protein complexes (w:w ratio 1:2.5) in antibody buffer (20–50 µg of crosslinked
material, see Note 9).
3. Shake for 2 h at room temperature.
4. Recover IgGsorb by microcentrifugation and resuspend in 0.5 mL antibody
buffer. Repeat this procedure five times.
